Life is exposed to relatively predictable daily changes in the environment, the most conspicuous of which is the daily light/dark (LD) cycle. Endogenous circadian (approximately 24 h) timing systems have evolved in organisms in response to daily cycles of abiotic (such as temperature cycles) and biotic factors (such as food availability cycles) to generate circadian rhythms in behaviour and physiology to anticipate and adapt to these fluctuations and temporally compartmentalise incompatible biological processes, such as anabolism and catabolism( Reference Bass and Takahashi 1 ). The circadian system therefore primes organisms to feed at specific times, and restricting food access to times at which feeding is typically low in model organisms produces many deleterious health consequences. Fruit flies fed at the ‘wrong’ time, for example, produce fewer eggs( Reference Xu, DiAngelo and Hughes 2 ), and mice fed during the light period only – the rest period for these nocturnal rodents – are prone to diabetes, the metabolic syndrome, obesity, and even impaired cognitive function( Reference Loh, Jami and Flores 3 – Reference Bray, Ratcliffe and Grenett 6 ).
The circadian system comprises networks of molecular clocks throughout body tissues. Although circadian rhythms are autonomous, self-sustaining and temperature compensated, the circadian system has remarkable plasticity, and feeding can modify circadian rhythms from the molecular to behavioural level( Reference Damiola, Le Minh and Preitner 7 , Reference Boulos, Rosenwasser and Terman 8 ). Indeed, peripheral tissue clocks such as the liver clock are particularly sensitive to the composition and timing of food consumed. Disorganisation of the circadian system and loss of timing relationships between circadian rhythms in particular are thought to contribute to the development of certain chronic diseases( Reference Mukherji, Kobiita and Damara 5 ). Hence, appropriate nutrition, where energy intake is aligned with energy expenditure and clear feeding/fasting cycles are synchronised with clock-regulated metabolic changes, helps maintain robust behavioural and physiological circadian rhythms and health( Reference Chaix, Zarrinpar and Miu 9 ).
Relatively recent environmental changes have predisposed many individuals to circadian system disruption. The advent of artificial lighting, jetlag induced by high-speed trans-meridian travel, shift work and around-the-clock access to energy-dense food are but a few factors that may conspire to disorganise the circadian system, and thereby adversely affect the health of people in modern societies( Reference Damiola, Le Minh and Preitner 7 , Reference Moreno, Vasconcelos and Marqueze 10 , Reference Sack, Auckley and Auger 11 ).
The purposes of this review were therefore to introduce the circadian system, highlight its influences on physiological responses to feeding, show how feeding in turn influences the circadian system and to provide implications for nutritionists and directions for future research.
The hierarchical circadian system
Central and peripheral clocks
The paired suprachiasmatic nuclei (SCN) in the anterior hypothalamus orchestrate circadian rhythms throughout body tissues using autonomic, behavioural and humoral mechanisms( Reference Ralph, Foster and Davis 12 , Reference Silver, LeSauter and Tresco 13 ). SCN cells contain cell-autonomous molecular clocks based on negative feedback loops that generate approximately 24-h rhythms in ‘clock’ gene transcription( Reference Welsh, Takahashi and Kay 14 ) (Fig. 1). As transcription factors, clock genes temporally segregate incompatible cellular processes by regulating the transcription of myriad clock-controlled genes, many of which are enriched for metabolic functions, and the same molecular clocks present in the SCN regulate rhythmic cellular processes in tissues throughout the body( Reference Partch, Green and Takahashi 15 ). That over half of protein-coding genes in mice have been shown to exhibit circadian transcription in certain conditions( Reference Patel, Ceglia and Zeller 16 ), and large proportions of proteins and metabolites follow suit( Reference Reddy, Karp and Maywood 17 , Reference Eckel-Mahan, Patel and Mohney 18 ), exemplifies the importance of clock control in metabolism. Post-transcriptional clock protein regulation confers another level of tissue-specific metabolic control( Reference Cardone, Hirayama and Giordano 19 – Reference Hirayama, Sahar and Grimaldi 22 ). Recently discovered non-transcriptional rhythms in peroxiredoxins, redox-sensitive proteins, are ubiquitous among organisms of all kingdoms, but how these are integrated with clock gene feedback loops is little understood( Reference O’Neill, van Ooijen and Dixon 23 ).
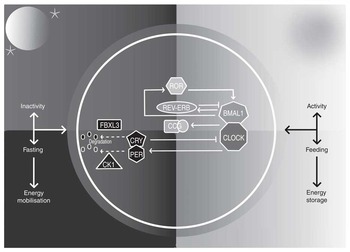
Fig. 1 The mammalian circadian clock. The molecular clock consists of ‘clock’ genes that form negative-feedback loops. The transcription factors circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) heterodimerise and activate clock-controlled genes (CCG). On activation by CLOCK-BMAL1, cryptochrome (CRY) 1–2 and period (PER) 1–3 proteins accumulate in the cytosol, multimerise and translocate into the nucleus and form inhibitory complexes, repressing CLOCK-BMAL1 and terminating CRY1–2 and PER1–3 transcription during the rest phase. As the rest phase progresses, PER-CRY complexes are degraded by F-box/LRR-repeat protein 3 (FBXL3), casein kinase 1 (CK1) ε and CK1δ. Inhibition of CLOCK-BMAL1 activity ends, completing the negative feedback loop. Auxiliary feedback loops are antiphasic to the core loop and regulate BMAL1 transcription. The nuclear receptors reverse-erythroblastosis (REV-ERB) α and β repress BMAL1 transcription, whereas RAR-related orphan receptor (ROR) α activates BMAL1 transcription. Auxiliary feedback loops add robustness, among other roles.
In the absence of time cues, the human circadian system has a period of approximately 24·2 h( Reference Czeisler, Duffy and Shanahan 24 ) and must therefore be re-set (entrained) daily to the 24-h day. The SCN are primarily entrained by light via a monosynaptic pathway from intrinsically photosensitive retinal ganglion cells in the inner retinae to the SCN( Reference Berson, Dunn and Takao 25 ). In turn, a multisynaptic pathway from the SCN to the pineal gland is a major route by which photoperiodic information is disseminated( Reference Moore 26 ). During darkness, the pineal gland synthesises melatonin, a hormone that increases sleep propensity and acts on its widely expressed receptors to provide photoperiodic information, and contributes to synchronisation of circadian rhythms in other tissues( Reference Zawilska, Skene and Arendt 27 ). Dim light melatonin onset (DLMO) can therefore be used as a proxy for the onset of the biological night in humans, with melatonin offset in the morning corresponding to the start of the biological day.
In addition to melatonin, the SCN help maintain appropriate phase relationships among peripheral clocks by regulation of other humoral factors – for example, the SCN produce their own secretions to support synchronisation of clocks in other tissues( Reference Kramer, Yang and Snodgrass 28 – Reference Kraves and Weitz 30 ). Further SCN secretions also contribute to the rhythmic release of hormones such as glucocorticoids by other tissues( Reference Kalsbeek, van Heerikhuize and Wortel 31 ), and glucocorticoids are particularly important entraining agents for many peripheral clocks. The demonstration that glucocorticoid receptor activation restores approximately 60 % of rhythmic gene transcripts in the mouse liver exemplifies this( Reference Reddy, Maywood and Karp 32 ). Another mechanism by which the SCN synchronise clocks throughout tissues is by regulating the circadian body temperature rhythm, as molecular clocks can be entrained by circadian temperature fluctuations by way of the heat shock pathway( Reference Buhr, Yoo and Takahashi 33 ).
The circadian system readies for feeding during the active phase
As it does with physical activity, the circadian system readies the body for daytime feeding. Human gastric emptying and gastrointestinal motility rates peak in the morning( Reference Goo, Moore and Greenberg 34 , Reference Rao, Sadeghi and Beaty 35 ), and studies in rodents have shown that clock regulation of bile acids and nutrient transporters optimises digestion during the active phase( Reference Han, Zhang and Jain 36 , Reference Hussain and Pan 37 ). Furthermore, daily rhythms in the gut microbiota of mice and humans fulfil time-of-day-specific functions, enhancing energy metabolism during the active phase and favouring detoxification during the rest phase( Reference Thaiss, Zeevi and Levy 38 ). The microbiota and circadian system have a complex bidirectional relationship, as disruption of the molecular clock disorganises rhythmic changes in the gut microbiota( Reference Liang, Bushman and FitzGerald 39 ), and germ-free mice have altered clock gene expression( Reference Leone, Gibbons and Martinez 40 ). Related to such changes, there are circadian rhythms in blood concentrations of many nutrients, such as glucose and lipids( Reference Morgan, Arendt and Owens 41 ). An important implication of circadian regulation of the gastrointestinal system is the importance of considering timing of nutritional tests, as exemplified by the recent demonstration that food allergy test results are contingent on the time of day( Reference Tanabe, Kitagawa and Wada 42 ).
The circadian system promotes energy substrate storage in appropriate tissues during the day. Insulin sensitivity has a bimodal daily peak during the active phase( Reference Scheer, Hilton and Mantzoros 43 ), and appetite for most foods is clock-controlled and lowest in the morning, perhaps to allow consolidated sleep despite diminishing energy availability( Reference Scheer, Morris and Shea 44 ). Diet-induced thermogenesis too has a circadian rhythm that peaks in the morning( Reference Morris, Garcia and Myers 45 ). These changes may be of particular relevance to the obesity epidemic, as they suggest that delayed bedtimes increase time for food consumption when appetite is high, and that consuming a higher proportion of dietary energy in the morning might encourage a negative energy balance, the principle determinant of decreasing body mass.
Feeding entrainment of clocks
Although the SCN clocks are primarily entrained by light, time-of-day-restricted feeding (TRF) studies, where food availability is restricted to a period of several hours, have shown that peripheral clocks are predominantly responsive to feeding. Indeed, rest phase TRF inverts gene expression profiles in many peripheral tissues including the heart, kidney, liver, pancreas, adipose tissue and the gastrointestinal tract( Reference Damiola, Le Minh and Preitner 7 , Reference Stokkan, Yamazaki and Tei 46 – Reference Hoogerwerf, Hellmich and Cornelissen 48 ). The time course of this entrainment varies depending on the organs in question, with the liver clock responding to feeding particularly rapidly. As a result, peripheral tissue rhythms can be uncoupled from SCN rhythms( Reference Damiola, Le Minh and Preitner 7 ). Interestingly, feeding shifts the liver clock more rapidly in SCN-lesioned mice, suggesting that the SCN counters internal desynchronisation – the loss of appropriate phase relationships between clocks that is thought to contribute to metabolic aberrations( Reference Saini, Liani and Curie 49 ). During ad libitum conditions, TRF does not appear to affect the phase of the SCN clock; however, the SCN clock phase may respond somewhat to TRF combined with energy restriction( Reference Mendoza, Graff and Dardente 50 ). Although few studies on the effects of TRF on the human circadian system have been published, circadian rhythms in core body temperature and heart rate were advanced after 3 d of morning v. evening TRF in healthy young men( Reference Krauchi, Cajochen and Werth 51 ).
Coupling between metabolism and clocks
Feeding entrainment of tissue clocks is predicated on reciprocal relationships between molecular clocks and metabolic sensors and regulators( Reference Eckel-Mahan and Sassone-Corsi 52 ). Feeding/fasting cycles produce changing nutrient availability, and hence periodic phosphorylation of energy sensors such as 5' AMP-activated protein kinase (AMPK), which promotes ATP production during reduced energy availability, and mechanistic target of rapamycin (mTOR), which promotes anabolic processes during increased energy availability. These regulators are coupled to molecular clock components, which in turn influence myriad metabolic processes integral to nutrient homoeostasis. AMPK, for example, phosphorylates and destabilises cryptochrome (CRY) 1 in peripheral cells( Reference Lamia, Sachdeva and DiTacchio 53 ) and interacts with SIRTUIN (SIRT) 1. In turn, SIRT1 modulates transcription factors including period (PER) 2( Reference Asher, Gatfield and Stratmann 54 ) as well as the ventromedial hypothalamic clock, a brain region that contributes to regulation of the circadian rhythm in feeding behaviour( Reference Orozco-Solis, Ramadori and Coppari 55 ). SIRT1 is one of a family of deacetylase enzymes that have many roles in metabolic regulation, and SIRT1 and SIRT6 appear to be particularly important to temporal partitioning of metabolism by controlling the transcription of distinct sets of genes with circadian expression profiles, with SIRT6 regulating the rhythmic transcription of genes involved in cholesterol and fatty acid (FA) metabolism( Reference Masri, Rigor and Cervantes 56 ).
Both tissue-specific and whole-body genetic disruption of the molecular clock produce diverse metabolic aberrations( Reference Paschos, Ibrahim and Song 57 , Reference Turek, Joshu and Kohsaka 58 ), and the molecular clock partly mediates beneficial effects of some nutritional interventions, such as the longevity-promoting effects of energy restriction( Reference Patel, Chaudhari and Gupta 59 ). These findings support recent observational studies that have associated SNP in clock genes with various facets of metabolic health. Regarding circadian locomotor output cycles kaput (CLOCK), for example, CLOCK SNP have been associated with non-alcoholic steatohepatitis, the metabolic syndrome, small dense LDL levels, obesity and diabetes( Reference Sookoian, Castano and Gemma 60 – Reference Uemura, Katsuura-Kamano and Yamaguchi 64 ). Perhaps the most studied of these associations is that of obesity: to date, eight common CLOCK SNP have been linked to obesity and three have been associated with energy intakes( Reference Valladares, Obregon and Chaput 65 ). Results of such small, candidate-gene association studies need support from large, unbiased, genome-wide association studies, however.
Food anticipatory activity and food-entrainable oscillators
Coupling between nutrient availability and the circadian system is also evident at the behavioural level. TRF in animals such as rats produces food anticipatory activity (FAA) – food-seeking behaviour at times during which food procurement is most likely. FAA is goal-directed towards places where food is available, and may thus be an adaptive strategy to enhance foraging success( Reference Boulos, Rosenwasser and Terman 8 ). Indeed, FAA is accentuated during energy restriction. As FAA is entrainable and persists during several days of food deprivation, FAA appears to be a true circadian rhythm.
Interestingly, FAA persists both following SCN ablation( Reference Stephan, Swann and Sisk 66 ) and disruption of the positive and negative arms of the molecular clock( Reference Storch and Weitz 67 ); therefore, the food-entrainable oscillators thought to underlie FAA must reside elsewhere. Candidate oscillators comprise various brain structures (including the cerebellum, dorsomedial nuclei, and dorsal striatal and mesocorticolimbic circuits( Reference Mendoza, Pevet and Felder-Schmittbuhl 68 – Reference Verwey and Amir 70 )), neurochemical pathways (including dopaminergic and melanocortinergic signalling( Reference Gallardo, Darvas and Oviatt 71 , Reference Sutton, Perez-Tilve and Nogueiras 72 )) and hormonal signals (including ghrelin and orexins( Reference LeSauter, Hoque and Weintraub 73 )).
Eating patterns: feeding/fasting matters
As metabolic rhythms are intertwined with nutrient availability, clear feeding/fasting cycles consolidate robust metabolic and behavioural rhythms. High-fat diets (HFD) blunt feeding/fasting cycles in mice, increasing the proportion of energy consumed during the rest phase, and hence dampen circadian rhythms in clock genes( Reference Kohsaka, Laposky and Ramsey 74 , Reference Hatori, Vollmers and Zarrinpar 75 ). Consistent with this, expression of adipose tissue clock genes such as PER2 is increased following weight loss in humans( Reference Pivovarova, Gogebakan and Sucher 76 ). Ad libitum access to HFD consistently and rapidly produces obesity in many animals, and endocrine rhythms are similarly blunted in obese humans( Reference Matkovic, Ilich and Badenhop 77 ). Whether obesity precedes dampened circadian rhythms has been contentious, but recent evidence indicates that HFD induce rapid re-organisation of gene transcription rhythms before overt increases in adiposity in mice( Reference Eckel-Mahan, Patel and de Mateo 78 ).
Compared with ad libitum feeding, TRF offsets HFD-induced blunted feeding rhythms in mice, and the result is superior metabolic health, including reduced adiposity, despite similar energy intakes( Reference Hatori, Vollmers and Zarrinpar 75 ). Comprehensive recent experiments have shown that, despite similar energy intakes and locomotor activity, various TRF schedules are beneficial during different nutritional ‘challenges’, such as HFD and high-fructose diets, and that beneficial metabolic effects of TRF are proportional to fasting duration( Reference Chaix, Zarrinpar and Miu 9 ). During HFD feeding, TRF produces nutrient sensor profiles (including AMPK and mTOR) that are more similar to mice fed normal chow( Reference Hatori, Vollmers and Zarrinpar 75 ). Furthermore, TRF counters HFD-induced reductions in cyclical changes in the gut microbiota, and stool metabolite analyses suggest that this effect of TRF contributes to metabolic health benefits of TRF( Reference Zarrinpar, Chaix and Yooseph 79 ). These studies used male C57/BL6 mice, animals with a particular susceptibility to diet-induced obesity. As such, it may be premature to extrapolate these findings to humans. Nevertheless, recent research found that eight obese adults with habitual eating periods exceeding 14 h experienced sustained weight loss and improved sleep when consumption of energy-containing foods and drinks was restricted to an 11-h period each day( Reference Gill and Panda 80 ). The latter study was clearly limited by its sample size, however.
In contrast to the beneficial effects of TRF during HFD feeding, TRF may not confer such striking metabolic advantages when mice are fed normal chow( Reference Hatori, Vollmers and Zarrinpar 75 ). The same may be true among lean humans consuming typical diets. Among fifteen healthy young adults, a cross-over trial found that evening TRF increased fasting glycaemia and impaired glucose tolerance v. an isoenergetic diet comprising three meals throughout the day( Reference Carlson, Martin and Stote 81 ). Another study of the same design associated TRF with increased hunger, blood pressure and cholesterol( Reference Stote, Baer and Spears 82 ). However, findings may have been confounded by circadian variations in these parameters, as measures were taken at different times of the day.
Although not described as TRF studies, breakfast skipping is conceptually akin to TRF. In a larger study of overweight and obese adults, breakfast skipping did not influence responses to weight-loss diets( Reference Dhurandhar, Dawson and Alcorn 83 ), and a careful study in lean young adults found that one of the only effects of 6 weeks of breakfast omission was increased afternoon glycaemic variability( Reference Betts, Richardson and Chowdhury 84 ). Subsequent research using the same protocol in obese adults also found few differences between groups, although insulin sensitivity was higher in breakfast eaters( Reference Chowdhury, Richardson and Holman 85 ). It is possible that breakfast omission altered the timing of peak insulin sensitivity, however. Therefore, it appears that TRF may not benefit metabolic health in all contexts. Certainly, further studies with larger sample sizes are needed. Important questions remain unanswered, such as what is the optimal TRF period and meal frequency, and under what circumstances?
Time-of-day-restricted feeding: meal timing matters
One factor that may be relevant to the efficacy of TRF is meal timing. Mice fed HFD during the rest phase tended to gain more fat mass than mice fed HFD during the active phase( Reference Arble, Bass and Laposky 4 ). Similarly, mice fed normal chow during the rest phase also gained more fat mass than mice fed during the active phase. Rest phase TRF also altered clock and metabolic gene expression profiles in peripheral tissues, blunted corticosterone rhythm amplitudes, reduced energy expenditure despite comparable locomotor activity and reduced lipid oxidation within 9 d( Reference Bray, Ratcliffe and Grenett 6 ). It is possible that deleterious metabolic effects of rest phase TRF are related to misalignment between energy intake and energy expenditure. Clock gene mutations alter circadian rhythm periods in organisms including humans( Reference Toh, Jones and He 86 ), and a transgenic hPER1 mutation in mice increases obesity risk by advancing peak feeding time relative to peak daily energy expenditure. Subsequently using TRF to synchronise feeding with peak energy expenditure mitigates obesity development in these animals( Reference Liu, Huang and Wu 87 ).
Ramadan confines eating to the rest phase and modifies circadian rhythms in hormone secretion – for example, the timing of the morning rise in cortisol and night-time melatonin peak are both delayed( Reference Bogdan, Bouchareb and Touitou 88 ). Some results of Ramadan studies appear to contradict rodent TRF study findings, however. Meta-analysis of thirty-five observational studies found a mean reduction in body mass of 1·24 kg during Ramadan, with differences between ethnicities and greater reductions in men. No effects on dietary macronutrient proportions were observed, and fasting duration was not associated with body mass changes( Reference Sadeghirad, Motaghipisheh and Kolahdooz 89 ). It was not possible to evaluate body composition, however, and carefully controlled human TRF experiments are needed to determine whether large differences in TRF timing produce similarly large metabolic changes to those seen in mice.
Time-of-day-restricted feeding: nutrient and energy distribution timing matters
We refer to nutrient intake timing as the timing of ingestion of specific nutrients and the distribution of energy assigned to eating occasions when the timing of eating occasions is otherwise similar. Studies of mice show that high-fat meal consumption at the end of the active phase increases adiposity, insulin, leptin, and triacylglycerolaemia v. consumption at the beginning of the active phase( Reference Bray, Tsai and Villegas-Montoya 90 ). Similarly, restricting fructose access to the rest phase increases adiposity and insulin resistance in comparison with restricting access to the active phase( Reference Morris, Araujo and Pohlman 91 ).
In overweight and obese women matched for energy intakes, those who consumed a larger proportion of daily energy early in the day lost more weight than those consuming more later in the day( Reference Jakubowicz, Barnea and Wainstein 92 ), consistent with other findings that earlier lunch consumption is associated with greater weight loss after 20 weeks( Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar 93 ). Similar associations have since been reported in severely obese adults following bariatric surgery( Reference Ruiz-Lozano, Vidal and de Hollanda 94 ). As diet-induced thermogenesis peaks in the morning, and breakfast consumption is associated with higher subsequent non-exercise activity thermogenesis, and hence energy expenditure( Reference Betts, Richardson and Chowdhury 84 ), it is plausible that assigning more of daily energy expenditure to earlier meals may encourage a negative energy balance during hypoenergetic diets. Further studies on how meal composition and energy availability affect responses to TRF will be valuable.
Eating patterns: consistency matters
Finally, eating patterns are very inconsistent in some adults( Reference Gill and Panda 80 ), and this may be relevant to metabolic health. In mice, fixing TRF to a 12-h period during twice-weekly 6-h LD cycle advances might be expected to uncouple LD cycle-entrained SCN rhythms from feeding-entrained peripheral clock rhythms and produce corresponding metabolic disorder. In these conditions, however, TRF mitigated the obesogenic effects of LD cycle shifts observed in ad libitum-fed mice, despite similar energy intakes. Hence, meal regularity and not just its timing relative to activity may be important to metabolic benefits of TRF( Reference Oike, Sakurai and Ippoushi 95 ). The mechanisms by which regular feeding schedules offset obesity in this study are unclear, however, and similar studies in humans are necessary to determine whether these findings are applicable to populations such as shift workers. It will also be interesting to clarify whether TRF needs to be implemented daily to be beneficial; some evidence suggests otherwise( Reference Halberg, Henriksen and Soderhamn 96 ).
Together, it appears that TRF may be a promising way to improve metabolic health in overweight and obese individuals. Consistent meal patterns and consuming meals shortly after physical activity may help optimise metabolic health. Furthermore, allocating a higher proportion of energy intake to earlier meals may promote a lower energy balance when diets are matched for energy intake. Nevertheless, many questions remain. It is important to determine how effective different TRF schedules are compared to one another and what factors determine inter-individual variability in responses.
Nutrient composition modifies clocks
The compositions of foods have been shown to influence many different circadian rhythms in rodents, from gene expression profiles to behavioural rhythms. HFD have sometimes but not always been found to influence peripheral tissue clock gene expression profiles in mice studies( Reference Kohsaka, Laposky and Ramsey 74 , Reference Yanagihara, Ando and Hayashi 97 ), and these discrepancies may have resulted from factors including diet composition. In support of this contention, higher-protein, lower-carbohydrate chow advanced expression rhythms of multiple clock genes in the kidneys and livers of mice, and increased mean expressions of brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (Bmal1) and Cry1 in comparison with standard chow( Reference Oishi, Uchida and Itoh 98 ). In humans, switching participants from higher-carbohydrate (55 %) and lower-fat (30 %) diets to isoenergetic lower-carbohydrate (40 %) and higher-fat (45 %) diets delayed and increased the amplitude of cortisol rhythms, changed inflammatory and metabolic gene expression profiles and altered PER gene expression rhythms in monocytes( Reference Pivovarova, Jurchott and Rudovich 99 ).
In addition to the proportions of dietary energy coming from the macronutrients influencing peripheral clocks, individual nutrients may influence the circadian system, even within certain types of nutrients. Using FA to exemplify this, palmitate, the most abundant SFA in animals, and DHA, a PUFA found plentifully in fish, differentially affected Bmal1 expression in a murine hypothalamic cell line( Reference Greco, Oosterman and Belsham 100 ). Moreover, manipulating dietary DHA and EPA content shifts liver clock gene expression profiles in mice in vivo ( Reference Furutani, Ikeda and Itokawa 101 ).
There are also several non-essential dietary compounds consistently shown to influence the circadian system. Alcohol is widely consumed in many societies and appears to be particularly disruptive to molecular, endocrine and behavioural circadian rhythms in humans and other animals( Reference Huang, Ho and Chen 102 – Reference Brager, Ruby and Prosser 106 ). Caffeine, the most-used psychoactive compound worldwide, is present in many foods and beverages and influences the amplitudes and phases of peripheral tissue clock gene expression rhythms in mice( Reference Sherman, Gutman and Chapnik 107 ). Evening caffeine consumption delays the human circadian system in vivo and lengthens clock gene expression periods in vitro ( Reference Burke, Markwald and McHill 108 ). Hence, careful use of caffeine can expedite circadian rhythm entrainment following jetlag( Reference Pierard, Beaumont and Enslen 109 ). However, even if subjective sleepiness is unaffected by its ingestion, caffeine impairs sleep following jetlag( Reference Beaumont, Batejat and Pierard 110 ). Caffeine has also been studied for efficacy in entraining individuals with chronic circadian system dysfunction. In a small study of blind individuals with non-24-h sleep/wake rhythm disorder, a disorder where light fails to synchronise the circadian system with the 24-h d, 150 mg of morning caffeine was insufficient to entrain circadian rhythms( Reference St Hilaire and Lockley 111 ). Dietary polyphenols are another group of compounds consistently shown to influence both molecular and behavioural circadian rhythms in some animals( Reference Ribas-Latre, Baselga-Escudero and Casanova 112 , Reference Pifferi, Dal-Pan and Menaker 113 ), and other novel nutritional supplements such as dietary polyamines( Reference Zwighaft, Aviram and Shalev 114 ) phase-shift the circadian system in rodents. Further research is needed to see whether such compounds might be useful in humans, however; if they are, what are the best times to consume them to maximise their impact, and what are the dose–response and phase–response curves of these compounds?
Conclusions and directions for future research
Growing interest in nutrition and the circadian system has produced many insights into the reciprocal relationships between the two in recent years. Findings from these studies have many implications. When assessing nutritional status and the efficacy of nutritional interventions, for example, test timing is an important consideration. More specifically, physiological measures should be taken relative to internal time (DLMO, for example) where feasible. Related to this, chronotype classifies individuals into morning or evening types according to their preference for when to be active and when to sleep. Where laboratory measures of internal time are impractical, chronotype can be estimated by simple questionnaires such as the Morningness–Eveningness Questionnaire and the Munich Chronotype Questionnaire Test( Reference Horne and Ostberg 115 , Reference Roenneberg, Wirz-Justice and Merrow 116 ). As chronotype influences the times at which various physiological processes are optimised, consideration of chronotype will be important for personalised nutrition recommendations. Recent studies have also begun exploring how clock gene SNP may influence responses to dietary interventions( Reference Garcia-Rios, Gomez-Delgado and Garaulet 117 ), and ultimately knowledge of circadian system gene variants may also help inform personalised nutrition.
Pressing questions remain unanswered, and there is a glaring need for human studies addressing these. Regarding eating patterns, whether TRF can accelerate entrainment in populations experiencing circadian disruption is a question of relevance to many. With respect to specific foods and supplements, are there dietary interventions with consistently beneficial effects on sleep? It is known that the composition of human breast milk varies daily( Reference Illnerova, Buresova and Presl 118 ), and perhaps infant formulae should reflect this.
Continuing collaboration between chronobiologists and nutritionists will further clarify interactions between nutrition and the circadian system, and ultimately has the potential to reduce the prevalence and burden of chronic diseases.
Acknowledgements
G. D. M. P. is supported by a Medical Research Council Doctoral Training Grant. P. J. G. is supported by a EuRhythDia grant (number 278397). J. E. C. and L. J. H. are supported by a Medical Research Council grant (number G1100235/1).
G. D. M. P. wrote the manuscript; J. E. C., P. J. G. and L. J. H. provided constructive feedback at all stages of its preparation.
The authors declare that there are no conflicts of interest.




