Introduction
Eustachian tube dysfunction refers to the failure of the Eustachian tube to adequately protect, ventilate, or drain secretions and pathogens away from the middle ear.Reference Llewellyn, Norman, Harden, Coatesworth, Kimberling and Schilder1,Reference Schilder, Bhutta, Butler, Holy, Levine and Kvaerner2 Insufficient drainage of the middle ear can result in otitis media with effusion (OME), defined as middle-ear fluid accumulation without signs or symptoms of acute infection.Reference Rosenfeld, Shin, Schwartz, Coggins, Gagnon and Hackell3 An inability of the Eustachian tube to equilibrate pressures within the middle-ear space and the nasopharynx results in negative middle-ear pressure.Reference Skoner, Skoner and Skoner4–Reference Teschner6 Signs of these common Eustachian tube dysfunction sequelae include middle-ear effusion, retraction or reduced mobility of the tympanic membrane, or a flat or left-shifted tympanogram.Reference Schilder, Bhutta, Butler, Holy, Levine and Kvaerner2,Reference Teschner6 Tympanometry is a highly sensitive (84–93 percent) tool for Eustachian tube dysfunction diagnosis.7 Eustachian tube dysfunction is prevalent in both children and adults, particularly those of lower socioeconomic status.Reference Shan, Ward, Goman, Betz, Reed and Poe8–Reference Roditi, Caradonna and Shin11
Reduction of oedema around the Eustachian tube opening through topical intranasal corticosteroids theoretically may improve the dysfunction.Reference Roditi, Caradonna and Shin11–Reference Rosenfeld17 Yet, conclusions from prior clinical trials of intranasal corticosteroids effects on Eustachian tube dysfunction have been conflicting, and it remains unclear whether patients without comorbid nasal symptoms would benefit from intranasal corticosteroids.Reference El-Anwar, Nofal, Khazbak, Sayed and Hassan18,Reference Crowson, Ryan, Ramprasad, Choi and Raynor19 Current international guidelines for OME advise against pharmacological therapies as the risk-to-benefit ratio is uncertain, particularly in children.Reference Rosenfeld, Shin, Schwartz, Coggins, Gagnon and Hackell3 Despite this, across specialties, intranasal corticosteroids remain one of the most prescribed treatments for Eustachian tube dysfunction patients, with or without additional nasal symptoms.Reference McCoul, Weinreich, Mulder, Man, Schulz and Shin20
Globally, management of Eustachian tube dysfunction continues to be controversial. To our knowledge, no systematic review and meta-analysis study has assessed randomised, controlled trials (RCTs) on the specific effects of intranasal corticosteroids in both paediatric and adult Eustachian tube dysfunction patients. The present study aims to (1) systematically review international literature for RCTs, evaluating the ability of intranasal corticosteroids to alleviate OME and negative middle-ear pressure in Eustachian tube dysfunction; and (2) conduct a meta-analysis of available data.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRIMSA’) guidelines, a systematic review was undertaken for investigation of this topic. A protocol was produced and registered on PROSPERO (CRD42021264211).
Search strategy
A standardised search query was created using the search items (“Eustachian Tube” or “Eustachian Tube Dysfunction”) crossed with (“Flonase” or “Fluticasone” or (“Nasal steroid” AND ”Administration, Intranasal”)) within the following electronic databases: PubMed, EMBASE, Web of Science, and The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL)) (Appendix 1). Neither study design filters, nor date limitations were applied to the search. References of included studies were scanned to identify any additional relevant records.
Eligibility assessment
Randomised, controlled trials assessing the effect of topical intranasal corticosteroid sprays on at least one of the stated primary outcomes in adult and children of any age clinically diagnosed with Eustachian tube dysfunction were included. As otitis media with effusion (OME) is a common complication of Eustachian tube dysfunction, clinical diagnoses of OME or middle-ear effusion were also accepted. No restrictions were set for control treatment. Studies that were non-RCTs, non-English, still unpublished, or that focused on the incorrect patient population (e.g. patulous Eustachian tube dysfunction, acute otitis media, rhinosinusitis) or incorrect intervention (e.g. orally administered corticosteroids) were excluded.
Initial pooled results underwent screening for duplicates and title or abstract eligibility. Eligible papers underwent a full text review to yield the final included references. Records were managed within the software Zotero, version 6.0.13 (Corporation for Digital Scholarship, Vienna, Virginia, USA). Screening and eligibility assessment were performed independently in a blinded, standardised manner through the website application Rayyan by 2 reviewers (TN, CT) using standardised eligibility forms (Appendix 2).Reference Ouzzani, Hammady, Fedorowicz and Elmagarmid21 Disagreements between reviewers were resolved by consensus. Consultation of third author was planned if warranted but was not found to be necessary.
Outcome measures
Primary outcomes included changes in middle-ear fluid and negative middle-ear pressure severity (assessed through tympanometry and/or otoscopy), as well as Eustachian tube dysfunction symptomatology. Additional outcomes of interest included pure tone audiometry, adverse events, ability to delay procedural treatment, cost-effectiveness, quality of life (QoL) and nasopharyngoscopy, although analysis of these outcomes was not a requirement for study inclusion.
Data extraction
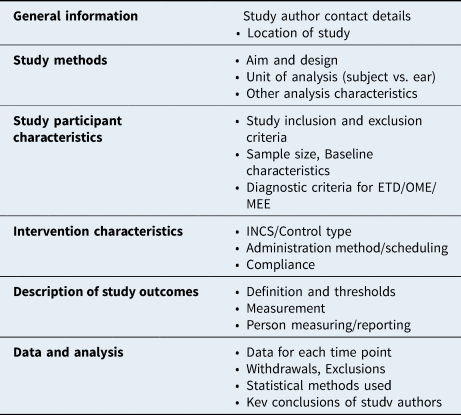
A slightly modified version of Cochrane's data collection form for RCTs was piloted and used to extract data on these outcomes (Appendix 2). One review author (TN) extracted data from included studies, and a second author (CT) checked extracted data for accuracy. Data from studies with multiple publications were planned to be extracted into one form and reported as a single study. The extraction form structure included collection of general information on the study, as well as data on study methods, participant characteristics, comparison and intervention characteristics, description of study outcomes, and summary of data and analysis (Table 1).
Table 1. Structure of data extraction forms. Tailored from Cochrane’s data collection form for randomized controlled trials. ETD = Eustachian tube dysfunction; OME = otitis media with effusion; MEE = middle-ear effusion; INCS = intranasal corticosteroids
Quantitative data synthesis and statistical analysis
A meta-analysis was planned for one or more of the outcomes of interest, conditional on the clinical and methodological heterogeneity of included studies.Reference Higgins and Thompson22 Narrative synthesis was to be implemented if extracted data was found to be overall insufficient for rigorous quantitative analysis.
Data was available from four RCTs to conduct a meta-analysis of proportions using R (version 4.1.3). The outcome measure was tympanogram normalisation, defined as proportion of study group (by ear) recovering completely on tympanometry (i.e. from Type B/C at baseline to Type A immediately upon completion of the intervention schedule).
Tympanometry data from 512 ears with baseline Eustachian tube dysfunction were pooled. A random-effects model was implemented based on the computed I2 value for included studies (I2 = 53.8 per cent [0.0 per cent; 84.7 per cent], moderate statistical heterogeneity). Comparison of normalisation rates between study arms was expressed as an odds ratio with 95 percent confidence interval (CI), where odds ratio >1 favours intranasal corticosteroids treatment over control intervention. Subgroup analyses were planned to assess intranasal corticosteroids impact on Eustachian tube dysfunction by characteristics such as intranasal corticosteroids type and dosage schedule, patient age, and patient comorbidities, however this was limited by the lack of available data. Due to significant heterogeneity in the collection and reporting of data for the other outcomes across the included references, a quantitative analysis was not feasible for other primary or additional outcomes of interest.
Qualitative data synthesis
Narrative synthesis was employed to report the tympanometry data from additional included studies not eligible for quantitative analysis, for which collected data contained high methodological and clinical heterogeneity. For qualitative synthesis, a broader measure of treatment impact, tympanogram improvement, was reported. This outcome was defined as proportion of study group (by subject) found to experience any post-intervention improvement on tympanogram – either partial resolution (i.e. from Type B at baseline to Type C post-intervention) or complete resolution (i.e. from either Type B or C at baseline to Type A post-intervention).
Quality assessment
A standard critical appraisal tool, the Cochrane revised risk-of-bias form for randomised trialsReference Sterne, Savović, Page, Elbers, Blencowe and Boutron23, was used to assess for outcome-specific risk of bias in the tympanometry results of all eligible studies (Appendix 3). Randomisation process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of reported results were individually assessed. Disagreements were planned to be resolved by consensus, although was not found to be necessary. Risk-of-bias assessment results were summarised using the robvis tool.Reference McGuinness and Higgins24
Results
Description of included studies
Study characteristics
Initial pooled results (n = 330) underwent title/abstract screening, and full reports were sought for potentially eligible papers (n = 21). Fifteen reports underwent full text review, as detailed in Figure 1. Based on study characteristics, eight RCTs were eligible for data synthesis (Figure 2). Study publication dates ranged from 1982 to 2020, and trials ranged in size from 59 to 217 participants. The majority of studies were conducted internationally (n = 6), while two studies were carried out within the United States. Four of the eight eligible studies, performed between 1982 and 2011 and randomising 312 subjects, reported data with clinical and methodological homogeneity that allowed for pooling and subsequent meta-analysis (highlighted in Figure 2).

Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRIMSA’) flow diagram used for identification of studies used; RCT = randomised, controlled trial.

Figure 2. Qualitative summary of included study characteristics, including assessed risk of bias (RoB) grading for each study trial. The shaded studies in the Study ID column indicate the four studies for which tympanometry data was pooled for meta-analysis (Barati 2011, Gluth 2011, Lildholdt 1982, Tracy 1998). *Indicates INCS treatment administered as adjunct to co-intervention. INCS = intranasal corticosteroids; RoB = risk of bias; Barati 2011 = Barati et al., 2011;Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 Bhargava 2014 = Bhargava and Chakravarti, 2014;Reference Bhargava and Chakravarti28 Swain 2020 = Swain et al., 2020;Reference Swain, Behera, Agrawala and Shajahan27 Tracy 1998 = Tracy et al., 1998;Reference Tracy, Demain, Hoffman and Goetz32 Gluth 2011 = Gluth et al., 2011;Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 Lildholdt 1982 = Lildholdt and Kortholm, 1982;Reference Lildholdt and Kortholm31 Rahmati 2017 = Rahmati et al., 2017;Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 Williamson 2009 = Williamson et al., 2009.Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29
Patient characteristics
All studies included patients with clinically diagnosed manifestations of Eustachian tube dysfunction. Most studies evaluated only children (n = 7), with varying age restrictions, while one study evaluated both children and adults.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 The mean age of included patients ranged between 3.8 yearsReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 and 41.7 years.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 In two of the eight studies, allReference Swain, Behera, Agrawala and Shajahan27 or nearly all (83.3 per cent)Reference Bhargava and Chakravarti28 paediatric patients had an additional comorbidity of adenoidal hypertrophy.
Intervention and control characteristics
Intranasal corticosteroid sprays assessed were mometasone,Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26–Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29 beclomethasoneReference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30–Reference Tracy, Demain, Hoffman and Goetz32 and triamcinolone acetonide.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 Duration of treatment ranged from 4 weeks to 24 weeks. In the majority of the included RCTs, intranasal corticosteroid intervention was assessed alone in comparison to placebo.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Swain, Behera, Agrawala and Shajahan27,Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29,Reference Lildholdt and Kortholm31
However, two of the included studies instead assessed intranasal corticosteroids in comparison to no treatment (withReference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 or withoutReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 underlying co-intervention administered to both intervention and comparison groups). In another included study, two control groups were assessed – one group provided with no treatment and one group provided with a placebo nasal spray.Reference Tracy, Demain, Hoffman and Goetz32 For this study, data were available only for both control groups combined; therefore, comparison data were extracted for both control groups (no treatment and placebo) in conjunction.
Quantitative analysis of results – tympanometric normalisation
Tympanometry data from four of the included trials were eligible for meta-analysis of odds ratios for post-intervention rate of complete tympanometric normalisation by ear (Figure 3).Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30–Reference Tracy, Demain, Hoffman and Goetz32

Figure 3. Meta-analysis of tympanometry data from four of the included trials; Pooled analysis of odds ratios (ORs) for proportion of study group participants with complete tympanogram normalization after course of treatment with INCS vs. control, presented with 95 percent confidence intervals. Tympanometric normalization, the outcome measure for ETD resolution, was defined as change in one ear from Type B/C (pathologic) to Type A (healthy) on tympanogram. Forest plot represents aggregated results of four included studies from the systematic review with similarly measured and reported tympanometric data. OR = odds ratio; 95%–CI = 95 per cent confidence interval. Barati 2011 = Barati et al., 2011;Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 Gluth 2011 = Gluth et al., 2011;Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 Lildholdt 1982 = Lildholdt and Kortholm, 1982;Reference Lildholdt and Kortholm31 Tracy 1998 = Tracy et al., 1998.Reference Tracy, Demain, Hoffman and Goetz32
In 512 pooled ears, there was no significant difference in the overall proportion of patients that recovered from type B/C tympanogram at baseline to type A tympanogram post intervention (odds ratio 1.21, 95 per cent confidence interval 0.65–2.24) when comparing Eustachian tube dysfunction patients receiving intranasal corticosteroids to those receiving control treatment. Tympanometry data obtained from included studies were not adequate for sub-group analysis.
Qualitative synthesis of results
Tympanometric improvement
Eight RCTs were determined eligible for analysis through the systematic review screening. A qualitative analysis was conducted in which tympanometry data from all eight studies (n = 771 subjects) were compiled regardless of heterogeneity in data measurement and reporting (Figure 4).Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26–Reference Tracy, Demain, Hoffman and Goetz32 Only five of the eight studies reported a statistical comparison between intranasal corticosteroids and controls for post-intervention tympanometric data.Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26,Reference Swain, Behera, Agrawala and Shajahan27,Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29,Reference Lildholdt and Kortholm31 Of these five studies, only one reported a significant difference (comparison between intranasal corticosteroids and placebo saline spray, p = 0.0002).Reference Swain, Behera, Agrawala and Shajahan27 Qualitatively, it seemed that neither studies with the oldest participantsReference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 nor those with the youngest participantsReference Lack, Caulfield and Penagos5 found intranasal corticosteroids to be more effective in treating Eustachian tube dysfunction, at least in terms of tympanometric improvement.

Figure 4. Comparing proportion of study group participants with any tympanogram improvement (by subject) after course of treatment. Improvement of tympanogram was used as the outcome measure for a qualitative analysis of ETD resolution, and included both partial resolution (change over the course of the intervention from tympanogram Type B to C) and complete resolution (change from tympanogram Type B to A or from Type C to A). Figure represents aggregated results of all eight studies deemed eligible for inclusion through systematic review. Error bars demonstrate 1 standard error (±68% CI around sample group proportion). *Marks studies collecting data with ‘ears’ as study unit (not independent points, standard error could not be calculated for this study data). INCS = intranasal corticosteroids; Barati (2011) = Barati et al., 2011;Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 Bhargava (2014) = Bhargava and Chakravarti, 2014;Reference Bhargava and Chakravarti28 Gluth (2011) = Gluth et al., 2011;Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 Lildholdt (1982) = Lildholdt and Kortholm, 1982;Reference Lildholdt and Kortholm31 Rahmati (2017) = Rahmati et al., 2017;Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 Swain (2020) = Swain et al., 2020;Reference Swain, Behera, Agrawala and Shajahan27 Tracy (1998) = Tracy et al., 1998;Reference Tracy, Demain, Hoffman and Goetz32 Williamson (2009) = Williamson et al., 2009.Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29
Adverse events
Of the eight studies, six discussed adverse events that emerged during the course of treatment, while two did not discuss this outcome.Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 There were overall minimal differences in adverse events between intranasal corticosteroids and control groups.
Qualitative synthesis of additional outcomes
A number of studies discussed changes in reported symptoms,Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Swain, Behera, Agrawala and Shajahan27,Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29,Reference Tracy, Demain, Hoffman and Goetz32 otoscopyReference Bhargava and Chakravarti28,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30,Reference Tracy, Demain, Hoffman and Goetz32 and pure tone audiometry.Reference Swain, Behera, Agrawala and Shajahan27,Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29,Reference Lildholdt and Kortholm31 Very few studies reported on QoL,28,29 cost-effectivenessReference Williamson, Benge, Barton, Petrou, Letley and Fasey29 and nasopharyngoscopy.Reference Bhargava and Chakravarti28 No study contained discussion of the ability of intranasal corticosteroids to postpone or reduce the need for surgical management (e.g. tympanostomy tube placement).
Risk-of-bias assessment
Risk-of-bias assessment was performed for the outcome of tympanometry across all included studies, using five domains (Figure 5). Three studies were assessed as having a low risk of bias.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Bhargava and Chakravarti28,Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29 Five of the trials were found to have some concerns in the assignment of intervention (Domain 2), either due to a lack of specification about analysis on an intent-to-treat basis, or due to lack of clarity on the awareness of participants and outcome assessors regarding intervention allocation.Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26,Reference Swain, Behera, Agrawala and Shajahan27,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30–Reference Tracy, Demain, Hoffman and Goetz32 Of these five trials, one was found to be at high risk of bias due to missing outcome data beyond that accounted for by loss to follow-upReference Lildholdt and Kortholm31 (Domain 3).

Figure 5. Risk of Bias assessment of included studies, performed for the outcome of tympanometry using a revised Cochrane risk of bias tool for randomized trials (RoB 2). Judgement was made on five domains; three studies were assessed as low risk of biasReference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25, Reference Bhargava and Chakravarti28, Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29 four studies as generating some concernsReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26, Reference Swain, Behera, Agrawala and Shajahan27, Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30, Reference Tracy, Demain, Hoffman and Goetz32, and one study as high risk of bias.Reference Lildholdt and Kortholm31 Barati 2011 = Barati et al., 2011;Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 Bhargava 2014 = Bhargava and Chakravarti, 2014;Reference Bhargava and Chakravarti28 Swain 2020 = Swain et al., 2020;Reference Swain, Behera, Agrawala and Shajahan27 Tracy 1998 = Tracy et al., 1998;Reference Tracy, Demain, Hoffman and Goetz32 Gluth 2011 = Gluth et al., 2011;Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 Lildholdt 1982 = Lildholdt and Kortholm, 1982;Reference Lildholdt and Kortholm31 Rahmati 2017 = Rahmati et al., 2017;Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 Williamson 2009 = Williamson et al., 2009.Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29
The remaining four trials were not found to have high-risk characteristics. However, due to the aforementioned concern, as well as additional concerns about the randomisation process and data reporting (Domains 1 and 5), these were judged as “some concerns” for bias.Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26,Reference Swain, Behera, Agrawala and Shajahan27,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30,Reference Tracy, Demain, Hoffman and Goetz32 Two of these four trials did not compare intranasal corticosteroids to placebos, and instead conducted a comparison to no treatment.Reference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30 This may be of concern, as intranasal corticosteroids must be administered as a spray intranasally, and lack of treatment was likely an indicator to study participants and outcome assessors as to how the intervention was allocated. This poor allocation concealment may have compromised the benefits of randomisation for these trials, and potentially lowered reliability of each study's conclusions.
Discussion
The aim of this study was to systematically review randomised, controlled trials evaluating the ability of intranasal corticosteroids to alleviate clinical signs (OME, negative middle-ear pressure) in patients with Eustachian tube dysfunction and conduct a meta-analysis of available data. Study results do not provide supportive evidence for the use of intranasal corticosteroids to reverse sequelae of Eustachian tube dysfunction in children and adults. On the basis of complete tympanometric normalisation, neither intranasal corticosteroids nor control interventions were favoured to a statistically significant degree when pooling tympanometric data from Eustachian tube dysfunction patients in four RCTs.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30–Reference Tracy, Demain, Hoffman and Goetz32
Corticosteroids also failed to demonstrate benefit for treatment of Eustachian tube dysfunction sequelae in previous systematic reviews and meta-analyses.Reference Llewellyn, Norman, Harden, Coatesworth, Kimberling and Schilder1,Reference Mehta, Ma, Nguyen, McRackan, Meyer and Lambert33,Reference Simpson, Lewis, van der Voort and Butler34 However, two of these studies focused on treatment of only adult Eustachian tube dysfunction patients (≥ 18 years old;Reference Llewellyn, Norman, Harden, Coatesworth, Kimberling and Schilder1 ≥ 16 years oldReference Mehta, Ma, Nguyen, McRackan, Meyer and Lambert33), and assessed a wide range of medical management types, with very little data compiled specifically regarding intranasal corticosteroids efficacy alone. The third of these previous studies assessed the use of steroids in children (≤ 12 years old) diagnosed with OME, however the majority of outcome data characterised oral steroid treatment rather than intranasal corticosteroids.Reference Simpson, Lewis, van der Voort and Butler34
Based on these data, current clinical guidelines have recommended against medical management for Eustachian tube dysfunction. Continued observation is recommended instead, with tympanostomy tube placement for at-risk patients (unilateral or bilateral OME persisting for ≥ 3 months and/or type B tympanogram).Reference Rosenfeld, Shin, Schwartz, Coggins, Gagnon and Hackell3
Generally, conservative medical management reduces both risk and cost relative to procedural treatments. However, previous data have shown that intranasal corticosteroids are one of two medical management strategies with the highest adult Eustachian tube dysfunction-associated direct costs.Reference McCoul, Weinreich, Mulder, Man, Schulz and Shin20 All studies except oneReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 demonstrated less than ideal rates of spontaneous resolution in the control group (16.7–52.3 per cent), despite evaluation over long periods of time (up to 24 weeks).Reference Bhargava and Chakravarti28 In paediatric patients, Eustachian tube dysfunction persistence without treatment can interfere with behavioural development and impairments in learning, language and speech.Reference Rosenfeld, Shin, Schwartz, Coggins, Gagnon and Hackell3,Reference Bluestone9,Reference Most35 Given that intranasal corticosteroids are not definitively effective, an investigation of alternative interventions is warranted.
Compared to intranasal corticosteroids, a one-time tympanostomy tube placement may be more effective for Eustachian tube dysfunction, as it requires no daily action and is more resistant to variability in compliance. For many patients, tympanostomy tube placement may only require a brief outpatient procedure. Notably, one of the senior authors performs myringotomy as an anecdotal predictor of response to tympanostomy. Additional interventions such as Eustachian tube insufflation and Eustachian tube balloon dilatation may also be effective, however more conclusive data are needed on these options.
Of note, this study primarily focused on studies of patients without comorbid nasal symptoms. Patients experiencing nasal symptoms presumably would benefit from use of intranasal corticosteroids to treat their comorbid nasal condition, but the decision to start intranasal corticosteroids is less clear for patients without comorbid symptoms. Therefore, while there may be an association with nasal comorbidities and Eustachian tube dysfunction, a strength of this study is that it may better address patients who experience Eustachian tube dysfunction without comorbid nasal symptoms.
Further discussion
Intranasal corticosteroids may be effective in paediatric Eustachian tube dysfunction with a primarily adenoidal pathogenesis
In two of the four studies that reported intranasal corticosteroids to be an effective treatment, a significant proportion (allReference Swain, Behera, Agrawala and Shajahan27 or nearly all (83.3 per cent)Reference Bhargava and Chakravarti28) of the paediatric patients had the additional comorbidity of adenoidal hypertrophy. While neither study was entered in the meta-analysis, the high rate of comorbid adenoid hypertrophy found in these studies may suggest a relationship between paediatric Eustachian tube dysfunction and adenoid hypertrophy. Adenoidal hypertrophy is the most common entity causing Eustachian tube obstruction in children, and inflammation of the adenoid pads is a theorised aetiology for Eustachian tube dysfunction. In children with nasal pathology, such as inflamed or enlarged adenoids, intranasal corticosteroid efficacy may be more related to reductions in adenoidal inflammation, which may improve Eustachian tube function.
Age does not seem to play a role in intranasal corticosteroid efficacy
The mean age of included patients ranged between 3.8 yearsReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 and 41.7 years.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 This includes paediatric patients on both sides of the threshold (around 7 years of age) for morphological maturity of the Eustachian tube, as well as adults.Reference Sadler-Kimes, Siegel and Todhunter36 Qualitatively, in terms of tympanometric improvement, it seemed that neither studies with the oldest participantsReference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25 nor those with the youngest participantsReference Rahmati, Safdarian, Shiroui, Zare and Sadeghi26 found intranasal corticosteroids to be more effective in treating Eustachian tube dysfunction. Of note, RCTs assessing the effect of intranasal corticosteroids in Eustachian tube dysfunction in adults are scarce. Only one study including the adult population was found for inclusion in the systematic review, identifying a clear deficit in current clinical evidence around this problem.
Limitations
Data regarding differences in adherence to the nasal spray regimen between placebo and intervention groups were only available for two of the eight included studies.Reference Williamson, Benge, Barton, Petrou, Letley and Fasey29,Reference Tracy, Demain, Hoffman and Goetz32 Intranasal corticosteroids are most effective when used consistently. Studies that reported on adherence found no significant difference between treatment groups. However, for other studies it is unknown how consistently the administration schedule was followed.
Additionally, it is unspecified how included Eustachian tube dysfunction patient diagnoses were distributed between acute (signs/symptoms < 3 months) versus chronic (signs/symptoms ≥ 3 months) for all included studies except one.Reference Tracy, Demain, Hoffman and Goetz32 While management is similar between acute and chronic Eustachian tube dysfunction, patients with acute symptoms may have been more likely to self-resolve during treatment in both placebo and intervention groups.Reference Schilder, Bhutta, Butler, Holy, Levine and Kvaerner2 The impact of this limitation was likely small in the context of this study, however may be important to keep in mind for future studies in this topic.
• Eustachian tube dysfunction is multifactorial and leads to insufficient drainage and pressure regulation of the middle-ear cavity, which can significantly affect quality of life in adult and paediatric populations
• Topical administration of anti-inflammatory corticosteroids is theorised to improve Eustachian tube dysfunction, however conclusions from prior clinical trials on the subject have been conflicting
• Current international guidelines advise against intranasal corticosteroids for Eustachian tube dysfunction and its common sequelae, yet many providers continue to prescribe intranasal corticosteroids for this condition
• To our knowledge, no systematic review and meta-analysis study has assessed randomised, controlled trials on the specific effects of intranasal corticosteroids in both paediatric and adult Eustachian tube dysfunction patients
• Current evidence on this topic is of mediocre quality and does not support the use of intranasal corticosteroids in Eustachian tube dysfunction
Overall, available data obtained through systematic review was small in quantity, extremely heterogenous, and on average mediocre in quality. This precluded any planned quantitative subgroup analysis and lessens the predictive power and generalisability of our findings. Trends in efficacy by study size, participant age distribution, intranasal corticosteroids type, and/or treatment duration were only able to be assessed qualitatively. Existing systematic review and meta-analyses, in conjunction with the current study, provide evidence that a significant gap remains in the literature. Larger, higher-quality RCTs are needed with thorough subgroup data collection to more rigorously address this still unresolved contention in Eustachian tube dysfunction medical management.
Conclusions
Study results do not provide supportive evidence for the use of intranasal corticosteroids in Eustachian tube dysfunction. Neither intranasal corticosteroids nor control interventions were favoured to a statistically significant degree when pooling tympanometric normalisation rates from Eustachian tube dysfunction patients in four RCTs.Reference Gluth, McDonald, Weaver, Bauch, Beatty and Orvidas25,Reference Barati, Omrani, Okhovat, Kelishadi, Hashemi and Hassanzadeh30–Reference Tracy, Demain, Hoffman and Goetz32 As study results do not provide supportive evidence for the use of intranasal corticosteroids in Eustachian tube dysfunction, current clinical recommendations of avoiding intranasal corticosteroids for treatment of Eustachian tube dysfunction sequela remain acceptable, and further investigation of alternative interventions is warranted.
The precise mechanism of action of intranasal corticosteroids on Eustachian tube dysfunction remains unclear. Larger, higher-quality randomised, controlled trials are needed to more rigorously identify potential variations in intranasal corticosteroids efficacy among Eustachian tube dysfunction patient subgroups. Authoritative clinical data is particularly lacking in the adult Eustachian tube dysfunction patient population as well as in comparing Eustachian tube dysfunction patients with comorbid nasal conditions (e.g., allergic and non-allergic rhinitis, inflammation of the adenoids) to those with alternative Eustachian tube dysfunction aetiologies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022215124000756.
Acknowledgements
We are deeply grateful for those who contributed to the successful completion of this project. Russell Goebel and the graduate student team with the Boston University MSSP Consulting Program provided critical support with data analysis. Research was supported by the Medical Student Summer Research Program (MSSRP) at Boston University Chobanian & Avedisian School of Medicine. Listed authors have no competing financial interests to report.








