INTRODUCTION
Being one of the most frequently occurring foodborne diseases in the western world, human salmonellosis continues to be a significant burden on public health, although overall numbers of infections have been decreasing during the past decade in Germany [Reference Frank1]. Less than 20% of reported cases are linked to outbreaks, but most Salmonella infections are sporadic [2] and the source of infection remains unidentified. In 2009, almost half of the 20 500 reported infections in Germany related to underage cases [2]. Due to their immature immune system, children are predisposed to higher incidences of gastrointestinal infections and are at higher risk of complications compared to adults [Reference Coburn, Grassl and Finlay3, Reference Shimoni4]. More knowledge about the epidemiology of salmonellosis could lead to additional interventions to reduce its burden. At present, most of our understanding still comes from outbreak investigations which are of limited use for explaining the epidemiology of sporadic infections, especially in children [Reference Haddock5].
Recent studies investigating specific risk factors for children suggested that contaminated environmental sources contribute more than contaminated food vehicles to the acquisition of Salmonella infections in children [Reference Haddock5–Reference Delarocque-Astagneau10]. This is probably due to the fact that children have a more limited dietary exposure to foodborne pathogens compared to adults and, due to particular behaviours especially in small children (crawling, putting things and hands in mouth), their environmental exposures also differ [Reference Jones11]. To elucidate risk factors for sporadic salmonellosis in children we analysed data from cases aged ⩽14 years that were collected during a case-control study between June 2008 and May 2010 in Lower Saxony, Germany. Within the entire study, data collection was performed for all ages, and data from children aged ⩾15 were incorporated in the previously published analyses of adults [Reference Ziehm12, Reference Ziehm13]. In the present paper, we investigated risk factors for sporadic salmonellosis in children aged 0–3 years and 4–14 years.
METHODS
Subjects
Sampling of case and control persons was performed between June 2008 and May 2010 from all Salmonella cases in Lower Saxony reported within the German infectious disease notification system according to the Protection against Infection Act. Local health departments (LHDs) contacted next-of-kin (usually mother or father) of minors with laboratory-confirmed Salmonella infection and asked them to participate in the study. To ensure that only sporadic cases were included, case-patients were not selected if they were part of an outbreak identified by the LHDs' on-site investigations or if they affirmed a question about the recent occurrence of diarrhoea among their contact persons. As controls, we recruited rotavirus cases via the same notification system. Controls were selected from all persons with laboratory-confirmed rotavirus infections, who were not associated with a reported outbreak. Subjects with laboratory confirmed co-infection of rotavirus and Salmonella were not found. In both cases and controls, reason for exclusion from the study was onset of diarrhoea during a hospital stay. If communication was not possible due to language problems or mental state or if consent could not be obtained, persons were also excluded.
Variables
The interviews were based on a standardized questionnaire, as described in detail by Ziehm et al. [Reference Ziehm12, Reference Ziehm13]. Informed consent was obtained from the parent or guardian of the underage case prior to administering the questionnaire. Data on various environmental exposures and food exposures during the 3 days preceding the illness both in cases and rotavirus controls were collected. In particular, it was assessed whether dogs, cats, rodents, reptiles, cattle, small ruminants, pigs or birds were kept in the same household or whether the index person had contact with these animals (e.g. in a petting zoo) 3 days prior to onset of symptoms. The questionnaire also collected information on special buying habits (e.g. local stores, farmers' markets) or preferences (barn eggs or floor eggs; meat from the counter or packed).
Several items from the questionnaire were combined for the analysis, for example the variable ‘consumption of raw egg’ comprised consumption of mayonnaise, raw dough, home-made gravy with raw egg and desserts containing raw eggs (e.g. tiramisu). Fried eggs, scrambled eggs, soft-boiled eggs and pancakes were summarized as ‘food items containing undercooked eggs’. The variable ‘food with pasteurized egg’ comprised cream cake, ready-made gravy with powdered egg (convenience) and ice cream.
Statistical analysis
Data were managed using Microsoft Access2003 (Microsoft Corporation, USA) as the storage database. After controlling for plausibility as described previously [Reference Ziehm12, Reference Ziehm13], all variables were provided as dichotomous variables. Univariable and multivariable risk factor analysis was performed using a statistical software package (Stata Statistical Software, release 12, StataCorp LP, USA). In Lower Saxony, only 15% of children aged <3 years visit daycare facilities, whereas from the age of 4 years onwards, 93% are attending kindergarten, pre-schools and schools for more than 25 hours per week [14]. Therefore, data were analysed separately for children aged 0–3 years and 4–14 years. Single risk factor analysis of 83 variables was performed by Mantel–Haenszel combined odds ratios (OR) for each variable, using season as the stratification variable, accompanied by Wald's 95% confidence interval (CI) and two-sided P values. Values of P ⩽ 0·05 were considered significant. Based on the outcome of the univariable analyses, variables with OR > 1 (stratified by seasonality) and P < 0·3 and more than four exposed subjects in each group were selected for the multivariable logistic regression analyses. Multivariable logistic regression models were adjusted for age and summer season to control for possible confounding effects. The cut-off value for step-wise backward elimination of non-significant variables from the model was P ⩾ 0·3. For the multivariable analyses, several composite variables were constructed, which combined several variables from the univariable analysis. For example the variable ‘animal contact’ was constructed by combining the variables ‘dog contact’, ‘rodent contact’, etc. This was to avoid covariation within the exposure variables as well as to ensure the power of the analyses due to lower frequencies in the stratified contingency tables. Models with a certain composite variable (‘reduced models’) were compared to full models via likelihood ratio tests. Both models comprised the same combination of all other variables, i.e. if a reduced model with the variable ‘keeping animals’ was compared to a full model with the variables ‘keeping dog’, keeping cat’ ‘keeping rodent’, both models contained all other variables (e.g. food items) in the same combination.
Ethics approval
The study was exempted from ethics approval by the ethics committee of the Hannover Medical School.
RESULTS
Based on our inclusion criteria, 1284 cases with sporadic salmonellosis were selected and 884 (69%) telephone interviews were conducted successfully. Consent could not be obtained in 169 cases (13%), 20 persons (2%) were unable to communicate due to language problems or mental state and 101 cases (8%) could not be contacted via telephone after six attempts on different days and at different time points. In the rotavirus controls, out of the 1015 participants that met the inclusion criteria, 510 (50%) were interviewed successfully, consent for interview was not given 181 times (18%), 25 (3%) people had communication problems and 298 cases (29%) could not be contacted via telephone. Overall, this resulted in an entire dataset of 1394 participants. The 0–3 years age group consisted of 738 children in total (331 cases, 407 controls) with 49% and 51% males in cases and controls, respectively. Mean ages ( ± s.d.) were 1·7 ( ± 1) years in cases and 1·7 ( ± 0·8) years in controls. The 4–14 years age group consisted of 656 children in total (553 cases, 103 controls), with 44% and 43% males in cases and controls, respectively. Mean ages ( ± s.d.) in this group were 8·1 ( ± 3) years in cases, and 6·1 ( ± 2·4) years in controls.
In both age groups, the highest overall OR were found for consumption of raw ground pork in the univariable analysis. Consumption of raw ground pork, however, was not included in the multivariable analyses, as there were fewer than four exposed subjects in controls of both age groups. In children aged 0–3 years, multivariable analysis revealed further significant associations with the disease for consumption of food items containing pasteurized eggs, poultry and black pepper as well as animal contact. Exposure to cattle, pigs, cats and dogs and animal contact without subsequent washing of hands were significantly (P < 0·05) associated with sporadic salmonellosis in this age group in the univariable analysis and therefore included in the multivariable analysis. Furthermore, exposure to rodents and reptiles (P < 0·3 in the univariable analysis for both variables) were also considered in the multivariable analysis. After comparison via ratio likelihood test, only one composite variable for animal contact was included in the final model. An overview of the results from the univariable and multivariable analyses of children aged 0–3 years is given in Table 1. In children aged 4–14 years, besides raw pork consumption, also consumption of uncooked pork sausage was significantly associated with sporadic salmonellosis. In the multivariable analysis we tested consumption of uncooked pork sausage together with age, summer season, and all other variables with P < 0·3 in the univariable analysis (animal contact without subsequent washing of hands, keeping a cat, dog contact, rodent contact, consumption of poultry, undercooked eggs, barn eggs and fresh herbs, handling eggs without subsequent washing of hands and handling meat without subsequent washing of hands, knives, cutting boards, etc.). Results from the univariable and multivariable analyses of children aged 4–14 years are given in Table 2.
Table 1. Univariable and multivariable analysis of selected exposures associated with sporadic salmonellosis in a case-control study in children aged 0–3 years in Lower Saxony, 2008–2010. Variables that were significantly (P ⩽ 0·05) associated with the disease appear in bold
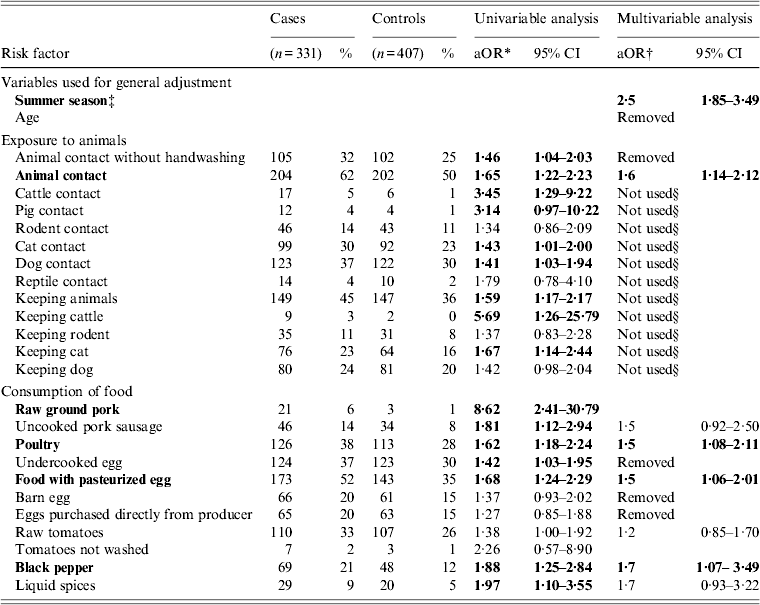
* Odds ratio stratified by season.
† Odds ratio adjusted for season and age.
‡ Onset of symptoms between April and September.
§ Variable integrated in composite variable ‘animal contact’.
Table 2. Univariable and multivariable analysis of selected exposures associated with sporadic salmonellosis in a case-control study in children aged 4–14 years in Lower Saxony, 2008–2010. Variables that were significantly (P ⩽ 0·05) associated with the disease appear in bold

* Odds ratio stratified by season.
† Odds ratio adjusted for season and age.
‡ Onset of symptoms between April and September.
§ Composite variable not tested in final model.
¶ Variable integrated in variable ‘rodent contact’.
∥ Preparation of meat without subsequent washing of hands, knives, cutting boards, etc.
# Handling of eggs without subsequent washing of hands.
DISCUSSION
A study performed 2004 in north-west Germany identified Salmonella spp. as the most frequently detected bacteria in medical care-seeking patients with acute gastroenteric symptoms and reported highest incidences in children aged 0–14 years [Reference Karsten15]. This illustrates that human salmonellosis continues to be a substantial burden on public health, especially in children. The present investigation therefore aimed at identifying risk factors for sporadic salmonellosis in children. Data were collected between June 2008 and May 2010 in a case-control study from a subset of Salmonella cases reported within the German infectious disease notification system according to the Protection against Infection Act in Lower Saxony [Reference Ziehm12, Reference Ziehm13]. Analysing data from cases aged ⩽14 years revealed highest OR for consumption of food items containing raw ground pork (‘Mett’ in German). These findings indicate that raw pork is a relevant source for sporadic Salmonella infections in children in Germany. Eating Mett is very popular in certain regions in Germany, especially the north and east. Mett is similar to steak tartare – raw pork is finely minced or ground, sometimes mixed with chopped onions, and then used as a spread on bread. Uncooked pork sausage is called ‘Mettwurst’ and consists of Mett with spices in a sausage skin. It is usually fermented or smoked, but only lightly. A statistically significant association between consumption of uncooked pork sausage and Salmonella infection was also confirmed by multivariable analysis of data from children aged 4–14 years. Salmonella spp. are prevalent in both pork sold by retail (0·4%) and ground pork (1·3%) in Germany [16], and consumption of food items containing raw ground pork has also been identified previously as a major source for Salmonella infections in adults of the same population [Reference Ziehm12, Reference Ziehm13].
To our knowledge in children, an association between raw pork consumption and sporadic salmonellosis has not yet been described. Rosner et al. [Reference Rosner17] identified consumption of raw or undercooked pork as the main driver of yersiniosis in children in Germany and state that exposure to raw minced pork was unexpectedly frequent even in very young children. Similarly, our findings in both age groups also suggest that raw pork products are fed regularly and deliberately to children aged <15 years of age in Lower Saxony, and strongly suggest that exposure to such food items accounts for sporadic salmonellosis in both age groups. Furthermore, in the 0–3 years age group, food items containing pasteurized eggs, such as cream cake, ready-made gravy with powdered egg (convenience) and ice cream were identified as risk factors via multivariable analysis. As pasteurization is supposed to inactivate Salmonella spp., this finding has to be interpreted carefully. Aside from pasteurized eggs, these food items can also include other potentially contaminated products and the possibility of secondary contamination should also be considered. Associations between Salmonella infection and egg consumption are well known [Reference Cowden18] and have resulted in both legal actions as well as measures to increase public awareness in the past. Routine testing in 2011 revealed that about 2% of all laying hen flocks in Germany host Salmonella spp., and 0·03% of all eggs sold to consumers are contaminated [16]. In contrast to these low percentages, egg consumption still seems to be a certain risk factor in children [Reference Delarocque-Astagneau10]. On the other hand, the low or even missing associations between salmonellosis and egg consumption in adults and older children, especially for raw or undercooked eggs (this study, [Reference Ziehm12]) are in line with the assumption that contaminated egg products are of decreasing relevance for Salmonella infections in Germany nowadays [Reference Frank1].
Regarding the type of eggs bought predominantly in the respective households, univariable analysis revealed a statistically significant association between barn eggs and Salmonella infection, which is difficult to interpret. Studies investigating the influence of the animals' housing system on Salmonella prevalence in laying hens yielded ambiguous results [Reference van Hoorebeke19], and Salmonella load at point of sale is usually not higher in barn eggs compared to eggs from other housing systems [16]. A possible explanation for our finding could be that barn eggs which are cheaper than free-range eggs or organic eggs, are used more often as ingredients in egg-containing food items, for example cakes or pastries. This would imply pooling of eggs and prolonged handling at room temperature during preparation [Reference Mokhtari20]. These procedures would also facilitate cross-contamination of other foods [Reference Bradford, Humphrey and Lappin-Scott21]. Similar to eggs, Salmonella load in chicken meat and other poultry products has been decreasing over the last decades [16]; however, there still seems to be a certain risk of infection via these food items for small children [Reference Jones11].
Aside from food items of animal origin, vegetables, sprouts, nuts and chocolate have recently been identified as sources for Salmonella infections [Reference Fatica and Schneider22, Reference Podolak23]. Testing a variety of produce and spices, our own analyses identified consumption of black pepper as a risk factor for sporadic salmonellosis in children aged 0–3 years. Although contaminated black pepper has been identified previously as the source of Salmonella outbreaks [Reference Gieraltowski24], we doubt that black pepper is responsible for the sporadic infections in our study. Spice pepper is usually not consumed by itself but in combination with either raw or cooked food items, which makes interpretation of this finding difficult and indicates that the risk potential of non-animal-related food items requires further research in the future. Besides foodborne infections, direct or indirect animal contact is a potential source for human salmonellosis. Domestic livestock as well as companion animals have been found to host and shed various Salmonella spp., often without clinical manifestation of the disease, thereby contributing to human infection [Reference Hoelzer, Moreno and Wiedmann25]. Our analysis revealed that exposure to animals is associated with sporadic salmonellosis in children aged 0–3 years, whereas in children aged 4–14 years, significant associations between salmonellosis and exposure to animals were not detected. This suggests an age-dependent susceptibility to direct zoonotic transmission, with smaller children being at higher risk of infection via animal exposure. Accordingly in adults, no association between animal exposure and salmonellosis was found [Reference Ziehm12]. In children aged 0–3 years, besides exposure to cattle, cats and dogs, pig contact seemed to be associated with Salmonella infection as well but the association lacked significance due to the low number of exposed subjects. Salmonella infections in children via contact with pigs are rarely described in literature [Reference Steinmuller26], although Salmonella spp. are regularly isolated from domestic livestock in Germany during routine testing [16]. In contrast to our expectations, exposure to reptiles was not significantly associated with the disease. It is estimated that about 90% of all pet reptiles host Salmonella spp. [Reference Woodward, Khakhria and Johnson27], thereby representing a substantial risk factor for human salmonellosis, especially in children [Reference Jones11, Reference Ward28]. It is now generally recommended not to keep reptiles in households with children aged <5 years [Reference Weiss29] and perhaps due to this advice, in both age groups overall exposures did not exceed 4% in the present study.
The impact of Salmonella transmission in private households or daycare centres, which has previously been identified as one of the predominant sources of Salmonella infection in children, especially in infants [Reference Chen6, Reference Delarocque-Astagneau10, Reference Delarocque-Astagneau30, Reference Younus31] was not assessed in the present study, because our control group consisted of children with laboratory-confirmed rotavirus infection and therefore, similar symptoms (e.g. diarrhoea) would have occurred in contact persons of both case and control groups. Using rotavirus cases as a control group has some strong advantages compared to using population-based controls only. Our controls were recruited from the same notification system as the Salmonella cases, which reduces the risk of selection bias due to differential access to medical care or socioeconomic status [Reference Tam, Rodrigues and O'Brien32]. Furthermore, compliance of cases and controls was similar, reflected for example in equal response rates for the interviews and similar overall interview duration. A certain limitation for using rotavirus cases as a control group is an expected selection due to individual pre-existing conditions which might promote infection and diagnosis of rotavirus. Therefore, the influence of pre-existing immunosuppressive conditions or medical treatment for Salmonella infections was not considered in our analysis. Overall, we mainly focused on possible associations of sporadic salmonellosis with animal contact or certain food items. Rotavirus infection is usually not considered a zoonosis [Reference Cook33] and transmission of the disease via animals as well as foodborne infections are thus unlikely. We therefore assumed our rotavirus control group to be an adequate control for the investigated risk factors. The positive associations that we found in the present study constitute new and interesting findings in regard to sources for sporadic salmonellosis in children in Germany. Raw ground pork consumption stood out as a major risk factor for the disease in both age groups. These results reflect previous findings in adults [Reference Ziehm12, Reference Ziehm13] and underline the significance of raw ground pork consumption for Salmonella infections among the German population. Across Europe, pork is a substantial contributor to human salmonellosis [Reference Hansen, Christensen and Aabo34] and the importance of these food items has only been recognized recently [Reference Jansen, Frank and Stark35]. To our knowledge, studies aimed at investigating an association between raw pork consumption and sporadic salmonelloses in children have not yet been conducted.
Recommendations
Our current findings certainly have major implications for public health: products containing raw pork meat (‘Mett’) are popular food items in Germany and public education should highlight their potential risk. Besides children, also immunocompromised or elderly persons, who are particularly vulnerable for a severe clinical course of salmonellosis should reduce their infection risk by avoiding the consumption of raw and briefly fermented pork products [Reference Jansen, Frank and Stark35]. At the same time, efforts to reduce both Salmonella load in pigs and other livestock as well as cross-contamination during slaughter and food processing should certainly be continued and intensified. Despite a further reduction of total numbers of Salmonella-positive pigs and pork products that could be achieved via good manufacturing strategies, there will probably always be transmission of Salmonella spp. to consumers, even when slaughterhouses, cutting plants and butchers work under optimum conditions [Reference Berends36, Reference Davies37]. In conclusion, communicating the potential risk of feeding small children with raw ground pork, not only to parents but also to general practitioners and people working in child-care might perhaps be at present the most effective intervention to mitigate the burden of the disease. Reducing the children's exposure to raw ground pork would at the same time decrease the number of infections with other pork-associated pathogens such as Y. enterocolitica [Reference Rosner17] and Shiga toxin-producing Escherichia coli [Reference Werber38].
ACKNOWLEDGEMENTS
We thank the participating health departments, the children and their parents who answered our questionnaires. We also thank Ina Holle and Nicola Jahn for their valuable technical assistance and Manuela Harries for stimulating discussions. This study was funded by means of the German Federal Ministry of Education and Research (BMBF) through the German Aerospace Center (DLR), grant number 01KI07128 (FBI-Zoo).
DECLARATION OF INTEREST
None.





