Among 230 million diabetic patients worldwide, 4·9 million are patients with type 1 diabetes (T1D), and the incidence of T1D is growing by 3–5 % each year worldwide(Reference Danne, Lange and Kordonouri1). T1D is a T-cell-mediated autoimmune disease resulting from the selective destruction of pancreatic β-cells. As of 2010, there is no known cure for this disease. Successful islet transplantation is a promising approach to T1D treatment. However, the lack of sufficient islets, loss of islet cell mass after islet isolation and potential autoimmune destruction of the transplanted islets prevent the widespread use of this procedure. In addition, islet transplantation is accompanied by significant side effects from immunosuppressive drugs(Reference Zhao, Lin and Darflinger2). Therefore, the search for novel and cost-effective agents that can prevent or treat T1D is extremely important to decrease the burden of morbidity and mortality from this disease.
Epigallocatechin gallate (EGCG) is a polyphenolic compound abundant in green tea(Reference Balentine, Wiseman and Bouwens3). Unlike other structurally related catechins, which primarily exist in conjugated forms in the blood after intestinal absorption(Reference Chow, Cai and Alberts4, Reference Lee, Maliakal and Chen5), EGCG is the only catechin primarily present in the plasma in a free form. While there are a number of case reports indicating that green tea consumption resulted in liver failure in humans and supraphysiological doses of green tea extracts induced hepatic injury in mice, dietary intake of EGCG supplements is considered safe as no adverse effect was observed in various human toxicity studies(Reference Chow, Cai and Hakim6, Reference Isbrucker, Bausch and Edwards7). A number of experimental and epidemiological studies reported that EGCG can reduce the risk of chronic diseases such as CVD(Reference Babu and Liu8), obesity(Reference Moon, Lee and Choi9, Reference Wolfram10) and cancers(Reference Wolfram10–Reference Ju, Hong and Zhou12). In addition, consumption of green tea extracts or EGCG was shown to have beneficial effects on blood glucose control in obese and diabetic humans(Reference Wolfram10, Reference Thielecke and Boschmann13), mice(Reference Wolfram, Raederstorff and Preller14) and rats(Reference Wolfram, Raederstorff and Preller14–Reference Igarashi, Honma and Yoshinari16). While EGCG was shown to exert the beneficial effects on some autoimmune diseases such as rheumatoid arthritis(Reference Ahmed, Pakozdi and Koch17), Sjogren's syndrome(Reference Hsu, Dickinson and Qin18) and encephalomyelitis(Reference Aktas, Prozorovski and Smorodchenko19), no study, to the best of our knowledge, has reported on whether this compound has an effect on autoimmune-related T1D. In the present study, we determined whether the dietary intake of EGCG can reduce the risk for the development of T1D in non-obese diabetic (NOD) mice and further examined whether it has a direct protective effect on pancreatic islets.
Materials and methods
Mice and experimental design
Female NOD/LtJ mice, 5 weeks old, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Female NOD mice are probably the most used animal model in the research for new therapies for T1D because the cumulative incidence of spontaneous diabetes is much higher in females than in males (75–95 % by 6–7 months of age in females v. 20–30 % at the same age in males)(Reference Krause, Tomer and Elias20, Reference Matarese, Sanna and Lechler21). Mice were fed ad libitum on an AIN-93G purified rodent diet (Dyets, Inc., Bethlehem, PA, USA) and kept in a room with a 12 h light–12 h dark cycle. Mice were randomly divided into two groups (n 12) and given either 0 or 0·05 % (w/v) of EGCG in drinking-water (Taiyo International, Inc., Minneapolis, MN, USA). This dose of EGCG is comparable to EGCG concentration in a typical cup of green tea that people usually drink(Reference Basu and Lucas22). Based on our records, the estimated daily consumption of EGCG was 60–90 mg/kg body weight, which is equivalent to 4·5–6·8 g/d by a 75 kg person. To ensure the stability of EGCG, the stock compound was stored at − 80°C, and the water bottle was sealed and kept away from light. Fresh EGCG was made and provided to mice every other day with the same batch of EGCG throughout the study. Food intake and body weight were measured biweekly, and water intake was recorded every 2 d. Every 3–5 weeks, non-fasting blood glucose was measured in blood samples from the tail vein using a glucometer (Kroger, Inc., Cincinnati, OH, USA). During the whole period of treatment, the general clinical condition and mortality of mice were monitored daily. Killing of animals was independently assessed by a veterinarian according to the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. Mice with body weight less than 25 % of their original body weight were killed and censused, and their blood and tissues were collected and included for further analysis. The animal protocol was approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University, Blacksburg, VA, USA.
Intraperitoneal glucose tolerance test
For glucose tolerance tests, mice at 31 weeks of age (n 5) were fasted for 12 h and then injected intraperitoneally with a single bolus of glucose (2 g/kg body weight)(Reference Ruohonen, Pesonen and Moritz23). Glucose levels in the blood collected from the tail vein were measured at time points of 0, 5, 15, 30, 60 and 120 min after glucose administration.
Plasma insulin and glycosylated Hb measurements
At 32 weeks of age, overnight-fasted mice were anaesthetised for collecting blood samples. Plasma insulin concentration was measured by ELISA (Mercodia, Inc., Winston-Salem, NC, USA), and glycosylated Hb levels were measured using an assay kit (Henry Schein, Inc., Melville, NY, USA).
Histopathological procedure and insulitis evaluation
Mice were killed, and the pancreas was removed and fixed in 10 % neutral buffered formalin, and then embedded in paraffin. Tissue sections at 500 μm apart from each other were deparaffinised, hydrolysed and stained with haematoxylin. Islets in each section were assessed as described previously(Reference Signore, Annovazzi and Giacalone24, Reference Zhang, Todorov and Lin25), and insulitis was graded as follows: score 0, no lymphocytic infiltration; score 1, less than 20 % infiltration; score 2, approximately 20–50 % infiltrated islet; score 3, approximately 50–80 % infiltrated islet; score 4, more than 80 % infiltration. For each mouse, five sections were scored, and twelve mice from each group were evaluated.
Plasma cytokine measurements
Cytokines in the serum were tested using a mouse cytokine array kit (Quansys Biosciences, West Logan, UT, USA), including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, monocyte chemoattractant protein-1, interferon-γ (IFN-γ), TNF-α, macrophage inflammatory protein-1α, granulocyte macrophage colony-stimulating factor and RANTES.
Cell viability assay
Human islets were obtained through the Islet Cell Resource Centers funded by the National Institutes of Health and the Islet Distribution Program at the Juvenile Diabetes Research Foundation International. The purity of islets for these studies was 80–90 %, and viability was 80–97 %. Islets (200 islets/well) were pre-incubated with various doses of EGCG for 12 h in Roswell Park Memorial Institute 1640 medium containing 5·5 mm-glucose and 10 % fetal bovine serum. The islets were then washed and treated with cytokines (IL-1β (5 ng/ml)+IFN-γ (10 ng/ml); R&D System, Inc., Minneapolis, MN, USA) in the continued presence or absence of EGCG for 48 h. Islet cell viability was determined by measuring the reduction of resazurin to fluorescent-labelled resazurin using a fluorescence CellTiter 96 aqueous assay kit (Promega, Madison, WI, USA).
Caspase-3 activity assay
Human islets (approximately 200 islets/well) were pre-incubated with various doses of EGCG for 12 h in Roswell Park Memorial Institute medium as stated earlier. The islets were then washed and treated with cytokines (IL-1β (5 ng/ml)+IFN-γ (10 ng/ml)) in the continued presence or absence of EGCG for 24 h. Cytosolic enzymatic activity of caspase-3 in cell lysates was measured essentially as described in the manufacturer's protocol (Promega). Caspase-3 activity was normalised to the cellular protein concentration and expressed as a percentage of increase over the control cells.
Statistical analysis
Data were analysed by one-way or two-way repeated-measures ANOVA where appropriate. Significant differences between treatments were analysed using Student's t test or Tukey's test for human islet data. The log-rank test was applied to compare survival distributions of the control and EGCG-treated groups. Data of immune cell infiltration into islets were subjected to the non-parametric Mann–Whitney U test. Differences were considered significant at P < 0·05.
Results
Dietary supplementation of epigallocatechin gallate delays the onset of type 1 diabetes in non-obese diabetic mice
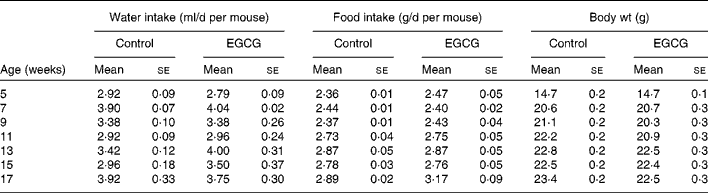
In the present study, we tested whether EGCG at a physiologically relevant dose has any beneficial effect on T1D. We found that EGCG (0·05 % in drinking-water) significantly ameliorated hyperglycaemia (Fig. 1(A)) and delayed the onset of T1D in NOD mice (Fig. 1(B)). The differences in blood glucose levels between the EGCG-treated and control groups became significant after the mice were 17 weeks old (P = 0·0001). At 32 weeks of age, eight out of the twelve mice (66·7 %) in the control group had overt diabetes (non-fasting blood glucose over 2500 mg/l), whereas only three out of the twelve mice (25·0 %) in the EGCG-treated group became diabetic (P = 0·013, statistical power 0·65). There was a significant interaction between EGCG and duration of treatment (P = 0·0016). Consistently, EGCG intake greatly reduced the mortality rate of diabetic mice from 58·3 to 8·3 % (Fig. 1(C); P = 0·007, statistical power 0·24). EGCG did not alter food and water intake as well as the body weight of NOD mice (Table 1), suggesting that the effect of EGCG on diabetic mice is not due to alternations in these parameters.
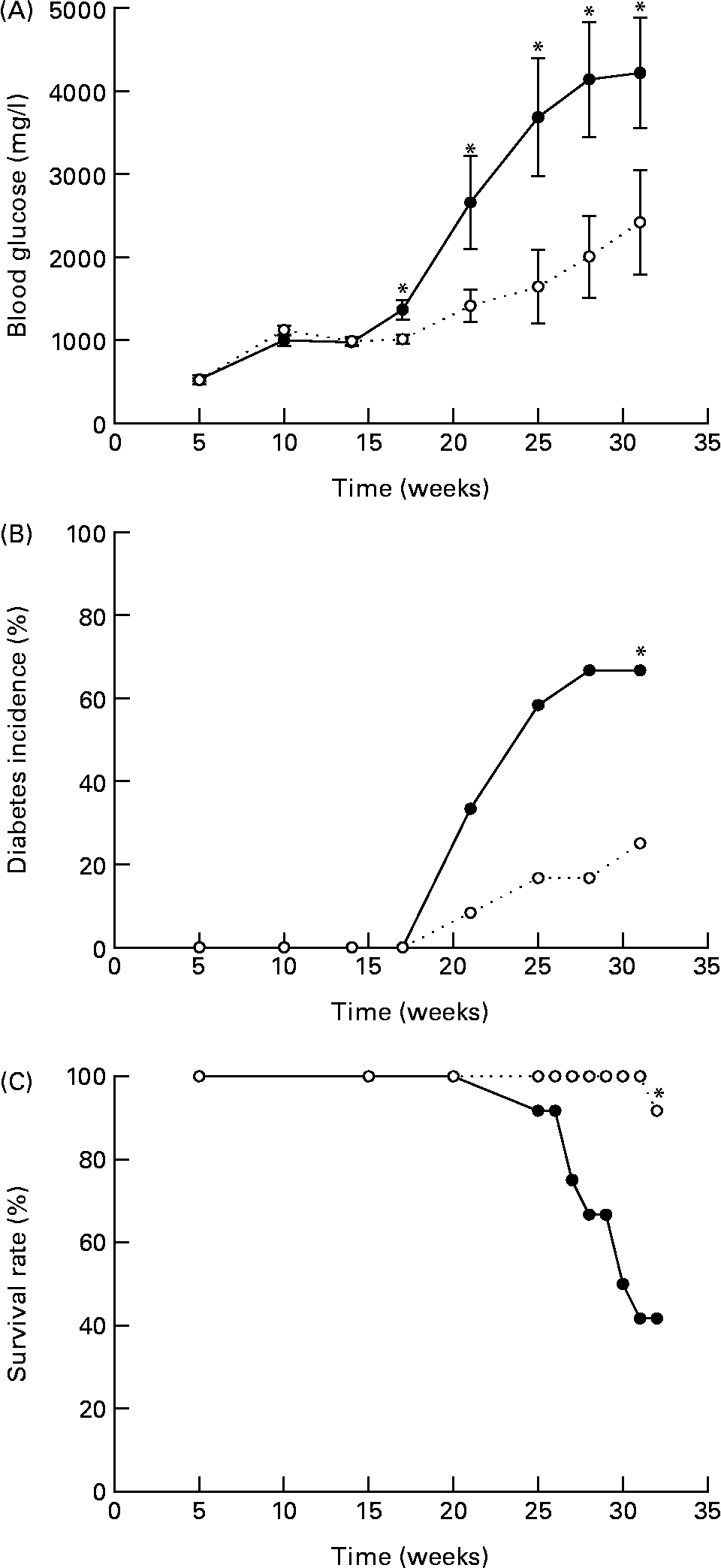
Fig. 1 Epigallocatechin gallate (EGCG) delays the onset of diabetes in non-obese diabetic (NOD) mice. Female NOD/LtJ mice (5 weeks old) were fed 0·05 % of EGCG in drinking-water. Age-matched control mice were given regular water. (A) Non-fasting blood glucose, (B) incidence of diabetes and (C) survival rate were recorded. Values are means, with standard errors represented by vertical bars (n 12). *P < 0·05 v. control. ![]() , Control;
, Control; ![]() , EGCG.
, EGCG.
Table 1 Epigallocatechin gallate (EGCG) has no effect on food and water intake and body weight in non-obese diabetic mice
(Mean values with their standard errors, n 12)
Epigallocatechin gallate improves glucose tolerance and lowers glycosylated Hb
To further confirm the anti-diabetic effect of EGCG in NOD mice, we performed the glucose tolerance test at 31 weeks of age and measured glycosylated Hb 1 week later. Mice fed EGCG showed significantly improved fasting blood glucose levels and glucose tolerance (Fig. 2(A); P = 0·001), while no significant time × treatment interaction was observed post-glucose injection. Consistently, blood levels of glycosylated Hb, a biomarker of blood glucose which can be interpreted as an average of blood glucose over a period of 3 months(Reference Aldasouqi and Gossain26), were significantly lower in the EGCG-treated mice than those in the control group (Fig. 2(B); P = 0·015). To determine whether the better glycaemic control by EGCG is the result of an improved islet function, we measured and compared plasma insulin levels in the control and EGCG-treated mice. We found that plasma insulin levels in the EGCG-treated mice were more than threefold higher than those in the control mice (Fig. 2(C); P = 0·003).

Fig. 2 Epigallocatechin gallate (EGCG) improves glucose tolerance and plasma glycosylated Hb (HbA1c) and increases plasma insulin level in non-obese diabetic mice. (A) For the glucose tolerance test, overnight-fasted mice were injected intraperitoneally with a bolus of glucose (2 g/kg body weight), followed by measurements of blood glucose at 0, 5, 15, 30, 60 and 120 min after glucose injection (n 5). (B) Blood levels of HbA1c and (C) plasma insulin concentration were measured at the end of the experiment with respective assay kits (control group, n 9; EGCG-treated group, n 12). Values are means, with standard errors represented by vertical bars. *P < 0·05 v. control. ![]() , Control;
, Control; ![]() , EGCG.
, EGCG.
Epigallocatechin gallate treatment has no effect on pancreatic insulitis
As T1D is an autoimmune disease, and several pro-inflammatory cytokines, such as IL-1β, IFN-γ and TNF-α, are believed to be important mediators leading to β-cell destruction in T1D(Reference Mandrup-Poulsen, Helqvist and Molvig27–Reference Li, El-Kholy and Rhodes32), we further assessed whether EGCG has a direct immune regulatory effect by evaluating islet insulitis and measuring a cohort of circulating immunoregulatory cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, monocyte chemoattractant protein-1, IFN-γ, TNF-α, macrophage inflammatory protein-1α, granulocyte macrophage colony-stimulating factor and RANTES). We did not observe any significant difference in the amount of infiltrated immune cells into the islets between the control and EGCG-treated mice (Table 2). EGCG treatment significantly increased plasma IL-10 (P = 0·036) and IL-12 (P = 0·002) levels, while all other cytokines were not significantly affected by EGCG (Fig. 3).
Table 2 Epigallocatechin gallate (EGCG) has no effect on pancreatic islet insulitis*
(Mean values with their standard errors)
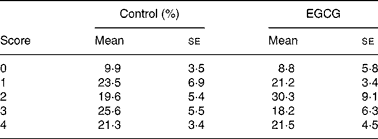
* Pancreatic sections were stained with haematoxylin and assessed for insulitis as described in the ‘Materials and methods’ section. For each mouse, five sections were scored, and twelve mice from each group were evaluated. A representative image for each grade of insulitis is shown.
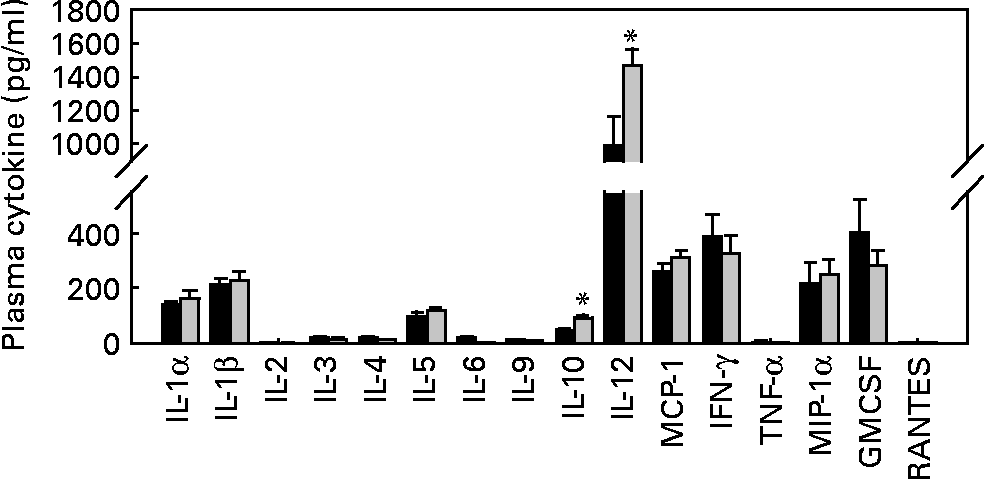
Fig. 3 Epigallocatechin gallate (EGCG) treatment increases plasma IL-10 and IL-12 concentrations but does not alter IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, monocyte chemoattractant protein-1 (MCP-1), interferon-γ (IFN-γ), TNF-α, macrophage inflammatory protein-1α (MIP-1α), granulocyte macrophage colony-stimulating factor (GMCSF) or RANTES levels. Blood was drawn from fasted mice, and plasma samples were used for measurements of various cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, MCP-1, IFN-γ, TNF-α, MIP-1α, GMCSF and RANTES (control group, n 9; EGCG-treated group, n 12). *P < 0·05 v. control. ■, Control; ![]() , EGCG.
, EGCG.
Epigallocatechin gallate promotes viability of human pancreatic islets exposed to pro-inflammatory cytokines
Since EGCG had no effect on pro-inflammatory cytokines and insulitis, we speculated that EGCG may have a direct protective effect on pancreatic islet β-cells exposed to these cytokines. We show that incubation of human islets with a cocktail of cytokines (IL-1β (5 ng/ml) and IFN-γ (10 ng/ml)) for 48 h reduced islet cell viability (P = 0·002) (Fig. 4(A)). However, addition of EGCG promoted islet viability in a dose-dependent manner, with 1 and 10 μm concentrations exerting a significant protective effect (P = 0·045 and 0·011, respectively), which was associated with a better three-dimensional configuration (Fig. 4(B)). These data suggest that EGCG may be a β-cell-protective agent with the potential to reduce the risk of T1D by preserving β-cell mass. Caspase proteins are critical components responsible for apoptosis, and caspase-3 is one of the key proteases involved in the convergence of disparate apoptotic signalling pathways. In parallel to increased cell viability, EGCG at the same concentrations potently inhibited caspase-3 activity in isolated human islets (Fig. 4(C); P = 0·023 and 0·012, respectively), confirming an anti-apoptotic effect of EGCG on human islets.

Fig. 4 Epigallocatechin gallate (EGCG) promotes human pancreatic β-cell viability. Human islets were pre-incubated in medium with or without various concentrations of EGCG (concentration of 5 μm was used for B) for 12 h, followed by the addition of cytokines (Cyto; IL-1β (5 ng/ml)+interferon-γ (10 ng/ml)). (A) Cell viability was determined, and (B) representative images of the control (C) and EGCG-treated islets were shown after 48 h. (C) Cellular caspase-3 activity was measured after 24 h of incubation. Values are means, with standard errors represented by vertical bars (n 4). Means values with unlike letters were significantly different (P < 0·05).
Discussion
In the present study, we found that EGCG at a dose of 0·05 % (w/v) in drinking-water effectively delayed the onset of diabetes in NOD mice. Consistently, EGCG administration improved glucose tolerance and lowered glycosylated Hb levels in NOD mice, which were concomitant with significantly improved plasma insulin levels and survival rates of diabetic animals. In addition, EGCG treatment significantly promoted human pancreatic β-cell survival. These findings provide evidence for the first time that EGCG may be a natural agent that can potentially be used to prevent T1D.
EGCG has been reported to exert the beneficial effects in three autoimmune diseases: rheumatoid arthritis(Reference Ahmed, Pakozdi and Koch17), Sjogren's syndrome(Reference Hsu, Dickinson and Qin18) and encephalomyelitis(Reference Aktas, Prozorovski and Smorodchenko19). These results suggest that EGCG may have an immunomodulatory effect. However, there is no study, to our knowledge, determining whether EGCG has a beneficial effect on the prevention or treatment of T1D, although the effect of EGCG on streptozotocin-induced diabetic mice has been examined(Reference Song, Hur and Han33, Reference Yun, Kim and Song34). T1D is a chronic autoimmune disease characterised by the T-cell-mediated destruction of pancreatic β-cells, resulting in absolute insulin deficiency(Reference Goto, Kida and Kaino35). In this context, the acute diabetic rodent model caused by injection of streptozotocin, which induces diabetes largely by causing glucose transporter-2-mediated β-cell damage, is not a closely relevant animal model of human T1D. Nevertheless, high doses of EGCG were directly injected into animals in these studies, which are unrealistic and far beyond those physiologically achievable through dietary consumption. In the present study, we present evidence that EGCG, provided in drinking-water at a dose (0·05 %) relevant to human consumption of green tea, can prevent the onset of diabetes in NOD mice. These mice are the most widely used and probably the best representative animal models of human T1D because they have far more similar characteristics of human T1D than pharmacologically induced models with respect to the immunoregulation and pathogenesis of diabetes(Reference Makino, Kunimoto and Muraoka36, Reference Yoshida and Kikutani37). Therefore, the present study using this rodent diabetic model may provide information applicable for further clinical trial in humans.
EGCG has been shown to exert an insulin-like effect in several in vitro studies(Reference Anton, Melville and Rena38). However, the hypoglycaemic effect of EGCG in NOD mice is not likely to be ascribed to this potential action because the observed insulin-mimetic effect of EGCG was only achieved at pharmacological doses of EGCG ( ≥ 50 μm), which is far beyond achievable plasma levels (0·6–1·8 μm) in both humans and animals through dietary ingestion of green tea extracts or pure EGCG supplements(Reference Riemersma, Rice-Evans and Tyrrell39, Reference Van Amelsvoort, Van Hof and Mathot40). EGCG has been reported to suppress intestinal absorption of glucose in rodents(Reference Skopec, Green and Karasov41), which could contribute to postprandial blood glucose control. However, we found that EGCG treatment also improved fasting blood glucose levels and intraperitoneal glucose tolerance, which only reflects a direct response of β-cells to circulating glucose(Reference Vialettes, Vague and Lassmann42–Reference Reimer and Russell44). Consistently, mice fed EGCG had about a threefold increase in circulating insulin levels compared with the control group. Therefore, the potential effect of dietary EGCG on intestinal events related to nutrient absorption plays no significant role in EGCG action in NOD mice. Rather, these data suggest that the anti-diabetic effect of EGCG may be at least partially due to the preservation of functional β-cell mass, an aspect that requires further investigation.
While pathogenic mechanisms and T-cell-mediated autoimmune process that destroy pancreatic β-cells in T1D are complex and are still not fully defined(Reference Sparre, Larsen and Heding45–Reference Tsai, Shameli and Santamaria48), it is clear from past studies that the infiltration of immune cells, such as T-helper type 1 cells and macrophages, into the islets and subsequent insulitis are hallmarks of the pathogenesis of T1D. Activated T cells and macrophages release several pro-inflammatory cytokines, such as IL-1β, IFN-γ and TNF-α, which are believed to be important mediators leading to β-cell destruction in T1D(Reference Mandrup-Poulsen, Helqvist and Molvig27–Reference Li, El-Kholy and Rhodes32). EGCG has been reported to modulate lymphocyte(Reference Kawai, Tsuno and Kitayama49, Reference Watson, Vicario and Wang50), macrophage(Reference Ichikawa, Matsui and Imai51, Reference Lyu and Park52) and dendritic cell functions(Reference Morel, Feili-Hariri and Coates53, Reference Proietto, O'Keeffe and Gartlan54), leading to the suppression of pro-inflammatory cytokine release and immune response. These results suggest that EGCG may protect the islets from immune-cell-mediated toxicity. However, the biological relevance of these in vitro findings is largely unknown because these studies used EGCG in doses (10–100 μm) far beyond levels physiologically attainable through dietary means(Reference Riemersma, Rice-Evans and Tyrrell39, Reference Van Amelsvoort, Van Hof and Mathot40).
In the present study, we first measured the circulating levels of inflammation-related cytokines, which are indicators of immune cell activity. While EGCG had no effect on most of the cytokines tested in the present study, it greatly increased plasma levels of IL-10, an anti-inflammatory cytokine that can reduce the risk of T1D in NOD mice(Reference Goudy, Song and Wasserfall55). This observation indicates that the effect of EGCG on T1D onset may be mediated by stimulating IL-10 production. Paradoxically, we observed that EGCG treatment also increased plasma levels of the pro-inflammatory cytokine IL-12, which was reported to enhance T1D development(Reference Aoki, Borchers and Ridgway56). In addition, we did not find that EGCG intake significantly modulated immune cell infiltration in the islets. Based on these results, we speculate that EGCG may not act through modulating immunity, but rather through protecting the infiltrated immune cell-mediated β-cell destruction, and thereby preserving β-cell mass and insulin secretion.
To test this speculation, we assessed the effect of EGCG on the viability of freshly isolated human islets exposed to the inflammatory milieu relevant to the pathogenesis of T1D. We found that EGCG promoted human islet viability and preserved its three-dimensional organisation, the typical islet cell aggregates that are crucial for preserving β-cell function(Reference Beattie, Montgomery and Lopez57, Reference Farilla, Bulotta and Hirshberg58). In parallel to increased cell viability, we showed that EGCG potently inhibited caspase-3 activity in cultured human islets, further confirming a direct anti-apoptotic effect of EGCG on pancreatic β-cells. These results suggest that EGCG treatment may directly exert a cytoprotective effect on the islets instead of suppressing immune cell infiltration, which results in improved islet mass and function. However, how EGCG exerts such a protective effect on islet cells is presently unknown. It is well recognised that activation of NF-κB is a crucial step for various cytokine-stimulated β-cell dysfunctions(Reference Kwon, Corbett and Rodi59, Reference Thomas, Darwiche and Corbett30). The NF-κB-mediated destruction of β-cells is executed at least partially through the induction of its downstream gene inducible NO synthase and subsequent NO production(Reference Storling, Binzer and Andersson60, Reference Li and Mahato61). The critical role of inducible NO synthase-derived NO in the pathogenesis of T1D has been demonstrated in β-cell-specific inducible NO synthase knockout and transgenic animals(Reference Heitmeier, Scarim and Corbett62–Reference Flodstrom, Tyrberg and Eizirik64). However, it is presently unknown whether EGCG at physiologically relevant doses can suppress the NF-κB pathway activated by pro-inflammatory cytokines in β-cells.
In summary, we provide evidence for the first time that dietary intake of EGCG can delay the development of T1D in NOD mice. This protective effect is probably due to the preservation of functional β-cell mass. In line with this finding, EGCG also exerts a cytoprotective effect on human pancreatic islets exposed to the inflammatory milieu relevant to T1D. However, further studies are needed to elucidate the mechanism for this EGCG action, which will provide valuable information for clinical trial to further evaluate its anti-diabetic potential in humans with T1D.
Acknowledgements
The present study was supported by grants from the American Diabetes Association Junior Faculty Award (to D. L.), Virginia Commonwealth Health Research Board (to D. L.) and the National Institutes of Health (1R21AT004694 to D. L.). We are grateful for the following Islet Cell Resource Centers for providing human pancreatic islets: City of Hope National Medical Center, Duarte, CA; Washington University at St Louis, MO; the University of Minnesota at Minneapolis, MN; the University of Miami, FL; the University of Illinois at Chicago, IL; the University of Alabama at Birmingham, AL; the University of Pennsylvania, Philadelphia, PA; the University of Wisconsin at Madison, WI; Northwestern University, Chicago, IL; Joslin Diabetes Center, Boston, MA; and for the Juvenile Diabetes Research Foundation Centers at the Southern California Islet Consortium; Washington University at St Louis, MO; University of Pittsburgh, Pittsburgh, PA; Massachusetts General Hospital, Boston, MA. D. L. is a Taishan Scholar and guest professor at Qingdao University, China. The contributions of authors were as follows: D. L. designed the study; Z. F., W. Z. and J. Y. performed the study; Z. F. and D. L. analysed the data and wrote the manuscript. The authors have no conflicts of interest.








