Lifestyle-related metabolic disorders such as hypertension, diabetes and dyslipidaemia play a crucial role in the development of atherosclerosis and CVD. Low-grade, but chronic inflammation is considered to be involved in the development of metabolic disorders. Among these, inflammation of adipose tissue is associated with obesity and directly promotes systemic low-grade inflammation and metabolic dysfunction( Reference Weisberg, McCann and Desai 1 – Reference Guilherme, Virbasius and Puri 3 ).
It has been reported that two naturally existing milk casein-derived bioactive tripeptides, isoleucine-proline-proline (IPP) and valine-proline-proline (VPP), produced during lactic acid bacteria fermentation or in casein hydrolysates, inhibit angiotensin-converting enzyme activity( Reference Nakamura, Yamamoto and Sakai 4 , Reference Mizuno, Nishimura and Matsuura 5 ), and foods containing these peptides reduce blood pressure in several clinical trials( Reference Cicero, Gerocarni and Laghi 6 , Reference Cicero, Aubin and Azais-Braesco 7 ). VPP and IPP have also been shown to induce the production of vasodilative substances including NO in human umbilical vein endothelial cells and isolated rat arterial vessels( Reference Hirota, Nonaka and Matsushita 8 ). Several recent clinical trials have shown that intake of casein hydrolysate containing VPP and IPP for 1 week improved vascular endothelial dysfunction without affecting systemic blood pressure in subjects with untreated stage-I hypertension( Reference Hirota, Ohki and Kawagishi 9 ). Moreover, an 8-week intervention using the same regimen has been shown to decrease central systolic blood pressure and arterial stiffness (brachial–ankle pulse wave velocity and carotid arterial compliance)( Reference Yoshizawa, Maeda and Miyaki 10 , Reference Nakamura, Mizutani and Ohki 11 ). Animal studies have revealed that in male apoE − / − mice, continuous intake of VPP, IPP or fermented milk, or casein hydrolysate containing these peptides, leads to a significant decrease in the development of atherosclerosis (intima to media thickness in the aortic arch)( Reference Nakamura, Hirota and Mizushima 12 ). In vitro studies have shown that VPP and IPP attenuated phorbol 12-myristate 13-acetate (PMA)-stimulated adhesion of monocytic cells (THP-1) to activated human umbilical vein endothelial cells via suppression of the PMA-induced up-regulation of β1 and β2 integrin activation( Reference Aihara, Ishii and Yoshida 13 ). The mechanism of action may involve the inhibition of c-Jun N-terminal kinase phosphorylation in THP-1. These data suggest a distinct anti-inflammatory effect of VPP on vascular inflammation and atherosclerosis.
In the present study, we focus on the role of the VPP peptide in modulating obesity-related chronic inflammation under high-fat diet (HFD) conditions in vivo. We demonstrate that oral administration of VPP exerts an anti-inflammatory effect on the adipose tissue of HFD-fed mice via the inhibition of the accumulation of pro-inflammatory macrophages in the stromal vascular fraction (SVF).
Materials and methods
Animals
Male C57BL/6J mice (7 weeks of age; day 0) were obtained from Oriental Yeast Company Limited. They were fed with normal chow (NC; CLEA Japan, Inc.) or a HFD (20 % beef tallow and 1·25 % cholesterol; CLEA Japan, Inc.). Mice fed with NC were given plain tap water (NC group) and those fed the HFD were given plain tap water (HFD group) or water containing 0·3 mg VPP/ml (HFD+VPP group) for 10 weeks. VPP was provided by Calpis Company Limited.
Both food and water were provided ad libitum throughout the course of the experiments. Mice were euthanised under anaesthesia induced by an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Blood samples were collected from the tail vein to measure blood glucose, insulin and lipid concentrations. Blood samples, epididymal adipose tissue and the liver were isolated and immediately frozen in liquid N2, and then stored at − 80°C until analysis.
All animal experiments were approved by the Ethical Committee for Animal Experimentation of Tokyo Medical and Dental University, Tokyo and conducted according to the institutional guidelines.
Flow cytometric analysis of circulating leucocytes
Leucocytes were prepared by haemolysing whole blood. The cells were incubated with anti-mouse antibodies (fluorescein isothiocyanate (FITC)-CD11b, phycoerythrin (PE)-CD11c, FITC-CD29, PE-CD18, FITC-CD49d, FITC-F4/80 (BioLegend, Inc.); Alexa Flour 647-CD204 (AbD Serotec)) for 45 min on ice. After washing, fluorescence intensity was measured from cell fractions containing 5000 cells using a FACSCalibur (BD Biosciences), and data were analysed with FlowJo software (Tree Star, Inc.). When fluorescence activity was detected, data were obtained from monocytes using gating definitions, as reported previously( Reference Webster, Bowles and Karim 14 – Reference Hagita, Osaka and Shimokado 16 ).
Flow cytometric analysis of cell populations in the stromal vascular fractions of adipose tissue
The SVF of each mouse was prepared as described previously( Reference Hagita, Osaka and Shimokado 16 ). Briefly, the SVF was isolated from the adipose tissue of mice in the control, HFD and HFD+VPP groups after a test period of 10 weeks. Adipose tissue was excised, minced and incubated in PBS with heparin (38·5 μg/ml; 5 U/ml) for 30 s to remove circulating blood cells, then the suspension was centrifuged at 1000 g for 8 min and the collected adipose tissue was incubated with type 2 collagenase in Tyrode's buffer (137 mm-NaCl, 5·4 mm-KCl, 1·8 mm-CaCl2, 0·5 mm-MgCl2, 0·33 mm-NaH2PO4, 5 mm-HEPES and 5 mm-glucose). The digested adipose tissue solution was centrifuged at 1000 g for 8 min, and pellets containing the SVF were resuspended in PBS followed by filtration through a 70 μm cell strainer (BD Biosciences). Isolated cells were incubated with anti-mouse antibodies (FITC-CD11b, PE-CD11c, FITC-F4/80 (BioLegend, Inc.); Alexa Flour 647-CD204 (AbD Serotec)) for 45 min on ice, followed by washing. Fluorescence intensity was measured from 5000 cell fractions using a FACSCalibur (BD Biosciences), and data were analysed with FlowJo software (Tree Star, Inc.). When fluorescence activity was detected, data were obtained from monocyte/macrophage subsets using gating definitions, as reported previously( Reference Hagita, Osaka and Shimokado 16 , Reference Brake, Smith and Mersmann 17 ). Cell populations in the SVF consisted of M1 macrophages (F4/80+/CD11c+ cells), M2 macrophages (F4/80+/CD204+ cells) and activated monocytes (CD11b+/CD11c+ cells), as reported previously( Reference Wu, Perrard and Wang 18 – Reference Lumeng, Bodzin and Saltiel 24 ).
Histological analysis of visceral adipose tissue
At the end of each experiment, epididymal adipose tissue of mice were dissected and weighed. A portion of the tissue was fixed with 10 % formalin in neutral buffer solution, and the fixed epididymal fat tissue was dehydrated through a graded ethanol series, embedded in paraffin, cut into 5 μm sections, and stained with haematoxylin and eosin. For immunohistochemical analysis, the 5 μm sections were deparaffinised and rehydrated, treated with 1 % of H2O2 in methanol, and subjected to antigen retrieval in a high-pressure steamer, followed by blocking in skimmed milk. Subsequently, the sections were incubated with anti-mouse F4/80 antibodies (BioLegend, Inc.) overnight at 4oC. After washing, they were probed with Histofine® Simple Stain Mouse MAX PO (Rat) (Nichirei Corporation) and then stained with the peroxidase substrate diaminobenzidine (Dako Denmark A/S), followed by counterstaining with haematoxylin and examination under a light microscope (Olympus IX71; Olympus Corporation) equipped with a SPOT RT Color-2000 digital camera (Diagnostic Instruments, Inc.). Crown-like structures were quantified in five random fields in each group of animals.
RNA isolation and quantitative real-time PCR analysis
Total RNA was isolated from frozen tissue samples using an RNeasy Lipid Tissue mini kit (Qiagen), and then mixed and reacted with a One-Step SYBR® PrimeScript® RT PCR Kit II (Takara Bio, Inc.). Real-time PCR analysis was performed with a LightCycler® 480 device (Roche Diagnostics). Relative mRNA transcript levels were calculated using the comparative cycle threshold method, which is based on the difference in cycle threshold values between the expression levels of target mRNA and 18S ribosomal RNA, which is used as an internal control. The primers used for real-time PCR analysis are described in Table 1.
Table 1 Primers used for real-time PCR analysis

CCL2, CC chemokine ligand 2; MCP-1, monocyte chemoattractant protein-1; CCR2, CC chemokine receptor 2; rRNA, ribosomal RNA.
Statistical analysis
Data are presented as means with their standard errors. Statistical analysis was carried out using the software Prism 5.0 (GraphPad Software, Inc.). The normality of data was assessed using the Kolmogorov–Smirnov test, and all data were normally distributed. Significant differences comparing more than two groups were analysed by the one-way ANOVA, and significant differences between two groups were analysed using the unpaired t test (two-tailed). A P value < 0·05 was considered to be statistically significant.
Results
Effect of valine-proline-proline on the physiological parameters of high-fat diet-fed mice
Male C57BL/6J mice (7 weeks of age) were fed with NC (NC group), a HFD and plain tap water (HFD group) or water containing 0·3 mg VPP/ml (HFD+VPP group) ad libitum for 10 weeks. Physiological variables of each treatment group are shown in Table 2. The body weight, epididymal fat weight and liver weight were not significantly different between the HFD and the HFD+VPP groups. There was also no significant difference in the concentrations of plasma total cholesterol, HDL- and LDL-cholesterol, TAG and glucose between the two groups.
Table 2 Body weight (BW), tissue weight and plasma parameters of normal chow (NC)-fed mice, high-fat diet (HFD)-fed mice and HFD+valine-proline-proline (VPP)-fed mice for a test period of 10 weeks (Mean values with their standard errors, n 7)

Effect of the oral administration of a high-fat diet supplemented with valine-proline-proline on integrin expression in peripheral blood monocytes
Activation of monocytes is closely correlated with systemic inflammatory status. We observed the expression levels of integrins in peripheral blood monocytes after mice were fed a HFD supplemented with or without VPP. As shown in Fig. 1, the HFD induced the relative expression levels of CD11b, CD11c, CD49d, CD18 and CD29. The induction of CD18 was statistically significantly decreased in mice administered with VPP.
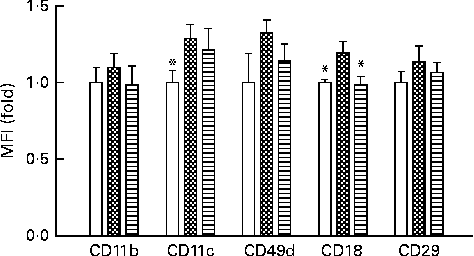
Fig. 1 Dietary fat and valine-proline-proline (VPP) modulates the expression levels of specific integrins on the surface of mouse peripheral blood monocytes. Flow cytometric analysis was used to assess the abundance of individual integrin species on the cell surface of monocytes from the peripheral blood of normal chow (NC)-fed mice (□), high-fat diet (HFD)-fed mice (![]() ) and HFD+VPP-fed mice (
) and HFD+VPP-fed mice (![]() ). Each group was treated for a test period of 10 weeks (n 5–8 animals per group), and specific integrins were identified using cognate monoclonal antibodies as described in the Materials and methods section. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the HFD group (P< 0·05). MFI, mean fluorescence intensity.
). Each group was treated for a test period of 10 weeks (n 5–8 animals per group), and specific integrins were identified using cognate monoclonal antibodies as described in the Materials and methods section. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the HFD group (P< 0·05). MFI, mean fluorescence intensity.
Effect of the oral administration of a high-fat diet supplemented with valine-proline-proline on the polarisation of monocytes and macrophages in the stromal vascular fractions of epididymal fat
Diet-induced obesity is associated with two phenotypic changes in adipose tissue macrophages: classically activated M1 macrophages and alternatively activated M2 macrophages.
After HFD feeding for 10 weeks, we examined the number of activated monocytes and M1 macrophages, identified as CD11b+/CD11c+ and F4/80+/CD11c+, respectively, in the SVF of epididymal fat tissue in the NC, HFD and HFD+VPP groups. The number of activated monocytes (CD11b+/CD11c+ cells) was significantly increased in response to the HFD in the SVF of epididymal fat tissue in the HFD group, when compared with the control group. Treatment of HFD-fed mice with VPP did not significantly reduce the number of activated monocytes. The number of M1 macrophages (F4/80+/CD11c+ cells) in the HFD group was significantly increased when compared with the control group. However, this increase was significantly reduced by the administration of VPP. By contrast, the number of M2 macrophages (F4/80+/CD204+ cells) did not differ among the three groups (Fig. 2).
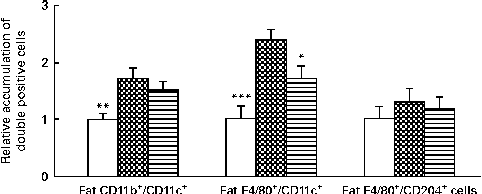
Fig. 2 Valine-proline-proline (VPP) attenuates high-fat diet (HFD)-induced macrophage infiltration into adipose tissue. After a test period of 10 weeks, stromal vascular fractions were isolated from the epididymal visceral adipose tissue of the different treatment groups (normal chow (NC)-fed mice (□), high-fat diet (HFD)-fed mice (![]() ) and HFD+VPP-fed mice (
) and HFD+VPP-fed mice (![]() )), and the relative amounts of activated monocytes (CD11b+/CD11c+ cells), M1 macrophages (F4/80+/CD11c+ cells) and M2 macrophages (F4/80+/CD204+ cells) were determined by flow cytometric analysis. Values are means (n 6–7 animals per group), with their standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: * P< 0·05, ** P< 0·01, *** P< 0·001.
)), and the relative amounts of activated monocytes (CD11b+/CD11c+ cells), M1 macrophages (F4/80+/CD11c+ cells) and M2 macrophages (F4/80+/CD204+ cells) were determined by flow cytometric analysis. Values are means (n 6–7 animals per group), with their standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: * P< 0·05, ** P< 0·01, *** P< 0·001.
Valine-proline-proline reduces macrophage infiltration into the adipose tissue of high-fat diet-fed mice
To directly observe the inflammation in adipose tissue, we counted the number of crown-like structures in the epididymal fat tissue of HFD-fed mice. The crown-like structures comprised F4/80+ adipose tissue macrophages surrounding an adipocyte( Reference Cinti, Mitchell and Barbatelli 25 , Reference Nishimura, Manabe and Nagasaki 26 ), which are rarely observed in those of standard diet-fed mice( Reference Weisberg, McCann and Desai 1 ). The number of crown-like structures in the HFD+VPP group was significantly decreased compared with that in the HFD group (Fig. 3(a)), which was consistent with the reduction in the number of pro-inflammatory M1 macrophages (Fig. 2).

Fig. 3 Valine-proline-proline (VPP) reduces adipose tissue inflammation and macrophage accumulation in the adipose tissue of high-fat diet (HFD)-fed mice. The formation of crown-like structures (CLS) in the epididymal adipose tissue of HFD-fed and HFD+VPP-fed mice for a test period of 10 weeks was analysed. (a) Histology of the epididymal adipose tissue of HFD-fed and HFD+VPP-fed mice. Epididymal fat tissue was stained with the anti-F4/80 antibody. The arrows indicate the CLS. (b) Quantification of the number of CLS in HFD-fed and HFD+VPP-fed mice. The number of CLS was counted as described in the Materials and methods section. Values are means (n 7 animals per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the HFD group (P< 0·05). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Valine-proline-proline suppresses inflammatory gene expression in adipose tissue
M1 macrophages in adipose tissue secrete pro-inflammatory cytokines, such as TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1), thus contributing to the induction of insulin resistance( Reference Lumeng, Bodzin and Saltiel 24 , Reference Gordon and Taylor 27 , Reference Mantovani, Sica and Sozzani 28 ). We then measured the expression levels of mRNA prepared from the adipose tissue of mice fed the HFD supplemented with or without VPP.
The expression of MCP-1, a monocyte or macrophage chemotactic factor, was significantly reduced in the HFD+VPP group when compared with the HFD group (Fig. 4). This result suggests a causative role of MCP-1 in the recruitment of M1 macrophages in adipose tissue. These VPP-mediated reductions in the expression of MCP-1 play a key role in the stabilisation of adipose tissue inflammation via M1 macrophages.
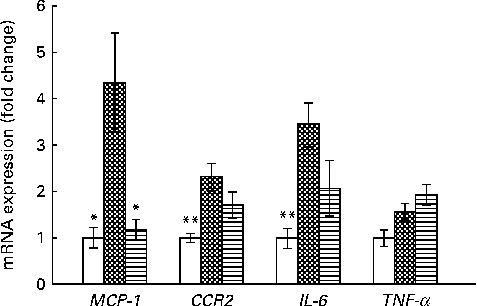
Fig. 4 Valine-proline-proline (VPP) suppresses the expression of pro-inflammatory cytokines and the chemokine receptor in adipose tissue. mRNA expression of inflammatory markers in epididymal adipose tissue from mice fed with normal chow (NC; □), a high-fat diet (HFD; ![]() ) and a HFD+VPP (
) and a HFD+VPP (![]() ) for a test period of 10 weeks. Values are means (n 5 animals per group), with their standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: * P< 0·05, ** P< 0·01. MCP-1, monocyte chemoattractant protein-1; CCR2, CC chemokine receptor 2.
) for a test period of 10 weeks. Values are means (n 5 animals per group), with their standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: * P< 0·05, ** P< 0·01. MCP-1, monocyte chemoattractant protein-1; CCR2, CC chemokine receptor 2.
Discussion
In the present study, oral administration of a naturally occurring tripeptide, VPP, diminished obesity-related chronic inflammation induced by HFD feeding in mice. We observed a local anti-inflammatory effect of VPP on adipose tissue via the inhibition of the accumulation of pro-inflammatory macrophages in the SVF, without any change in body weight and epididymal fat weight. Anti-inflammatory properties of food-derived peptides have been reported in several previous studies( Reference Nakamura, Hirota and Mizushima 12 , Reference Aihara, Ishii and Yoshida 13 , Reference Hernández-Ledesma, Hsieh and de Lumen 29 – Reference Cam and de Mejia 31 ). However, to our knowledge, the present study is the first aimed at examining the anti-inflammatory effect exerted by the oral administration of such peptides on adipose tissue inflammation and pro-inflammatory macrophages.
The HFD enhances the expression levels of integrins in monocytes and increases the amount of MCP-1 in the peripheral blood. In addition, it leads to the accumulation of activated monocytes and M1 macrophages in visceral adipose tissue( Reference Hagita, Osaka and Shimokado 16 , Reference Wu, Perrard and Wang 18 , Reference Wu, Gower and Wang 32 ). In the present study, we examined the number of M1 macrophages that infiltrated into epididymal fat tissue, as a function of diet and casein-derived peptides. An important question is whether the increase that we observed, in the levels of M1 macrophages infiltrating into adipose tissue in HFD-fed mice, is due to a phenotypic change from M2 to M1 macrophages, or whether circulating pro-inflammatory monocytes are recruited into adipose tissue directly from the bloodstream( Reference Stout, Jiang and Matta 33 , Reference Lumeng, DelProposto and Westcott 34 ). In the present study, we found that HFD feeding resulted in an increase in M1 macrophages (F4/80+/CD11c+ cells) in adipose tissue, as reported previously, whereas the number of M2 macrophages (F4/80+/CD204+ cells) was not affected, even in the HFD group. This strongly suggests that the net increase in the infiltration of M1 macrophages may be due to the direct recruitment of circulating pro-inflammatory monocytes or macrophages into adipose tissue. The administration of VPP significantly decreased the number of M1 macrophages but not M2 macrophages in epididymal fat tissue, in response to the HFD treatment. This indicates a role of VPP in regulating the recruitment of pro-inflammatory cells and, therefore, in the regulation of diet-induced inflammatory changes in adipose tissue.
Previous studies have indicated that the MCP-1/CC chemokine receptor 2 (CCR2) pathway contributes to macrophage polarisation in adipose tissue( Reference Weisberg, Hunter and Huber 35 ). In agreement with that study, we found that the expression levels of MCP-1, CCR2 and IL-6 in adipose tissue were increased in response to the HFD treatment, and that VPP reduced the HFD-dependent expression levels of MCP-1. These data suggest that VPP modulates HFD-induced MCP-1/CCR2 signalling in adipose tissue. However, the effect of VPP on the reduction in M1 macrophage infiltration was relatively small when compared with its effect on the expression levels of MCP-1. In the present study, we failed to observe a systemic effect of VPP on the amount of MCP-1 in the plasma of HFD-fed mice (data not shown). Therefore, another mechanism independent of MCP-1 may also contribute to the activation of M1 macrophages( Reference Inouye, Shi and Howard 36 , Reference Kirk, Sagawa and McDonald 37 ).
Monocyte–endothelial interactions, including tethering, rolling, firm adhesion and extravasation, form one of the crucial steps leading to atherosclerosis, which is mediated by various adhesion molecules expressed on leucocytes and endothelial cells. In the present study, we investigated whether the expression levels of integrins in monocytes were altered in mice fed the HFD supplemented with or without VPP. We found that the expression level of CD18 was significantly decreased by the administration of VPP (Fig. 1). It has previously been reported that mRNA levels of intercellular adhesion molecule-1 (ICAM-1) were increased specifically in the visceral adipose tissue of male wild-type mice after 3 weeks of HFD feeding( Reference Brake, Smith and Mersmann 17 ). In addition, increased leucocyte–endothelial cell interaction was observed in the microcirculation of visceral adipose tissue in ob/ob and HFD-induced obese mice, as well as up-regulated expression of ICAM-1, E-selectin and P-selectin on endothelial cells (CD31+ cells) of visceral adipose tissue in ob/ob mice( Reference Nishimura, Manabe and Nagasaki 38 ). The β2-integrin, also known as CD18, forms a dimer with the α-integrins CD11a, CD11b and CD11c, and the heterodimer binds to the cognate ligands ICAM-1 (CD11a and CD11b) and vascular cell adhesion molecule-1 (CD11c) on the endothelial surface, thus contributing to the adhesion between monocytes and endothelial cells. As we have previously found that IPP and VPP significantly reduced PMA-stimulated THP-1 cell adhesion to activated human umbilical vein endothelial cells via β2-integrin in THP-1 cells( Reference Aihara, Ishii and Yoshida 13 ), the present observations generalise these findings to the microvasculature of adipose tissue in a whole animal model.
Previous studies have shown that VPP was absorbed intact through the intestine, and moved into the systemic circulation( Reference Masuda, Nakamura and Takano 39 – Reference Kawaguchi, Nakamura and Kamiie 42 ). After entering the plasma, these peptides become accessible to adipose tissue. The previous reports have also shown the successful detection of IPP and VPP in the aorta of spontaneously hypertensive rats after the oral administration of these peptides( Reference Masuda, Nakamura and Takano 39 , Reference Kawaguchi, Nakamura and Kamiie 42 ), and cyanine 3 (Cy3)-labelled derivatives of both peptides were localised in the endothelial cells of arterial vessels in rats( Reference Kawaguchi, Nakamura and Kamiie 42 ). Therefore, VPP may accumulate in the microvascular vessels of adipose tissue, and exert an in situ anti-inflammatory effect.
In conclusion, the results of the present study highlight the possibility that administration of VPP can ameliorate chronic inflammation induced by a HFD, at least in adipose tissue. The underlying mechanism by which VPP supplementation leads to the decrease in the accumulation of pro-inflammatory macrophages in the SVF involves the inhibition of the expression levels of pro-inflammatory cytokines and chemokines. Further studies are needed to elucidate the precise molecular mechanisms by which VPP negatively regulates inflammation. Ultimately, such understanding may eventually lead to the development of a preventive strategy for chronic inflammation-related diseases.
Acknowledgements
The present study was supported in part by Calpis Company Limited, Tokyo, Japan.
The authors' contributions are as follows: K. A. and M. Y. designed the research; K. A. and M. O. conducted the research and analysed the data; K. A. and M. Y. wrote the manuscript; K. A., M. O. and M. Y. reviewed and edited the manuscript. All authors read and approved the final manuscript.
K. A. is affiliated with Calpis Company Limited. The rest of the authors declare that there are no conflicts of interest.








