Introduction
Land-use change and agricultural expansion endanger the integrity of the most diverse terrestrial ecosystems of our planet, the tropical forests (Lewis et al. Reference Lewis, Edwards and Galbraith2015). Different strategies have been proposed to achieve long-term conservation of biodiversity in human-modified tropical landscapes (Arroyo-Rodríguez et al. Reference Arroyo-Rodríguez, Fahrig, Tabarelli, Tischendorf, Benchimol, Cazetta, Faria, Leal, Melo, Morante-Filho, Santos, Arasa-Gisbert, Arce-Peña, Cervantes-López, Cudney-Valenzuela, Galán-Acedo, San-José, Vieira, Slik, Nowakowski and Tscharntke2020, Soley & Perfecto Reference Soley and Perfecto2021). While some strategies stress the importance of protected forest remnants to conserve biodiversity, others stress the importance of wildlife-friendly agricultural matrices (i.e., land-sharing versus land sparing; Grass et al. Reference Grass, Loos, Baensch, Batáry, Librán-Embid, Ficiciyan, Klaus, Riechers, Rosa, Tiede, Udy, Westphal, Wurz and Tscharntke2019). Contrasting strategies have generated much debate, but the emerging view is that they should be complementary (Grass et al. Reference Grass, Loos, Baensch, Batáry, Librán-Embid, Ficiciyan, Klaus, Riechers, Rosa, Tiede, Udy, Westphal, Wurz and Tscharntke2019, Arroyo-Rodríguez et al. Reference Arroyo-Rodríguez, Fahrig, Tabarelli, Tischendorf, Benchimol, Cazetta, Faria, Leal, Melo, Morante-Filho, Santos, Arasa-Gisbert, Arce-Peña, Cervantes-López, Cudney-Valenzuela, Galán-Acedo, San-José, Vieira, Slik, Nowakowski and Tscharntke2020). Thus, the problem is not deciding which strategy is the best, but how to combine them most effectively for a given context and conservation target. To be able to do this, we need to increase our understanding of the value that agricultural covers in tropical landscapes have as habitat for different groups of organisms.
In the tropics many important cash crops can be grown under a canopy of trees (e.g., coffee, cocoa, cardamom, allspice, etc.), and these shaded agroecosystems have received much attention as potential habitat for biodiversity (Martin et al. Reference Martin, Osen, Grass, Hölscher, Tscharntke, Wurz and Kreft2020). Yet, different groups of organisms are not evenly represented in these studies. Among animals, there is a strong bias towards birds, mammals, and some insect taxa, while other groups, such as amphibians and reptiles, tend to be underrepresented (Palacios et al. Reference Palacios, Agüero and Simonetti2013). Many species of amphibians and reptiles are highly sensitive to habitat loss and disturbance, which are main drivers of their population declines worldwide (Nori et al. Reference Nori, Lemes, Urbina-Cardona, Baldo, Lescano and Loyola2015, Doherty et al. Reference Doherty, Balouch, Bell, Burns, Feldman, Fist, Garvey, Jessop, Meiri and Driscoll2020). Furthermore, these animal groups are rarely considered in conservation planning and the geographic distributions of many species are not covered by protected areas (Nori et al. Reference Nori, Lemes, Urbina-Cardona, Baldo, Lescano and Loyola2015). This stresses the importance of assessing the conservation value of agroforests and other anthropogenic vegetation covers to help maintain the herpetofauna in human-modified landscapes (Palacios et al. Reference Palacios, Agüero and Simonetti2013, Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015).
Shaded agroecosystems, even those producing the same crop, can vary tremendously in the ways they are managed, which in turn affects their value as habitat for different groups of organisms (Deheuvels et al. Reference Deheuvels, Rousseau, Soto Quiroga, Decker Franco, Cerda, Vílchez Mendoza and Somarriba2014, Santos-Heredia et al. Reference Santos-Heredia, Andresen, Zárate and Escobar2018, Bennett et al. Reference Bennett, Sillett, Rice and Marra2021). Furthermore, habitat requirements differ greatly among taxa and functional groups, with their responses to shaded agroecosystems varying accordingly (e.g., Faria et al. Reference Faria, Paciencia, Dixo, Laps and Baumgarten2007, Borkhataria et al. Reference Borkhataria, Collazo, Groom and Jordan-Garcia2012, Bennett et al. Reference Bennett, Sillett, Rice and Marra2021). Thus, it is not surprising that studies of herpetofauna in shaded agroecosystems report variable responses (Palacios et al. Reference Palacios, Agüero and Simonetti2013). For example, while the decrease in abundance of some arboreal and terrestrial anurans seems to be directly related to loss of canopy cover in shade-coffee plantations (Pineda & Halffter Reference Pineda and Halffter2004), this same vegetation change may promote the abundance of certain lizard species (Macip-Ríos & Muñoz Reference Macip-Ríos and Muñoz2008). Similarly, some studies have found higher species richness in forest compared to shaded cocoa plantations (Lieberman Reference Lieberman1986, Heinen Reference Heinen1992), whereas others have found the contrary (Whitfield et al. Reference Whitfield, Bell, Philippi, Sasa, Bolaños, Chaves, Savage and Donnelly2007). Such inconsistencies underscore the need to carry out context-specific studies to guide conservation actions. Knowing which characteristics of an agroecosystem affect amphibian and reptile populations and communities has important management implications, since these variables could be manipulated to favor target taxa (Wanger et al. Reference Wanger, Saro, Iskandar, Brook, Sodhi, Clough and Tscharntke2009, Rodríguez Leiva et al. Reference Rodríguez Leiva, Jiménez Romero, Peralta Tercero and Montalván Castellón2014).
Our objective was to determine if rustic cocoa plantations under very low use-intensity could be suitable habitat for amphibians and/or reptiles in the anthropogenic landscapes of a Neotropical biodiversity hotspot, the Lacandona rainforest. To address our objective we asked three questions: (i) How do the cocoa and forest habitats differ in environmental variables that are relevant for herpetofauna?; (ii) How do these habitats differ in the abundance, number of species and assemblage composition of amphibians and reptiles?; (iii) Which environmental variables are more strongly related to the abundance and/or the number of species within each habitat? Land-use change is dramatically threatening the ecological integrity of the Lacandona region in southern Mexico, and it is urgent to design landscapes that can be productive whilst conserving the immense biodiversity of the region (Zermeño-Hernández et al. Reference Zermeño-Hernández, Pingarroni and Martínez-Ramos2016). We hope that our results can help managers and conservationists design such landscapes for amphibians and reptiles.
Methods
Study site
This study was carried out in the Lacandona rainforest region, in the Mexican state of Chiapas. We worked in the community of Playón de la Gloria, located in the Marqués de Comillas municipality (16°9’20’’N, 90°53’50’’W; Figure 1). The climate is warm and humid, with mean annual rainfall of 2500-3500 mm (a wetter period occurs between June and November) and mean annual temperature of 24–26°C (INE 2000). The Lacandona rainforest is geomorphologically complex, giving rise to a heterogeneous vegetation, which in turn harbors one of the highest biodiversities in Mesoamerica; in 1978 the Montes Azules Biosphere Reserve (331 200 ha) was established to conserve this biodiversity (INE 2000).

Figure. 1. Maps (a) of Mexico showing the location of the state of Chiapas, and of Chiapas showing the location of the Marqués de Comillas municipality. Satellite image (b) showing the area covered by rustic cocoa plantations (white polygon in the center of image) and the forest south of it. Sample plots in cocoa and forest are represented by grey and white circles, respectively. Also shown is the Lacantún river, with the Montes Azules Biosphere Reserve west of it.
Unfortunately, rates of deforestation and land-use change outside the Reserve have been high for over 50 years, and remaining forest cover in the Marqués de Comillas municipality is ∼ 50% (33% old-growth forest and 17% secondary forest; Zermeño-Hernández et al. Reference Zermeño-Hernández, Pingarroni and Martínez-Ramos2016). The landscape consists of a mosaic of land covers, including some large forest remnants, many smaller forest fragments, human settlements, successional vegetation, and agricultural covers. The latter are of many types (e.g., cattle pastures, annual crops, oil-palm plantations, shade cocoa, etc.), varying greatly in vegetation structure and management intensity, and thus in their value as potential habitat for animals (Zermeño-Hernández et al. Reference Zermeño-Hernández, Pingarroni and Martínez-Ramos2016).
Our study was carried out in an area of ca. 100 ha covered by rustic cocoa plantations, and in the abutting large forest remnant (ca. 2000 ha; Figure 1). The forest remnant was a local reserve where hunting and logging were restricted. The cocoa area consisted of approximately 16 plantations, 4–7 ha each, owned by different farmers (see Zárate et al. Reference Zárate, Andresen, Estrada and Serio-Silva2014). During the 1980’s, cocoa trees were planted by clearing some understory and midstory vegetation but leaving most of the original forest canopy intact. Plantations were highly active until the 1990’s when a fungal disease (Moniliophthora forreri) caused yields to drop considerably (Zárate et al. Reference Zárate, Andresen, Estrada and Serio-Silva2014). At the time we carried out this research, the level of cocoa harvest was low, and additional management was limited to some pruning of cocoa trees, removal of a few shade trees (see Santos-Heredia et al. Reference Santos-Heredia, Andresen, Zárate and Escobar2018), and occasional harvest of timber and non-timber forest products (MdJ C-L, pers. obs.). Thus, the cocoa plantations that we studied represented the ‘friendly’ end of the gradient between biodiversity-friendly and intensively managed shaded agroecosystems.
Study design and measurement of environmental variables
We established 24 plots (12 in cocoa and 12 in forest), 25 m x 25 m, separated ≥ 100 m from each other and from the edge between the two vegetation types (Figure 1). In each plot, we measured 12 environmental variables that, based on the literature and our own experience, were considered important for amphibians and/or reptiles: (1) leaf litter depth, (2) percentage of leaf litter cover, (3) shrub density, (4) density of herbaceous plants, (5) maximum canopy height, (6) proportion of humus presence, (7) illuminance, (8) density of trees (including cocoa trees) with DBH (diameter at breast height) ≥ 10 cm, (9) density of fallen trunks and postrate lianas with diameters ≥ 10 cm, (10) presence of water bodies, (11) air temperature in the understory, and (12) air relative humidity in the understory. See Supplementary Material Appendix S1 for detailed descriptions on how each variable was measured.
Sampling of amphibians and reptiles
We sampled amphibians and reptiles in the 24 plots during the rainy season, in June and July 2015, using visual encounter surveys (VES; Crump & Scott Reference Crump, Scott, Heyer, Donnelly, McDiarmid, Hayek and Foster1994). In each plot we established three parallel strip transects 25 m long and 3 m wide, along two of the plot’s borders and in its center. Each strip transect was searched for amphibians and reptiles in the understory (up to 2 m above the ground) by the same team of three people working together (Vonesh et al. Reference Vonesh, Mitchell, Howell, Crawford and Dodd2009). The team leader (MdJ C-L) had several years of experience sampling herpetofauna in different habitat types in the study region, which decreases the probability of introducing sampling bias due to differences in detectability with VES in both habitats. Searching time per plot was 45 min during the day (between 09.00 and 12.00; 15 min per strip transect) and 45 min at night (between 19.00 and 21.00), to cover the periods of maximum activity (Jones Reference Jones, Coperrider, Boyd and Stuart1986). During the study period, each plot was sampled twice, with at least 15 days between samplings, for a total sampling effort of 180 min per plot. Taxonomy follows the Amphibian Species of the World 6.1 database (Frost Reference Frost2021) and the Reptile Database (Uetz et al. 2021).
Data analyses
Due to the spatial arrangement of our study plots, i.e., all the cocoa plots in one site covered by several cocoa plantations, and all the forest plots in one large forest site, all our statistical inferences are limited to comparing these two sites and cannot be generalized to “all” cocoa plantations versus forest comparisons. It is important that the reader keeps this limitation in mind when considering our results and conclusions.
All analyses were carried out in R 3.6.3 (R Core Team 2020), except where otherwise noted. First, we explored whether environmental variables differed between habitats by fitting linear models with habitat as predictor. Percentage of leaf litter cover, frequency of humus presence, presence of water bodies, and air relative humidity were logit-transformed, while shrub density was log-transformed. Package ‘tidyverse’ (Wickham et al. Reference Wickham, Averick, Bryan, Chang, McGowan, François, Grolemund, Hayes, Henry, Hester, Kuhn, Pedersen, Miller, Bache, Müller, Ooms, Robinson, Seidel, Spinu, Takahashi, Vaughan, Wilke, Woo and Yutani2019) was used for these analyses. Models were fitted using the ‘lm’ function from base R (R Core Team 2020).
To analyze differences in abundance between habitats, we ran multivariate abundance analyses using the package ‘mvabund’ (Wang et al. Reference Wang, Naumann, Wright and Warton2012). This analytical method performs a generalized linear model for the abundance of each species in a community, and then provides both a multivariate test as well as univariate tests for each species. To avoid model-fitting problems, for these analyses we excluded species for which only one (singletons) or two individuals (doubletons) were captured overall.
The number of species was analyzed both at the community (i.e., at the level of habitat) and the assemblage levels (i.e., at the level of plots within habitats), using the complete dataset. At the community level we compared species richness in the cocoa versus forest with rarefaction and extrapolation curves with 95% confidence intervals (bootstrap with 1000 repetitions) using the program iNEXT (Hsieh et al. Reference Hsieh, Ma and Chao2016). At the assemblage level we used a generalized linear model to assess the differences between habitats (fixed factor) in mean number of species per plot using a Poisson error distribution with a log-link function.
To analyze assemblage composition (i.e., species composition and the abundance of each species) we first built rank-abundance curves on the full dataset for descriptive purposes. Then, to assess the statistical effect of habitat we performed Canonical Correspondence Analyses (CCA) on abundance matrices (excluding singletons and doubletons) using the ‘vegan’ package (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O’Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2020). CCA uses the chi-square distance, where each term has a weight that is the inverse of its total absolute frequency. Thus, a CCA emphasizes abundance differences occurring in rare species because they have a relatively higher weight than differences occurring in common species. For this analysis we excluded one of the forest sites where no amphibians were recorded.
To analyze the effect of environmental variables on abundance and species number within each habitat, we used the whole dataset to carry out generalized linear models with a multi-model inference approach based on the Akaike (AIC) information criterion (Anderson Reference Anderson2008). To reduce collinearity, we standardized predictor variables prior to modelling. Both abundance and species number were modelled using a Poisson distribution. Due to the high number of predictor variables (12) relative to our sample size (12 plots), we focused on additive effects only and built all possible models by combining a maximum of two predictors. Then we performed model averaging to obtain best estimates of model parameters and to make inferences on the relevance of predictors (Anderson Reference Anderson2008, Galipaud et al. Reference Galipaud, Gillingham and Dechaume-Moncharmont2017). Those predictors whose 90% confidence interval did not include zero were included in the final model.
Results
Environmental variables in cocoa versus forest
The cocoa and the forest habitats had similar values for nine of the environmental variables (all p values ≥ 0.1) and differed in the other three (Supplementary Material Figure S1). Rustic cocoa had higher tree density (F 1,2 = 56.71, p < 0.001), litter cover (F 1,2 = 13.93, p = 0.001) and litter depth (F 1,2 = 5.29, p = 0.031) than the forest. The humus layer showed a tendency of occurring more often in cocoa than forest (F 1,2 = 3.57, p = 0.072).
Cocoa versus forest: abundance, number of species and assemblage composition
We recorded 1665 individuals belonging to 53 species and 20 families; 438 were amphibians of 18 species and 9 families, and 1217 were reptiles of 35 species and 11 families (Table 1). Only three species of amphibians (17%) were singletons or doubletons, while this was the case for 17 reptile species (49%). The species that we found represent 60% and 55% of all amphibian and reptile species, respectively, reported for the southeastern part of the Lacandona rainforest region (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Martínez-Ramos, Arroyo-Rodríguez, González-Hernández, González-Zamora, Zárate and Reynoso2014, Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015, Russildi et al. Reference Russildi, Arroyo-Rodríguez, Hernández-Ordóñez, Pineda and Reynoso2016). Of all species recorded, 7 amphibian and 10 reptile species are in a threat category according to the Mexican government (NOM-059-SEMARNAT-2010) and/or the IUCN Red List (SEMARNAT 2019, IUCN 2021) (Table 1).
Table 1. Amphibian (Anura and Urodela) and reptile (Squamata) species recorded in two habitats, rustic cocoa and forest, in the Lacandona region, Mexico. For each species, the total absolute (#) and relative (%) abundance recorded per habitat is given; when more than one individual was recorded, the number of plots in which they were found is given in parentheses next to the absolute abundance values. Also shown are the results of the univariate generalized linear model for herpetofauna (only for species with three or more individuals registered) that were part of the multivariate abundance analysis (see text): deviance value (Dev) and the associated probability values (p) for differences in absolute abundance between habitats. The IUCN/Mexico column indicates the conservation status according to the IUCN Red List, and to the Mexican Government (NOM-059-SEMARNAT-2010), respectively [IUCN categories: NE, Not Evaluated; LC, Least Concern; NT, Near Threatened; VU, vulnerable; Mexican categories (Mex): NoT, Not Threatened; SP, Special protection; Thr, Threatened]. The last column shows the name codes assigned to each species

Abundance
A total of 295 amphibians were recorded in cocoa, whereas less than half that number was recorded in forest (143 individuals). The same pattern, but less pronounced, was observed for reptiles, with 652 individuals in cocoa versus 565 in forest. The multivariate abundance analyses showed that the abundance of both amphibians and reptiles was higher in the rustic cocoa habitat than in forest (amphibians: Dev = 42.3, Res.df = 21, p = 0.012; reptiles: Dev = 38.6, Res.df = 22, p = 0.034; Figure 2a,b). These analyses also revealed that, at the level of individual species, three amphibians (Incilius campbelli, Hyalinobatrachium viridissinum, Lithobates brownorum) and two reptiles (Scincella cherriei, Coniophanes bipunctatus) had higher absolute abundance in cocoa than in forest. Only one species, the reptile Coleonyx elegans, was significantly more abundant in forest (Table 1).
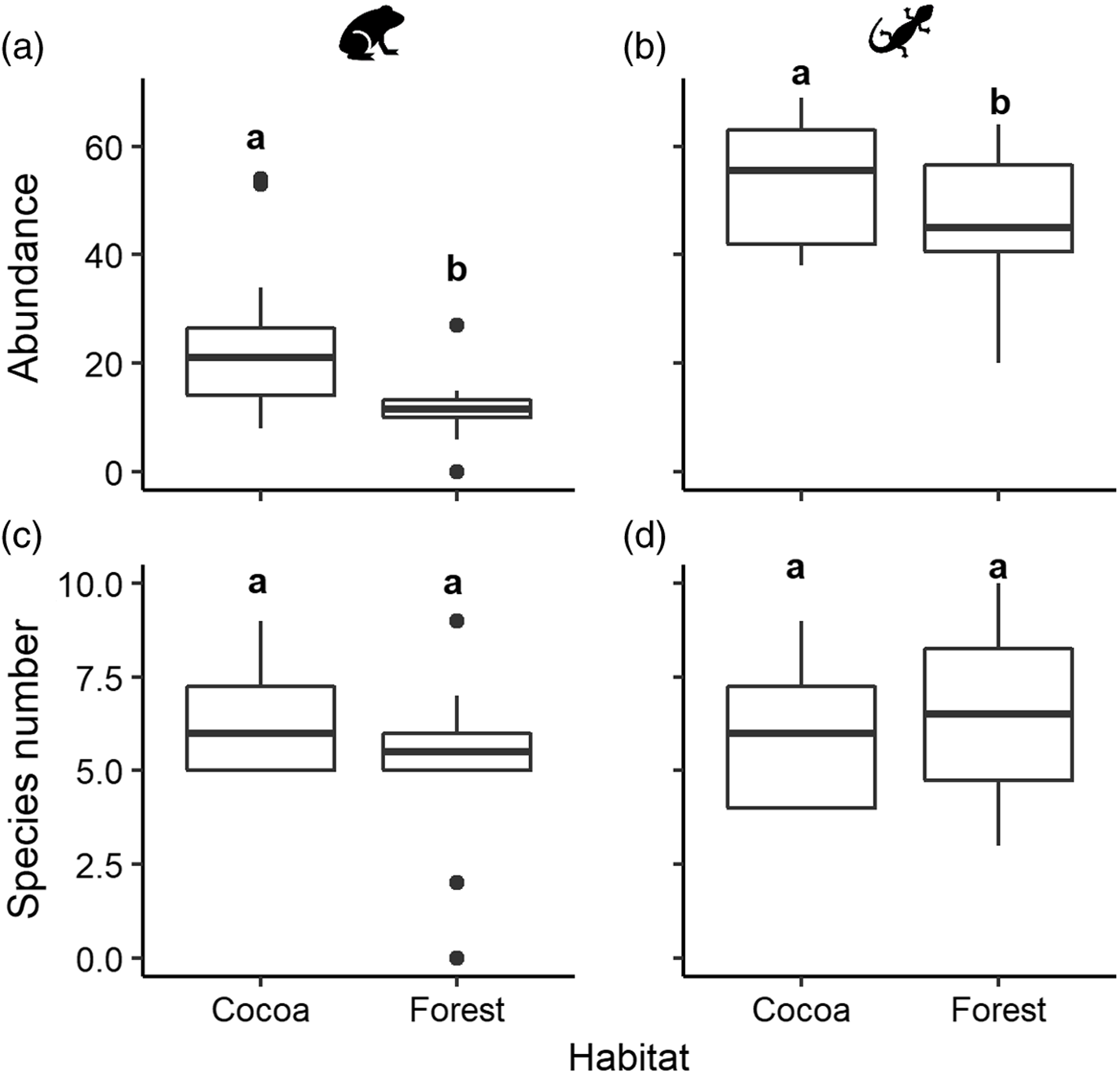
Figure. 2. Boxplots of the abundance (a and b) and number of species (c and d) observed per plot in two habitats, cocoa (n = 12 plots) and forest (n = 12 plots), for amphibians (a and c) and reptiles (b and d). Different letters above box-plots indicate statistical differences determined with multivariate abundance analyses (abundance) and generalized linear modelling (species number). Animal icons used with permission from Microsoft.
Number of species
In the cocoa habitat we found 15 species of amphibians and 26 of reptiles; species numbers were very similar in forest, with 15 amphibian and 27 reptile species. Species richness of amphibians and reptiles at the community level (both rarefied and extrapolated) did not differ between habitats (Supplementary Material Figure S2). At the assemblage level, the analyses also showed that the mean number of species recorded per plot did not differ between habitats (amphibians: χ2 = 20.4, df = 22, p = 0.2; reptiles: χ2 = 16.3, df = 22, p = 0.7; Figure 2c,d).
Assemblage composition
The two most abundant species were the same in the two habitats, both for amphibians (Craugastor laticeps and Incilius campbelli) and reptiles (Anolis uniformis and Scincella cherriei; Table 1; Supplementary Material Figure S3). The amphibian community showed a more even distribution of species abundances than the reptile community, but distributions for each taxon were similar in the two habitats (Supplementary Material Figure S3). Three amphibian species were only recorded in cocoa, one was a singleton but the other two had abundances of 6 (Lithobates brownorum) and 15 individuals (Hyalinobatrachium viridissimum). Similarly, three other amphibian species were only found in the forest, but with low abundances (1–3 individuals; Table 1). For reptiles, eight species were only found in cocoa (all singletons except Coniophanes bipunctatus with five individuals), while nine species were unique to forest (eight with 1–4 individual, and one with six, Coleonyx elegans). The Canonical Correspondence Analyses showed that habitat had a significant power, albeit low, for explaining differences in the structure of the amphibian and reptile assemblages (amphibians: F 1,21 = 1.83, p = 0.02, R 2 = 0.08; reptiles: F 1,22 = 1.84, p = 0.009, R 2 = 0.077), emphasizing differences in the abundance of the rare species mentioned above (Figure 3).

Figure 3. Canonical correspondence analysis for 12 plots of rustic cocoa plantations (circles) and 12 plots of forest (triangles), based on the composition of their amphibian (a) and reptile assemblages (b). Species are shown by a three-letter abbreviation; see Table 1 for full species names. Animal icons used with permission from Microsoft.
Relationships with environmental variables within each habitat
The multi-model inference approach revealed that some environmental variables were associated with the abundance of amphibians and reptiles, but that the identity of these variables varied between groups and habitats (Table 2). On the other hand, none of the environmental variables explained the number of species of amphibians or reptiles in cocoa or forest (Supplementary Material Table S1).
Table 2. Model-averaged coefficients estimates and standard errors (in parentheses) of environmental predictor variables used to model the mean abundance of amphibians and reptiles found per plot in two habitats, cocoa and forest. Those predictors whose 90% confidence interval did not include zero were included in the final model and are shown in bold. Also shown is the amount of deviance explained (% dev. expl.) by each final model. Details on how variables were quantified can be found in Supplementary Material Appendix S1.

In the cocoa habitat, the abundance of amphibians was positively related to canopy height and the presence of a humus layer (85% of deviance explained by the model), while the abundance of reptiles was negatively related to the relative humidity (42% of deviance explained; Table 2). In the forest habitat, the abundance of amphibians showed a negative association with the presence of water bodies, but the model only explained 24% of the deviance. The abundance of reptiles in forest was also related negatively to the presence of water bodies and positively to illuminance (74% of deviance explained; Table 2).
Discussion
Our findings support the idea that shade cocoa plantations under rustic low-intensity management can be suitable habitat for amphibians and reptiles in human-modified tropical forests. Supporting this idea, we found that most environmental variables we measured were similar in both habitats and that cocoa plantations had the same number of species of reptiles and amphibians as the forest. Interestingly, the abundance of both groups was higher in the cocoa, likely due to more leaf litter in this habitat. Importantly, the cocoa plantations in the Lacandona region not only served as habitat for common generalist species, but also for several species associated with old forest and secondary forest (intermediate successional stage) in the study region (e.g., the anurans Craugastor laticeps, C. palenque, and Incilius campbelli, the salamanders Bolitoglossa mulleri and B. rufescens, the lizards Anolis capito, A. uniformis, Lepidophyma flavimaculatum, and the snakes Botriechis schlegelii and Porthidium nasutum (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Martínez-Ramos, Arroyo-Rodríguez, González-Hernández, González-Zamora, Zárate and Reynoso2014, Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015; Russildi et al. Reference Russildi, Arroyo-Rodríguez, Hernández-Ordóñez, Pineda and Reynoso2016). Overall, these results have important management implications for designing landscapes that can be productive while conserving these ecologically relevant animal groups.
Our study underscores the fact that agroecosystems whose vegetation is complex and structurally similar to that of the native vegetation are likely to ensure adequate environmental conditions and resources for the local herpetofauna (Macip-Ríos & Muñoz Reference Macip-Ríos and Muñoz2008, Orozco-Aguilar 2016, Wanger et al. Reference Wanger, Iskandar, Motzke, Brook, Sodhi, Clough and Tscharntke2010). In the cocoa habitat, most environmental variables (9 of 12) had similar values to those in forest (Supplementary Material Figure S1). In another study in the same sites, it was also found that tree species richness did not differ between habitats (Zárate et al. Reference Zárate, Andresen, Estrada and Serio-Silva2014). These vegetation similarities were most likely one of the main reasons why both habitats had similar number of amphibian and reptile species and only small differences in assemblage composition (Supplementary Material Figure S3).
Species number is a metric that may be less sensitive to habitat changes (Thompson et al. Reference Thompson, Nowakowski and Donnelly2016, Hillebrand et al. Reference Hillebrand, Blasius, Borer, Chase, Downing, Erikssoon, Filstrup, Harpole, Hodapp, Larsen, Lewandowska, Seabloom, Van de Waal and Ryabov2018), particularly when those changes are more subtle, as between the rustic cocoa and forest we studied. Other studies comparing herpetofauna in shade cocoa and forest found a similar pattern for one or both groups (Faria et al. Reference Faria, Paciencia, Dixo, Laps and Baumgarten2007; Wanger et al. Reference Wanger, Iskandar, Motzke, Brook, Sodhi, Clough and Tscharntke2010). More generally, studies comparing biodiversity metrics in conserved forest with those found in disturbed forests (e.g., forest fragments, secondary forests, restoration forests, rustic agroforests, logged forests, etc.) often find that species number is a response variable that maintains, or quickly recovers, its magnitude (e.g., Thompson & Donnelly Reference Thompson and Donnelly2018, Acevedo-Charry & Aide Reference Acevedo-Charry and Aide2019). On the other hand, metrics of species composition may be more sensitive to subtle habitat differences (Díaz-García et al. Reference Díaz-García, López-Barrera, Toledo-Aceves, Andresen and Pineda2020). In shaded-cocoa, changes in assemblage composition of herpetofauna have been related to an increase in the relative abundance of a few common species (Lieberman Reference Lieberman1986, Heinen Reference Heinen1992, Faria et al. Reference Faria, Paciencia, Dixo, Laps and Baumgarten2007). In our study, while we found an increase in the abundance of common species in cocoa, their relative abundances did not change very much between the habitats. Instead, we found a weak effect of habitat on assemblage composition, mostly driven by rare species that were only present (or more frequent) in one of the habitats.
Unlike species number (which did not vary) and assemblage composition (which varied subtly), the abundance of amphibians and reptiles differed strongly between habitats. Abundances were higher in cocoa than in forest, though the pattern was somewhat different between groups: while reptiles increased by 15%, amphibian abundance increased by 106% (Table 1). Also, the increase in amphibians was more generalized than in reptiles. Five of the seven most abundant species of amphibians had higher abundances in cocoa (Incilius campbelli, Hyalinobatrachium viridissinum, Craugastor laticeps, Craugastor loki, and Bolitoglossa rufescens). In reptiles, higher abundance was mostly driven by the two most common species (Anolis uniformis and Scincella cherriei), while most other species were rare in both habitats. In agroforests worldwide, increases in abundance are more frequent than decreases in the case of reptiles, but equally frequent in amphibians (reviewed by Palacios et al. Reference Palacios, Agüero and Simonetti2013). While most agroforests have edge habitat that can favor the proliferation of a few reptile species, only agroforests that maintain certain structural elements (e.g., understory cover) are able to maintain similar or increased amphibian abundances (Palacios et al. Reference Palacios, Agüero and Simonetti2013 and references therein).
So, what does the rustic cocoa habitat have that favors herpetofauna in the Lacandona region, in particular amphibians? A few environmental characteristics differed between both habitats, and they were probably responsible for the abundance increases in cocoa. Tree density, leaf litter depth and litter cover had higher values in cocoa (Supplementary Material Figure S1). Higher tree density in the cocoa habitat is caused by the planted cocoa trees (Zárate et al. Reference Zárate, Andresen, Estrada and Serio-Silva2014). In turn, the high density of cocoa trees produces a copious leaf litter. Other studies have also reported more leaf litter in shade-cocoa plantations and have associated this characteristic to increased herpetofaunal abundance (Lieberman Reference Lieberman1986, Heinen Reference Heinen1992, Wanger et al. Reference Wanger, Iskandar, Motzke, Brook, Sodhi, Clough and Tscharntke2010). As suggested by these studies, amphibians and reptiles are likely to find abundant trophic and non-trophic resources in the dense litter and may experience a better and/or less variable microclimate (Whitfield et al. Reference Whitfield, Bell, Philippi, Sasa, Bolaños, Chaves, Savage and Donnelly2007). Contrary to the cocoa habitat, other types of disturbed forests in the region have been found to harbor decreased herpetofaunal abundances (forest fragments, Russildi et al. Reference Russildi, Arroyo-Rodríguez, Hernández-Ordóñez, Pineda and Reynoso2016; secondary forests, Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015), further highlighting the high conservation value of this agroforest.
In addition to the environmental variables that differed between habitats, we also found that within each habitat different environmental variables were related to the abundance of amphibians and reptiles. In cocoa the relationship of environmental variables was relatively weak for reptiles (42% of deviance explained) but strong for amphibians (85% of deviance explained). The abundance of reptiles in cocoa was negatively related to relative humidity, which points to the fact that this group of animals favors less humid microenvironments. On the other hand, the abundance of amphibians was positively related to canopy height and the presence of a humus layer. Similar to leaf litter (see above), the humus layer likely provides abundant resources and microclimatic conditions which are ideal for amphibians (Whitfield et al. Reference Whitfield, Bell, Philippi, Sasa, Bolaños, Chaves, Savage and Donnelly2007). Higher canopies are associated with the presence of large native trees in the cocoa plantations (Zárate et al. Reference Zárate, Andresen, Estrada and Serio-Silva2014). Large trees often have unique morphological characteristics, such as cavities, buttresses, large crowns, and heavy epiphyte loads, all of which provide abundant and diverse resources for many groups of animals (Pinho et al. Reference Pinho, Peres, Leal, Tabarelli, Dumbrell, Turner and Fayle2020). The presence of large trees may be of disproportionate importance for the conservation of animals in disturbed habitats. For example, in small rainforest fragments, the presence of forest-dependent arboreal mammals is directly related to the abundance of large trees (Arroyo-Rodríguez et al. Reference Arroyo-Rodríguez, Mandujano, Benítez-Malvido and Cuende-Fanton2007). Thus, maintaining a diverse cover of native trees in cocoa agroforests, including large individuals, is a management guideline that would probably not only favor amphibians, but other groups of organisms (e.g., birds; Bennett et al. Reference Bennett, Sillett, Rice and Marra2021).
Contrary to the results for the cocoa habitat, in the forest habitat the relationship of environmental variables was strong for reptiles (74% of deviance explained) but weak for amphibians (24% of deviance explained). The abundance of reptiles was positively related to illuminance and negatively to the presence of water bodies. These relationships are not surprising, considering that rainforest reptiles often bask in open areas (Wanger et al. Reference Wanger, Iskandar, Motzke, Brook, Sodhi, Clough and Tscharntke2010), which might have higher illuminance levels, and prefer drier microhabitats. The abundance of amphibians in forest was also negatively related to the presence of water bodies. This result was unexpected given that other studies have stressed the importance of the presence of water bodies for the maintenance of amphibians in disturbed habitats (Wanger et al. Reference Wanger, Saro, Iskandar, Brook, Sodhi, Clough and Tscharntke2009, Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015, Díaz-García et al. Reference Díaz-García, López-Barrera, Toledo-Aceves, Andresen and Pineda2020). However, given the low explanatory value of the model, we interpret this result as meaning that the presence of water bodies was not very important in determining the abundance of amphibians in forest. This may be because three of the four most abundant species recorded in forest have direct development (Craugastor laticeps, C. loki and Eleutherodactylus leprus), which means that they do not need water bodies to reproduce. Overall, our results on the effect of environmental variables confirm that different variables may be shaping amphibian and reptile communities within versus across habitats. Thus, to maximize our understanding both patterns should be examined, as already pointed out in a previous study (Wanger et al. Reference Wanger, Iskandar, Motzke, Brook, Sodhi, Clough and Tscharntke2010).
Finally, to properly contextualize our findings, it is important to remember that, for two main reasons, the cocoa habitat we studied probably represents one of the extremes in the “wildlife friendliness” gradient of agroecosystems. First, the cocoa plantations were under very low management intensity (see Methods). Low management intensity, of course, favors biodiversity, but it may not meet the requirements of human sustenance (Bennett et al. Reference Bennett, Sillett, Rice and Marra2021). Indeed, many of the shade-cocoa plantations that existed in the region have been replaced by different crops, mostly cattle pasture and annual crops. They are also being replaced by monoculture agroforests, such as oil palm plantations, which are known to have heavily impoverished herpetofaunas (Gallmetzer & Schulze Reference Gallmetzer and Schulze2015). Intensifying management of the cocoa plantations to make them profitable could decrease the quality of this agroecosystem as habitat for amphibians and reptiles. However, studies have also shown that, if well designed, agroforests or silvopastoral systems can be profitable while providing habitat for animals (e.g., Gordon et al. Reference Gordon, Manson, Sundberg and Cruz-Angón2007, Deheuvels et al. Reference Deheuvels, Rousseau, Soto Quiroga, Decker Franco, Cerda, Vílchez Mendoza and Somarriba2014, Montoya-Molina et al. Reference Montoya-Molina, Giraldo-Echeverri, Montoya-Lerma, Chará, Escobar and Calle2016, Bennett et al. Reference Bennett, Sillett, Rice and Marra2021).
Second, landscape composition and configuration most likely played an important role in favoring the maintenance of amphibian and reptile populations in the cocoa habitat. The area of the cocoa plantations was relatively large (100 ha), and, most importantly, it abutted with a large forest area (> 2,000 ha). Both the amount of forest cover in the surrounding landscape and the proximity to forest, are crucial characteristics that are associated positively to the persistence of native biodiversity in anthropogenic tropical landscapes (Acevedo-Charry & Aide Reference Acevedo-Charry and Aide2019, Grass et al. Reference Grass, Loos, Baensch, Batáry, Librán-Embid, Ficiciyan, Klaus, Riechers, Rosa, Tiede, Udy, Westphal, Wurz and Tscharntke2019, Arroyo-Rodríguez et al. Reference Arroyo-Rodríguez, Fahrig, Tabarelli, Tischendorf, Benchimol, Cazetta, Faria, Leal, Melo, Morante-Filho, Santos, Arasa-Gisbert, Arce-Peña, Cervantes-López, Cudney-Valenzuela, Galán-Acedo, San-José, Vieira, Slik, Nowakowski and Tscharntke2020).
In conclusion, our study indicates that rustic shade cocoa plantations in the Lacandona region can be considered an ecologically-friendly agroecosystem for amphibians and reptiles, including species that are considered forest specialists. Thus, in addition to the high conservation value of forest fragments (Russildi et al. Reference Russildi, Arroyo-Rodríguez, Hernández-Ordóñez, Pineda and Reynoso2016) and secondary forests (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Urbina-Cardona and Martínez-Ramos2015), an agricultural matrix containing cocoa agroforests could play an important role in the long-term conservation of herpetofauna in the anthropogenic landscapes of this Mesoamerican biodiversity hotspot. However, the potential conservation value of shade cocoa and other types of agroforests for tropical biodiversity will only be maximized, when these agroecosystems replace less suitable agricultural covers, such as cattle pastures and annual crops, rather than replacing forest (Martin et al. Reference Martin, Osen, Grass, Hölscher, Tscharntke, Wurz and Kreft2020).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467422000219
Acknowledgements
For field assistance we thank Don Isidro López Lira and Florentino López Bouchot. We are thankful to CONACyT for providing a graduate-study fellowship to MdJ C-L (658855). We thank the Posgrado en Ciencias Biológicas of the Universidad Nacional Autónoma de México (UNAM) for all support provided while MdJ C-L was a student of the Master’s program.
Financial support
Research was funded through a CONACyT research grant to E.A. (SEP-CONACyT 2010-152884).
Conflicts of interest
The authors declare none.
Ethical statement
This study complies with ethical standards. Herpetofaunal sampling was approved by the Mexican government secretariat in charge of the environment (Secretaría de Medio Ambiente y Recursos Naturales; permit: Nuím/SGPA/DGVS/02132).








