The role of vitamin D in skeletal health has been known for many years(Reference Lips1). Recently potential new effects of vitamin D have been studied, including a possible role in the prevention of certain types of cancer, multiple sclerosis, diabetes mellitus and various other conditions(Reference Holick2).
Vitamin D deficiency is common in non-Western immigrant groups living in northern Europe and the USA(Reference Awumey, Mitra, Hollis, Kumar and Bell3–Reference Meyer, Falch, Søgaard and Haug6). Little is known about vitamin D status before emigration. Some reports suggest that vitamin D status is higher in their countries of origin(Reference Rashid, Mohammed, Stephens, Warrington, Berry and Mawer7), probably due to higher sun exposure, whereas others indicate low vitamin D status also in Asian countries(Reference Arya, Bhambri, Godbole and Mithal8–Reference Lips12). A comparison of vitamin D status in individuals who have emigrated and individuals who remain in their country of origin is needed in order to provide information about the effect of moving to northern latitudes.
We have previously reported poor vitamin D status among immigrants from Sri Lanka living in Oslo, Norway, who participated in a population-based study(Reference Holvik, Meyer, Haug and Brunvand13). We have now performed a population-based study in Kandy, Sri Lanka enabling us to compare vitamin D status in Sri Lankans living at latitudes 7° north and 60° north in these two cross-sectional studies.
Subjects and methods
Participants
The study in Sri Lanka was performed in August–December 2005 in Kandy, Central Province of Sri Lanka; a city with 110 000 inhabitants. Kandy has a tropical climate with two Monsoons with a lot of rain (May to July and November to January). As most Sri Lankans participating in the preceding Oslo Immigrant Health Study in 2002 had a Tamil background, the study in Sri Lanka aimed to include only Tamils. The age was restricted to 30–60 years. First, all Tamils identified by family name from the 2004 electoral list and residing within the Kandy Municipal Council city limits were selected. Of these, a random sample was selected, men and women separately. The sample included all ages above 18 years as age was not included in the electoral list. All selected were invited individually at a house visit after verifying age. Out of the 399 individuals we attempted to contact, twenty-five were inaccessible or had moved from the address. Of those contacted, twenty-six were above age 60 years and sixty-eight were below age 30 years. The remaining 280 individuals (139 men and 141 women) were invited to participate. Of these, 103 men (74·1 %) and 130 women (92·2 %) took part in the study. Due to logistic problems blood samples were available for determination of serum 25-hydroxyvitamin D (s-25(OH)D) in 196 of the 233 participants.
Comparison was made with individuals born in Sri Lanka and currently living in Oslo, Norway. As previously described, the population-based Oslo Immigrant Health Study was carried out in 2002 under the joint collaboration of the Norwegian Institute of Public Health and the University of Oslo. All individuals born between 1942 and 1971 in Sri Lanka (aged 31–60 years) residing in Oslo were invited to participate. In addition, individuals born in Turkey, Iran, Pakistan, and Vietnam were invited(Reference Holvik, Meyer, Haug and Brunvand13), but they are not included in the present paper; neither are data from the additional young cohort born between 1972 and 1982. The participation rate among the Sri Lankans in Oslo was 50·9 %. All individuals included between 3 April and 10 June had their s-25(OH)D levels analysed (n 242). April to June is the period when 25(OH)D is expected to increase from the low winter status. Due to latitude, practically no cutaneous vitamin D production occurs in Oslo during October to March.
Data collection
In Kandy, Sri Lanka, data were collected by an interviewer-administered questionnaire, a clinical examination and the collection of a non-fasting blood sample.
As reported elsewhere(Reference Holvik, Meyer, Haug and Brunvand13), the participants in Oslo, Norway filled in a self-administered questionnaire, underwent a clinical examination and had a non-fasting venous blood sample drawn.
Blood sample analyses
The samples from Sri Lanka were placed at 0–4°C in a vaccine carrier immediately after collection and transported to a laboratory and centrifuged at 2000 rpm for 6 min to separate serum within 6 h of collection. Separated serum was stored as three separate samples below − 50°C in 1 ml NUNC CryoTubes (NUNC A/S, Roskilde, Denmark). It was sent by express mail to Oslo in dry ice, and at arrival it was still deep frozen and was stored at − 70°C until analysed.
As previously reported from the Oslo Immigrant Health study(Reference Holvik, Meyer, Haug and Brunvand13), s-25(OH)D was measured by RIA (DiaSorin, Stillwater, MN, USA), and the same method was used in the samples from Sri Lanka. This assay measures the sum of s-25(OH)D2 and s-25(OH)D3. The intra- and inter-assay CV were 6 and 14–15 %, respectively. The detection limit was 6 nmol/l. All analyses were performed at the Hormone Laboratory (Aker University Hospital, Oslo, Norway).
For comparison we also present previously published data on s-25(OH)D from the population-based Oslo Health Study 2000–1, restricted to 580 individuals aged 45–60 years with country of birth Norway (quoted as ethnic Norwegians). Subjects and methods are described in our previous publication(Reference Meyer, Falch, Søgaard and Haug6).
Comparisons of means were done by t test and χ2 tests were used for comparisons of proportions. Although not normally distributed, means and t tests are presented for 25(OH)D, as medians and non-parametric tests gave similar results. Multivariate adjusted means were calculated by ANOVA.
Results
A total of 196 Sri Lankans aged 30–60 years living in Kandy, Sri Lanka and 242 Sri Lankans aged 31–60 years living in Oslo, Norway were included in the analysis. Those living in Sri Lanka were somewhat older, included a larger proportion of women, and had had on average a shorter duration of education than those living in Norway (Table 1).
Table 1 Background characteristics and vitamin D status in Sri Lankans living in Kandy, Sri Lanka and Oslo, Norway
(Mean values and standard deviations)
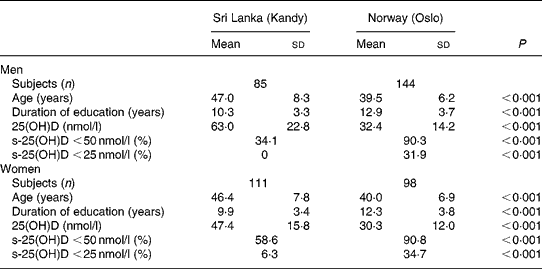
25(OH)D, 25-hydroxyvitamin D; s-25(OH)D, serum 25(OH)D.
Sri Lankans living in Norway had substantially lower s-25(OH)D (mean 31·5 nmol/l) compared with those living in Sri Lanka (mean 54·2 nmol/l). The prevalence of s-25(OH)D < 50 nmol/l was 1·9 times higher (95 % CI 1·6, 2·2) and the prevalence of s-25(OH)D < 25 nmol/l was 9·3 times higher (95 % CI 4·4, 9·6) in Norway compared with Sri Lanka. Mean s-25(OH)D was substantially higher in men compared with women in Sri Lanka (P < 0·001), whereas there was no sex difference among the Sri Lankans living in Norway (P = 0·25). There was no significant relationship between age and s-25(OH)D in any of the groups. s-25(OH)D was related to season in Sri Lanka. Men examined in August–September had a mean level of 43·3 nmol/l increasing to 55·8 nmol/l in October, 70·7 nmol/l in November and 87·0 nmol/l in December. The corresponding values in women were 36·7 nmol/l in August–September, 48·0 nmol/l in October, 59·4 nmol/l in November and 50·2 nmol/l in December. Out of seven women in Sri Lanka with s-25(OH)D < 25 nmol/l, five were examined in August–September. In Oslo, mean s-25(OH)D increased from 30·5 nmol/l in April to 38·9 nmol/l in June in men (P = 0·034) whereas there was no statistically significant change in women.
After controlling for season, education was not related to s-25(OH)D in Sri Lanka, and neither was there any association with education in Norway.
Only one individual in Sri Lanka reported daily use of a vitamin D-containing supplement. In Norway, thirty-seven individuals (20 %) reported daily use of cod liver oil or other fish oil supplement. Such supplements typically contain 5–10 μg vitamin D, but the dose was not registered in the study. The use of these supplements was positively associated with s-25(OH)D, and so was frequency of fatty fish intake in men.
With the exception of an inverse relationship between BMI and s-25(OH)D in men in Sri Lanka, there were no statistically significant associations between BMI and s-25(OH)D or physical activity level and s-25(OH)D. Adjusting for age, sex, education and BMI left mean s-25(OH)D nearly unaltered (32·4 nmol/l in Sri Lanka and 53·3 nmol/l in Oslo).
When compared with ethnic Norwegians living in Oslo(Reference Meyer, Falch, Søgaard and Haug6), Sri Lankans living in Oslo had dramatically lower s-25(OH)D, but also the Sri Lankans in their home country had considerably lower levels (Fig. 1). Mean s-25(OH)D was 74·6 (sd 24·3) nmol/l in ethnic Norwegian men and 76·5 (sd 24·1) nmol/l in ethnic Norwegian women, and only 14·6 % of the men and 11·2 % of the women had s-25(OH)D < 50 nmol/l.

Fig. 1 Distribution of serum 25-hydroxyvitamin D (25(OH)D) in Sri Lankans living in Kandy, Sri Lanka (n 196) or Oslo, Norway (n 242) and ethnic Norwegians (n 580).
Discussion
We found substantially higher s-25(OH)D in Sri Lankans living in Kandy, Sri Lanka (latitude 7° north) compared with Sri Lankans living in Oslo, Norway (latitude 60° north). s-25(OH)D was strongly related to season in Sri Lanka with substantially lower levels at the beginning of the study in August–September than at the end of the study in November–December. The explanation for this is not clear, but a main contributor might be climate as the present study was preceded by the south-west Monsoon which is wet, hot and humid. Temperature starts to decrease in October and it stays cooler until February. Unfortunately we do not have information on individual sun exposure, but it is plausible that this increased during our study period. An alternative explanation would be that intake of foods rich in vitamin D increased. However, it is not realistic that this could explain an increase in s-25(OH)D from 43 to 87 nmol/l as seen in men. Another possibility could be that those examined at the start of the study have permanently lower levels of s-25(OH)D than those examined later. On the other hand, when taking season into account, there was no association between education and s-25(OH)D-levels. Finally, the assessment of s-25(OH)D could change over time. However, this is unlikely, as all samples were treated equally and stored at − 50°C before being sent to Norway where all analyses were done in the same laboratory.
At the end of the study, s-25(OH)D was 87 nmol/l in men and women reached a mean 25(OH)D of 59 nmol/l in November. In a study from rural Gambia, mean s-25(OH)D was 81 nmol/l in premenopausal women and 91 nmol/l in early postmenopausal women(Reference Aspray, Yan and Prentice14), and a study from Punjab reported median s-25(OH)D of 74 nmol/l(Reference Rashid, Mohammed, Stephens, Warrington, Berry and Mawer7). Levels in this range thus seem to be realistic achievable s-25(OH)D levels for highly pigmented individuals living near the equator. Interestingly, the mean level in ethnic Norwegians living in Oslo at latitude 60° north was of the same magnitude(Reference Meyer, Falch, Søgaard and Haug6). In ethnic Norwegians, the attainment of such a high mean level may be attributed to a positive attitude towards sun exposure, an efficient cutaneous vitamin D production in the light-skinned population, as well as a higher usual intake of fatty fish and vitamin D-containing supplements including cod liver oil. The general attitude towards sun exposure is negative in Sri Lanka. A fairer complexion is more desirable than a darker one. However, women are not covered for religious reasons and none of the participants in the present study were Muslims. However, we found that women in Sri Lanka were more prone to vitamin D deficiency than men (probably indicating less sun exposure), whereas there was no sex difference among Sri Lankans in Oslo.
The Sri Lankans living in Oslo, Norway had a very high prevalence of vitamin D insufficiency. This is supported by another recent study from Oslo which found that Sri Lankan Tamils not taking vitamin D supplements had a mean s-25(OH)D of 24 nmol/l in late winter, and 90 % had levels below 50 nmol/l(Reference Holvik, Madar, Meyer, Lofthus and Stene15). Thus, it is clear that the limited UV availability at latitude 60° north deteriorates vitamin D insufficiency further in dark-skinned immigrants from tropical and sub-tropical latitudes. Whether the attitude toward sun exposure has changed after migration to Norway is not known. These results indicate that the poor vitamin D status commonly found in non-Western immigrants living at northern latitudes should not be regarded as normal levels for these ethnic groups. However, also in women in Sri Lanka, s-25(OH)D was rather low in August–September when five out of twenty-nine women (17 %) had 25(OH)D < 25 nmol/l. But this is still a much lower prevalence compared with 35 % of Sri Lankan women in Oslo.
A strength of the present study was that the serum samples from both study sites were taken from population-based samples. A limitation was the lack of data concerning fish consumption in the Sri Lanka study. However, due to the inland location of the city of Kandy with a considerable distance to the ocean and high prices of seafood, fish consumption is probably low. We cannot exclude the possibility for selection bias in the Oslo data due to an attendance rate of about 50 %. However, a comprehensive analysis of non-attendance does not suggest this to be a major problem (http://www.fhi.no/dav/C1E43891DD.pdf).
In order to study how migration influences vitamin D status, the best would be to study individual changes in s-25(OH)D based on vitamin D assessment before and after migration. However, we are not aware of any such study. The vitamin D status in the study population in Kandy is not necessarily representative for the whole of Sri Lanka due to differences in UV exposure (variation in length of the rainy season, different UV exposure due to different altitudes), dietary differences including a higher fish consumption in coastal areas, etc.
We conclude that vitamin D status among Sri Lankans living in Kandy, Sri Lanka, was considerably higher than that among Sri Lankans living in Oslo, Norway. The low vitamin D status commonly observed in non-Western immigrant groups living at northern latitudes should not be regarded as normal levels for these groups. However, also in Sri Lanka we found a profound seasonal variation with the lowest levels in August and September after the Monsoon.
Acknowledgements
H. E. M. and S. U. B. T. planned the study. S. U. B. T. collected the data and C. M. L. was responsible for the vitamin D analyses. H. E. M. and K. H. analysed the data and drafted the article. S. U. B. T. and C. M. L. reviewed and commented on the manuscript. There are no conflicts of interest. The vitamin D analyses were funded by Anders Jahres fond til vitenskapens fremme. The study in Sri Lanka obtained ethical clearance from the Higher Degrees and Research Ethics Committee of the University of Peradeniya, Kandy, Sri Lanka. The study in Norway was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. Informed, written consent was collected from the participants in both studies.




