Worldwide, the population is ageing(Reference Suzman and Beard1). Europe is no exception; in 2017, 19·4% of the population was aged 65 years or older and this is expected to increase to 29·1% by 2080(2). The proportion of those aged 80 years and older is expected to more than double from 5·5% in 2017 to 12·7% by 2050(2). With this ageing population comes considerable societal challenges, as declining health status and disease can lead to disability and dependence(Reference Christensen, Doblhammer and Rau3). One such challenge is protein-energy malnutrition (PEM).
What is protein-energy malnutrition?
PEM, often referred to simply as malnutrition, is a condition resulting from inadequate intake of energy (kJ) and/or protein, or an inability to absorb and/or digest adequate energy and/or protein. PEM has many associated physiological and psychological consequences; these include a decline in physical and/or mental functionality, which can result in reduced quality of life, poor disease outcomes and more frequent and longer hospital stays(Reference Skates and Anthony4, Reference Sobotka5). Within the developed world, there has been much discussion about how PEM should be defined(Reference Corish and Kennedy6, Reference Meijers, van Bokhorst-de van der Schueren and Schols7). Although a gold-standard definition has yet to be agreed among the scientific community(Reference Cederholm, Bosaeus and Barazzoni8), the current consensus is that PEM can be broadly defined as a state resulting from lack of uptake or intake of nutrition leading to altered body composition (decreased fat free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease(Reference Sobotka5). Expert bodies have recommended that diagnostic criteria for PEM are agreed; this would enable comparison of the results of research studies and facilitate standardisation of clinical practice(Reference Corish and Kennedy6, Reference Meijers, van Bokhorst-de van der Schueren and Schols7). To comply with this recommendation, the European Society for Clinical Nutrition and Metabolism, in 2015, released a consensus statement that specified the minimum criteria that should be used to identify malnutrition(Reference Cederholm, Bosaeus and Barazzoni8). This consensus stated that malnutrition is present in individuals with BMI <18·5 kg/m2, in those with a combination of weight loss (either >10% of body weight over an indefinite period or >5% over 3 months) and low BMI (BMI <20 kg/m2 in those under 70 years and <22 kg/m2 in those over 70 years) and in those with low fat free mass index (<15 kg/m2 for women and <17 kg/m2 for men)(Reference Cederholm, Bosaeus and Barazzoni8). Very recently (September 2018) a global consensus on the criteria to diagnose malnutrition in adults in clinical settings has been proposed(Reference Cederholm, Jensen and Correia9, Reference Jensen, Cederholm and Correia10). This Global Leadership Initiative on Malnutrition recommends a two-step approach to the diagnosis of malnutrition; firstly, screening for risk of malnutrition and, secondly, assessment for diagnosis and grading the severity of malnutrition. Non-volitional weight loss, low BMI and reduced muscle mass are recommended as phenotypic criteria and reduced food intake or assimilation, and inflammation or disease burden are recommended as aetiologic criteria. To diagnose malnutrition, at least one phenotypic criterion and one aetiologic criterion should be present. Phenotypic metrics to grade severity into Stage 1 (moderate) and Stage 2 (severe) malnutrition are proposed. It is recommended that the aetiologic criteria are used to guide intervention and anticipated outcomes. Overlaps with syndromes such as cachexia and sarcopenia should be considered and the diagnostic construct should be reconsidered every 3–5 years.
Prevalence of protein-energy malnutrition in older adults
Due to discrepancies surrounding the definitions and diagnostic criteria used to identify malnutrition, there has been considerable variation in the prevalence of malnutrition reported between and within the hospital, rehabilitation, residential care and community settings. This issue is further aggravated by inconsistency in the terminology and outcomes being measured. Many studies interchangeably use the terms ‘malnutrition risk’ and ‘malnutrition’ to mean the same thing. Use of a malnutrition screening tool will invariably result in higher prevalence as these tools estimate the risk of malnutrition whereas a full assessment of a patient at risk of malnutrition using diagnostic measures of malnutrition will result in a lower prevalence. Moreover, the use of different diagnostic criteria and screening tools within each setting further aggravates this issue. Despite these concerns, it is generally agreed that approximately 5–10% of community-dwelling older adults, 50% of those in rehabilitation, 20% in residential care and 40% in the hospital are malnourished(Reference Kaiser, Bauer and Ramsch11). Malnutrition screening tools have a valuable role in identifying those who are at risk of malnutrition. However, it is imperative that those who are identified as at risk are referred to a nutritionally qualified healthcare professional with the competence to undertake a full nutritional assessment that incorporates consideration of the aetiology of the diagnosis of malnutrition and the implementation of an appropriate intervention(Reference Kondrup, Allison and Elia12). There is clear evidence that early identification of malnutrition risk followed by timely and appropriate intervention is associated with better nutritional care and lower malnutrition incidence(Reference Eglseer, Halfens and Lohrmann13).
The Malnutrition in the Elderly project
The ageing of the European population and its known association with PEM has stimulated research into this topic. The Malnutrition in the Elderly (MaNuEL) project is a Joint Programming Initiative under the A Healthy Diet for a Healthy Life theme that ran from early 2016 until September 2018. The focus of the project is on PEM in older persons aged 65 years and older and the project consortium comprises twenty-two research groups from seven countries (Austria, France, Germany, Ireland, Spain, The Netherlands and New Zealand) in addition to a stakeholder advisory board of experts in geriatric nutrition, representative of all relevant European expert societies. The project had four main objectives: to gain knowledge; to strengthen evidence-based practice; to build a better research network and to harmonise research and clinical practice across Europe. The design and objectives have previously been explained in detail(Reference Visser, Volkert and Corish14) but a brief outline is given below.
Knowledge gain
The MaNuEL project has summarised current knowledge through the use of systematic literature reviews and meta-analyses. These also aim to identify knowledge gaps where further research needs to be conducted.
Strengthen evidence-based practice
MaNuEL has synthesised current knowledge about the screening and identification of malnutrition among older persons in order to make evidence-based recommendations on the most appropriate methods for use in older adults in the different settings in which older adults live. The project also attempted to identify effective nutrition interventions to prevent or reverse malnutrition. In addition, the work of MaNuEL will contribute to recommendations on appropriate methods to assess the determinants of malnutrition that will be used to highlight important risk factors for malnutrition among different subgroups of older adults.
Build capacity
MaNuEL has worked to develop a competent network of researchers who have a track record of collaboration on the topic of malnutrition in older adults across Europe and globally, which will contribute to future collaborative projects.
Harmonise research and clinical practice
The MaNuEL project promotes the harmonisation of research, policy, education and clinical practice with regard to malnutrition in older adults. Particular emphasis is being placed on harmonising the screening and assessment of malnutrition in older persons in clinical practice with evidence-based research. Furthermore, harmonisation of data collection, databases and data analyses has occurred within the MaNuEL project. Combined, these efforts will facilitate future collaboration among partner countries and optimise ongoing work in the area.
Design of the Malnutrition in the Elderly knowledge hub
The MaNuEL Knowledge Hub comprised five connected work packages (Fig. 1): (i) defining malnutrition; (ii) screening for malnutrition; (iii) determinants of malnutrition; (iv) interventions to prevent and treat malnutrition; and (v) policies and education regarding malnutrition screening and interventions. The final, sixth work package focuses on the management of the overall project.
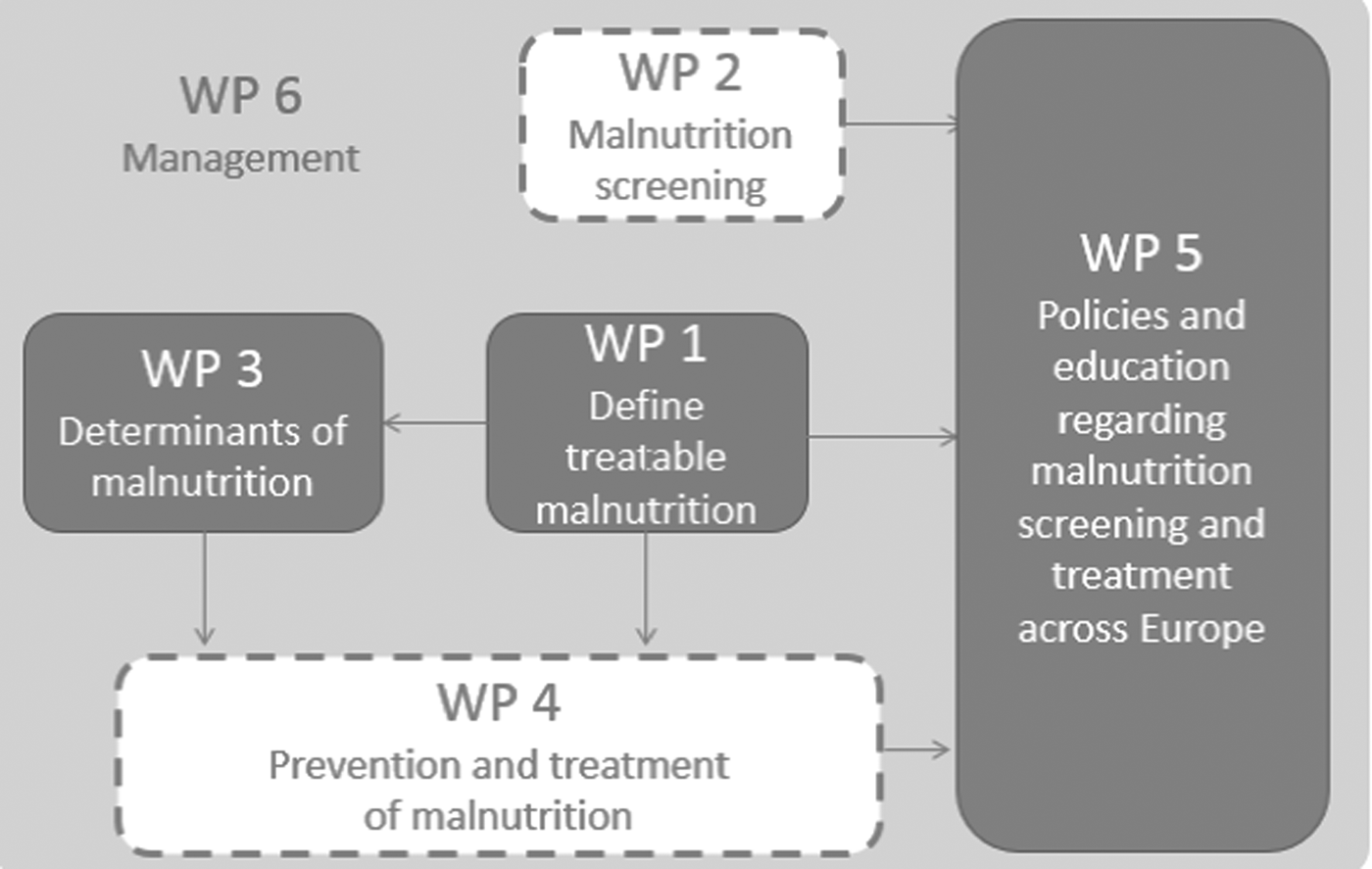
Fig. 1. Structure of the Malnutrition in the Elderly project. WP, work package.
The aim of the present paper is to report on the published findings that resulted from the compilation of existing data with regard to the screening and determinants of malnutrition in older adults(Reference Power, Mullally and Gibney15–Reference Bardon, Streicher and Corish18).
Malnutrition screening: why is it important and which tools are most appropriate to use?
Malnutrition screening is useful in identifying those who are malnourished or at risk of malnutrition(Reference Power, Mullally and Gibney15, Reference Skipper, Ferguson and Thompson19). Screening should be a quick process, conducted using a tool which has been validated in the population in which it is to be used(Reference Power, Mullally and Gibney15, Reference Skipper, Ferguson and Thompson19) and that is easy to administer. It is important that screening is conducted regularly so that the risk of malnutrition can be identified in a timely manner and appropriate interventions implemented. For older people, the frequency of malnutrition screening is dependent on the setting in which they reside. Within the hospital setting, it is recommended that screening takes place on a weekly basis(Reference Kondrup, Allison and Elia12). For older community-dwelling adults (>75 years), the UK National Institute for Health and Care Excellence recommends that screening for malnutrition should take place annually in general practitioner surgeries or where there is a clinical concern(20).
Current recommendations on which screening tool should be used favour a simplistic approach. For example, European Society for Clinical Nutrition and Metabolism recommends the Mini Nutritional Assessment for older adults in hospital, long-term care and community settings(Reference Kondrup, Allison and Elia12). Such an approach fails to consider the heterogeneity between these specific sub-groups of older adults with regard to the aetiology of their frailty status, level of dependence and concurrent morbidity. For example, the aetiology of malnutrition risk in a community-dwelling older person is likely to be different from that of a hospitalised older person or an older person who is in long-term residential care. Furthermore, the barriers to nutrition screening may, certainly in part, relate to their suitability for use in different settings. It is likely, therefore, that screening tools should be setting dependent.
Work package 2 of the MaNuEL project aimed to, firstly, create a database and review existing tools used to screen for malnutrition in older adults and, secondly, create a scoring system to rate these screening tools and identify those most appropriate to screen for malnutrition in older adults across all settings (community, hospital, rehabilitation, residential care). The review of screening tools identified forty-eight tools which are in use to screen for malnutrition in older populations, only thirty four of which have been validated for use in this population(Reference Power, Mullally and Gibney15). Some of these tools have not been designed or are inappropriate for use among older populations. Furthermore, the use of a large number of screening tools has led to poor comparability between studies and the wide disparity in the reported prevalence rates of malnutrition risk in older adults. Some studies have used screening tools to report the prevalence of malnutrition whereas the diagnosis of malnutrition requires more in-depth assessment as highlighted in the Global Leadership Initiative on Malnutrition consensus(Reference Cederholm, Jensen and Correia9, Reference Jensen, Cederholm and Correia10). The review highlighted that validation studies were predominantly conducted in hospital and community settings while validation studies in the rehabilitation and long-term care settings were lacking(Reference Power, Mullally and Gibney15). In all settings, many studies were poorly conducted(Reference Power, Mullally and Gibney15).
To create a scoring system to rate malnutrition screening tools, MaNuEL used a consensus panel of project partners and experts in geriatric malnutrition. The scoring system has been explained in detail previously(Reference Power, de van der Schueren and Leij-Halfwerk16). However, a brief description is as follows: the scoring system comprised three equally weighted domains based on the quality of the validation studies published, the evidence for the parameters used within the tool to indicate malnutrition risk in older adults and the practicability of the tool. Each domain could score a maximum of 15 points, giving an overall maximum score of 45 points. Within each domain, different weightings were agreed for each question, based on the evidence for their importance in screening for malnutrition in older adults. The scoring system was applied to each of the forty-eight tools identified within the review.
Using the combined score from the three domains, five screening tools were identified which presently appear to be best to use with older adults across community and healthcare settings. The highest scoring malnutrition screening tools for older adults were(Reference Power, de van der Schueren and Leij-Halfwerk16): (i) DETERMINE your health checklist in community-dwelling older adults (26 points); (ii) Nutritional Form for the Elderly for older persons in the rehabilitation setting (26 points); (iii) Short Nutritional Assessment Questionnaire-Residential Care for older adults within long-term care or residential settings (28 points); (iv) Malnutrition Screening Tool and the Mini Nutritional Assessment-Short Form for hospitalised older adults (both scoring 26 points).
The score within each domain for the different screening tools is presented in Table 1(Reference Power, de van der Schueren and Leij-Halfwerk16).
Table 1. Highest scoring malnutrition screening tools within each healthcare setting(Reference Power, de van der Schueren and Leij-Halfwerk16)
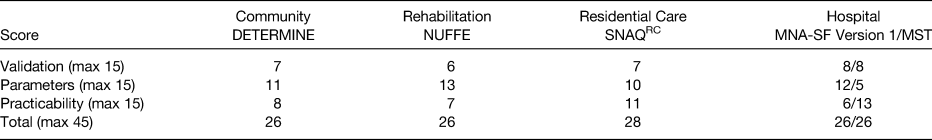
DETERMINE, Determine Your Health Checklist; MNA-SF, Mini Nutritional Assessment Short Form; MST, Malnutrition Screening Tool; NUFFE, Nutritional Form for the Elderly; SNAQRC, Short Nutritional Assessment Questionnaire Residential Care.
All tools scored low for validation, most scored reasonably well for the parameters included within the screening tools and variable results were obtained for the practicability of the tools. Further aspects of appraising malnutrition screening tools are inter-rater and intra-rater reliability analyses to measure the agreement of results of screening between two administrators or by the same administrator at different time points. Presently, little evidence exists on the reliability of malnutrition screening tools(Reference Power, de van der Schueren and Leij-Halfwerk16). Such studies are required and if conducted in a standardised and thorough manner, the inclusion of a reliability domain would add value when scoring malnutrition screening tools.
Based on the work of MaNuEL work package 2, it is recommended that more screening tools should not be developed but that we should concentrate on improving the evidence for those which already exist(Reference Power, de van der Schueren and Leij-Halfwerk16).
Determinants of malnutrition
Malnutrition is more prevalent in older adults compared with their younger counterparts(Reference Kyle, Unger and Mensi21). There are many social, environmental and health-related factors which can contribute to the development of malnutrition in this population group(Reference Volkert22). Although there are a plethora of studies which have set out to establish the determinants of malnutrition in older adults, by and large, these studies are cross-sectional in design and their ability to accurately identify those factors which predict and are not solely associated with malnutrition is poor. Hence, the results of these studies are highly heterogeneous(Reference Boulos, Salameh and Barberger-Gateau23–Reference Kvamme, Gronli and Florholmen30) and have done little to clarify accurately the true determinants or predictors of malnutrition in older people. There are only a small number of longitudinal studies that have been conducted among older populations to explore the factors that can predict malnutrition prior to its development(Reference Schilp, Wijnhoven and Deeg31–Reference Mamhidir, Ljunggren and Kihlgren34). A number of systematic reviews have been published on this topic in recent years(Reference Tamura, Bell and Masaki35–Reference Fávaro-Moreira, Krausch-Hofmann and Matthys37). These are summarised in Table 2. These reviews predominantly comprise cross-sectional studies, thus emphasising the need for high-quality longitudinal studies in older populations that investigate the broad range of factors which could influence the development of malnutrition(Reference van der Pols-Vijlbrief, Wijnhoven and Schaap36). Identification of determinants is vital to enable nutrition and healthcare strategies to be implemented that could potentially reduce the incidence of malnutrition in the older population.
Table 2. Published systematic literature reviews on the determinants of malnutrition in older adults
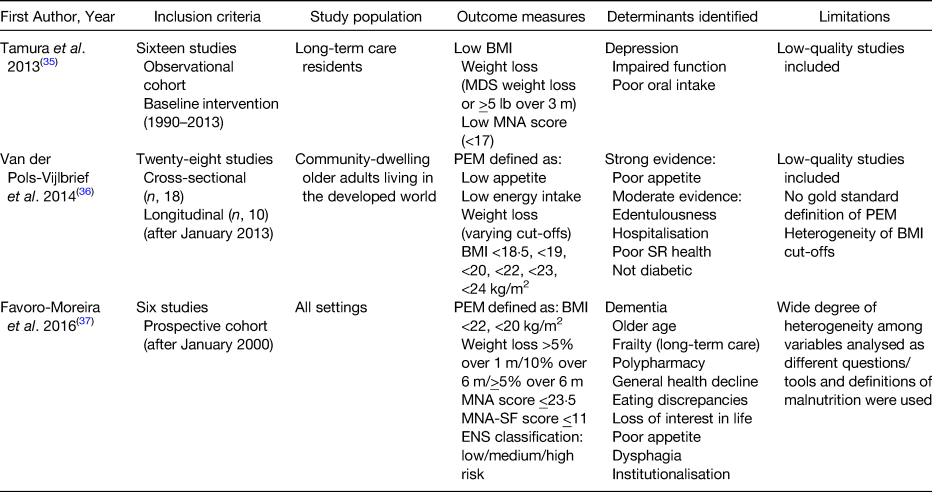
ENS, Elderly Nutrition Screening; m, month; MDS, minimum data set; MNA, Mini Nutritional Assessment; PEM, protein-energy malnutrition; SR, self-rated.
Work package 3 of the MaNuEL project conducted a multi-cohort meta-analysis of longitudinal studies from partner countries. This is the first meta-analysis of its kind and, therefore, provides significant progress in identifying the determinants of malnutrition in older adults(Reference Streicher, van Zwienen-Pot and Bardon17). This meta-analysis only included studies of longitudinal design and removed participants who were malnourished at baseline; therefore, distinguishing the determinants of malnutrition from the associated consequences of the condition. Six studies suitable for inclusion were identified; namely, ErnSiPP (Nutritional Situation of Community-dwelling Older Adults in Need of Basic Care; Germany(Reference Kiesswetter, Pohlhausen and Uhlig38)), ActiFe (Activity and Function in the Elderly; Germany(Reference Denkinger, Franke and Rapp39)), KORA-Age (Cooperative Health Research in the Region of Augsburg; Germany(Reference Peters, Döring and Ladwig40, Reference Holle, Happich and Lowel41)), LASA (The Longitudinal Aging Study Amsterdam, the Netherlands(Reference Hoogendijk, Deeg and Poppelaars42)), TILDA (The Irish Longitudinal Study on Ageing; Ireland(Reference Cronin, O'Regan and Finucane43, Reference Whelan and Savva44)) and LiLACS NZ (Life and Living in Advanced Age, a Cohort Study; New Zealand(Reference Hayman, Kerse and Dyall45)). These datasets are representative of the European population. A uniform definition of malnutrition was applied. All studies used similar assessment methods to collect their data(Reference Streicher, van Zwienen-Pot and Bardon17).
The inclusion criteria for this study involved participants aged >65 years and free from malnutrition (defined as BMI <20 kg/m2 or previous unplanned weight loss) at baseline. Participants provided BMI information at baseline and follow-up. The time-span and amount of unplanned weight loss varied to a small extent depending on the original study design: ErnSIPP and ActiFe, >3 kg over the previous 3 months; LASA, >4 kg over previous 6 months; LiLACS NZ and KORA-Age- >5 kg in previous 6 months; TILDA, >4·5 kg over the previous year. Those who were deceased or lost to follow-up were excluded from the analysis.
This meta-analysis identified six determinants of incident malnutrition; these were increasing age, being unmarried/separated/divorced status (vs. married but not widowed status), difficulties walking 100 m, difficulties climbing a flight of stairs, hospitalisation in the year prior to baseline and hospitalisation during the follow-up period. Across these six datasets, the determinants of malnutrition appear to be predominantly from the demographic and physical functioning domains.
Interestingly, disease-related (with the exception of hospitalisation), food intake, lifestyle, psychological and social factors had no association with incidence of malnutrition in this meta-analysis. Furthermore, across all six studies, appetite falls in the year prior to baseline, living alone, polypharmacy, smoking status and social support consistently showed no association with incident malnutrition. Although further meta-analyses are warranted to confirm these factors as the true determinants of malnutrition in older adults, the meta-analysis study methods are strong; a uniform definition of incident malnutrition was applied across all datasets, a wide range of harmonised potential determinants was assessed and a standardised protocol for data analysis was implemented. Furthermore, this meta-analysis included six longitudinal cohorts of older adults from Western populations. The findings from this meta-analysis should be incorporated into strategies to identify older persons vulnerable to developing malnutrition. This is particularly relevant for determinants which are potentially modifiable (e.g. mobility limitations).
Although datasets were only included in the meta-analysis if there was a high degree of similarity between their assessment methods and variables of interest, it should be acknowledged that some differences between the cohorts included in the meta-analysis existed. The baseline characteristics of the study cohorts are displayed in Table 3. The incidence of malnutrition at follow-up ranged from 4·6% in KORA-Age to 17·2% in the LiLACS NZ cohort (both over a 3-year follow-up). For the majority of people, the incidence of malnutrition was attributed to weight loss irrespective of the cohort. This highlights that recent weight loss should form an essential component in the identification and assessment of malnutrition in older people and supports the recently published Global Leadership Initiative on Malnutrition consensus(Reference Cederholm, Jensen and Correia9, Reference Jensen, Cederholm and Correia10).
Table 3. Characteristics of each study population(Reference Streicher, van Zwienen-Pot and Bardon17)
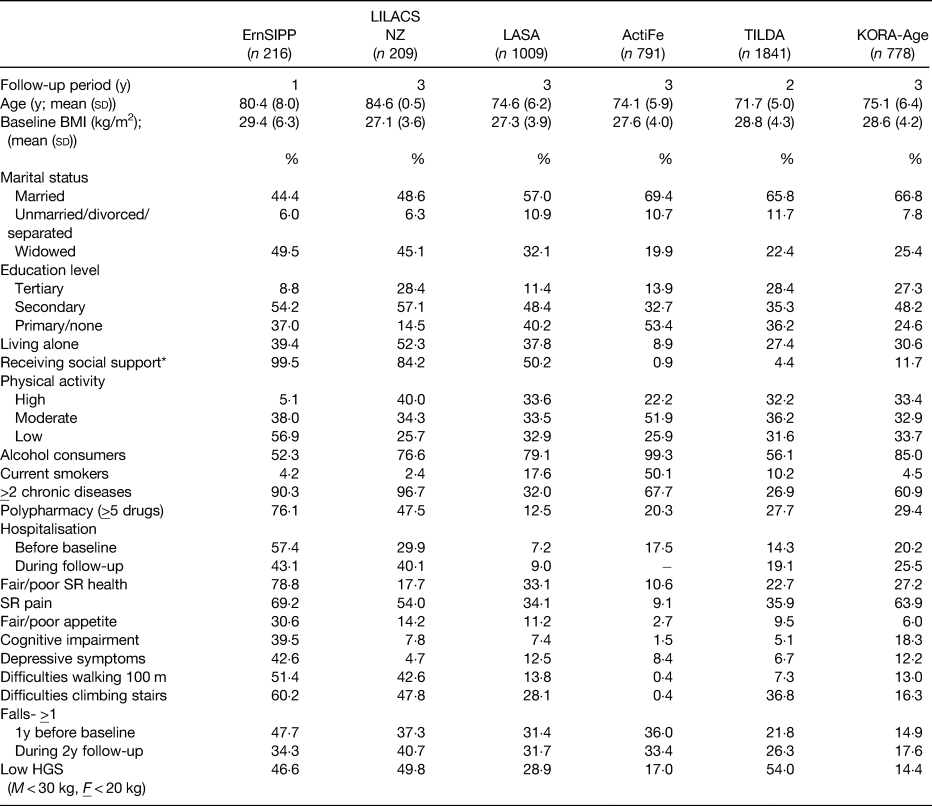
HGS, handgrip strength; SR, self-rated; y, year; ErnSiPP, Nutritional Situation of Community-dwelling Older Adults in Need of Basic Care, Germany; LiLACS NZ, Life and Living in Advanced Age, a Cohort Study, New Zealand; LASA, The Longitudinal Aging Study Amsterdam, the Netherlands; ActiFe, Activity and Function in the Elderly, Germany; TILDA, The Irish Longitudinal Study on Ageing, Ireland; KORA-Age, Cooperative Health Research in the Region of Augsburg, Germany.
* Receiving help completing any of the following: getting groceries, making a hot meal or doing household chores.
Sex-specific predictors of incident malnutrition in older Irish adults
Further analysis was conducted on the TILDA dataset to investigate whether determinants of incident malnutrition are sex-specific. Ireland is no exception to the rest of Europe with an increasing older population. The proportion of the Irish population aged 65 years and older is projected to increase from 19% (2016) to 29% by 2041(46, Reference McGill47). For the purpose of this analysis, the data were stratified by sex. Regression models were developed to identify the predictors of incident malnutrition from baseline to the 2-year TILDA follow-up. The same inclusion criteria and definition of malnutrition (BMI <20 kg/m2 and/or weight loss >10% calculated over the 2-year follow-up) that was used in the meta-analysis was applied to this work. In summary, the mean age of the TILDA sample (n 1841) was 72 years (sd 4·99) at baseline and 49·8% were male (n 916). Incident malnutrition was present in 10·7% (10·4% in males, 11% in females, P = 0·649) of the participants at 2-year follow-up. As a group, the independent predictors of incident malnutrition were being unmarried/divorced/separated, hospitalisation in the year prior to follow-up, difficulties walking 100 m and difficulties climbing stairs. Following stratification by sex, hospitalisation in the year prior to follow-up, falling during the 2-year follow-up period and difficulties climbing stairs independently predicted incident malnutrition in older males. Among female participants, cognitive impairment and receiving social support independently predicted the development of malnutrition at 2-year follow-up. These findings indicate that declining health and functional status predict older males’ risk of developing malnutrition while a declining ability to self-care appears to influence the development of malnutrition in older females(Reference McGill47).
This work adds to the limited number of longitudinal studies assessing sex-specific predictors of incident malnutrition(Reference Schilp, Wijnhoven and Deeg31) and is the first analysis to be conducted in an older, Irish, community-dwelling population.
Conclusions
The MaNuEL project has made an important contribution to the knowledge surrounding malnutrition in older adults, particularly in European populations. The most appropriate screening tools for use among older adults in various settings have been identified, the determinants of incident malnutrition have been distinguished from factors associated with the condition and sex-specific predictors of incident malnutrition have been identified in a large, Irish population dataset. This knowledge should now be incorporated into policies and strategies aimed at identifying the risk of malnutrition and preventing the development of malnutrition through appropriate and timely interventions.
Acknowledgements
C. C. and L. B. would like to acknowledge the contributions of the MaNuEL workpackage 2 and workpackage 3 members to the work summarised for the present paper, in particular, Ms Lauren Power and Professor Marian de van der Schueren (work package 2) and Ms Melanie Streicher, Professor Dorothee Volkert and Professor Eileen Gibney (work package 3).
Financial Support
This work was supported by the EU Joint Programming Initiative ‘A Healthy Diet for a Healthy Life’ Malnutrition in the Elderly (MaNuEL) knowledge hub. The funding agencies supporting the work are (in alphabetical order of participating Member State): Austria: Federal Ministry of Science, Research and Economy; France: Ecole Supérieure d'Agricultures; Germany: Federal Ministry of Food and Agriculture represented by the Federal Office for Agriculture and Food; Ireland: Department of Agriculture, Food and the Marine, and the Health Research Board (grant number 15/RD/HDHL/MANUEL); Spain: Instituto de Salud Carlos III and the SENATOR trial (FP7-HEALTH-2012-305930); The Netherlands: The Netherlands Organisation for Health Research and Development.
Conflicts of Interest
None.
Authorship
C. C. and L. B. contributed to the first draft. Both C. C. and L. B. revised subsequent drafts and approved the final draft.






