Introduction
The interplay between diet and brain health is receiving ever more attention(Reference Marx, Lane and Hockey1–Reference Medawar, Huhn and Villringer3). In tandem, over the past two decades, the importance of the bidirectional communication between the gut microbiota (bacteria, viruses, fungi, protozoa, archaea) and the brain has also surfaced, positioning this community of microorganisms as an accessible target to alter brain function and behaviour. It is now well-established that diet is a potent manipulator of the gut microbiota, with the capacity to alter both microbial abundance and functionality(Reference Rothschild, Weissbrod and Barkan4,Reference Johnson, Vangay and Al-Ghalith5) , and therefore its potential to improve brain health, is emerging as a plausible therapeutic intervention strategy(Reference Long-Smith, O’Riordan and Clarke6). While several dietary foods and patterns have been shown to modify the gut microbiota, dietary fibre and fermented foods with their capacity to act as substrates for microbial digestion and to supply live microorganisms as well as the associated enzymatically converted food components, respectively, have been identified as key modulators of the gut microbiota and subsequent signalling to the brain(Reference Berding, Vlckova and Marx7–Reference Stiemsma, Nakamura and Nguyen9).
Increasing attention is now given to the concept of a diet-microbiota-gut-brain axis(Reference Berding, Vlckova and Marx7) with an appreciation that it is not just what microbes are present in the gut but what they are doing and how they interact with diet to affect host health(Reference Sonnenburg and Bäckhed10). Undigested fibre is a major energy source for the gut microbiota and short-chain fatty acids (SCFAs) that are produced upon gut microbial fermentation of fibre influence cognitive and emotional responses of the host(Reference O’Riordan, Collins and Moloney11,Reference Dalile, Van Oudenhove and Vervliet12) . Similarly, fermented foods produced through enzymatic conversion of food components by live microorganisms also house a large community of microbes at the time of consumption (e.g. kefir, kimchi)(Reference Lang, Eisen and Zivkovic13) that can increase gut microbiota diversity(Reference Le Roy, Kurilshikov and Leeming14,Reference Wastyk, Fragiadakis and Perelman15) . These new or expanded members of the gut microbiota can subsequently produce neuroactive metabolites (e.g. SCFAs, polyphenolic, tryptophan, bile, gamma-aminobutyric acid (GABA)). Intriguingly, there is a growing appreciation that, behaviourally, both food types have been shown to benefit cognitive and emotional processing(Reference Aslam, Green and Jacka16–Reference Balasubramanian, Schneider, Gunnigle and Cotter20), effects which are suggested to be mediated via the microbiota-gut-brain axis(Reference Berding, Carbia and Cryan19,Reference Marx, Scholey and Firth21) . It is therefore worth evaluating the relative efficacy of fibre and fermented foods to alter brain functioning and signalling along the microbiota-gut-brain axis to inform future interventional research and move toward personalised medicine approaches for brain health management. Currently, there are very few studies investigating the biological basis explaining the beneficial actions of fibre and fermented foods(Reference Wastyk, Fragiadakis and Perelman15,Reference Balasubramanian, Schneider, Gunnigle and Cotter20) . While the studies that have been conducted provide tentative evidence of the scope of influence that fibre and fermented foods have on human health, it is clear that observational and interventional studies directly/systematically evaluating each food component on the microbiota-gut-brain axis are, in general, lacking. In this narrative review, we evaluate the differential neuroactive potential of dietary fibre and fermented foods to modulate cognitive and emotional functioning in humans. We further discuss possible pathways through which fibre and fermented foods can act on the brain via the microbiota-gut-brain axis. Future directions for the field and the therapeutic potential of fibre and fermented foods to improve brain health are highlighted.
Fibre
The widely-adopted definition from Codex Alimentarius defines fibre, natural and synthetic, as ‘carbohydrate polymers with ten or more monomeric units, which are not hydrolysed by the endogenous enzymes’(Reference Alimentarius22). Although the terms are often used interchangeably, prebiotics only represent the subset of fibres that are ‘substrates that are selectively utilised by host microorganisms conferring a health benefit’(Reference Gibson, Hutkins and Sanders23). While most prebiotics are dietary fibres, not all fibres are prebiotics, nor are all prebiotics fibres. Indeed, certain polyphenols and omega-3 fatty acids also exhibit prebiotic-like effects on the gut microbiota(Reference Gibson, Hutkins and Sanders23,Reference Alves-Santos, Sugizaki and Lima24) , although not being classed as dietary fibres. Despite the large body of research examining the impact of fibre supplements on the microbiota-gut-brain axis, whole dietary approaches more closely resemble the variety of foods that humans consume each day, particularly in terms of the fibre delivery matrix. Therefore, only studies that examine fibre from dietary sources, including whole grains, legumes, fruits and vegetables, are included in this review.
Dietary fibre and the gut microbiota
Fructo- and galacto- oligosaccharides, inulin and pectins from plants, animal tissue, or food-borne microbes are important energy sources for the gut microbiota(Reference Sonnenburg and Sonnenburg25). Fermentation of dietary fibre increases the abundance of Bifidobacterium and lactobacilli(Reference So, Whelan, Rossi and Morrison26). It has been proposed that roughly 30 % of the dietary fibre found in grain products is accessible for microbial metabolism, while estimates suggest that 75–90 % of fibres from fruits and vegetables are metabolised by the gut microbiota(Reference Nyman, Asp and Cummings27,Reference Wisker, Daniel and Rave28) . However, insoluble fibres can increase faecal bulk to reduce gut transit time, which also shapes gut microbiota composition(Reference Procházková, Falony and Dragsted29).
The gut microbiota is highly responsive to both acute and chronic fibre intake. A dramatic restructuring of the gut microbiota, including a significant alteration of over 25 bacterial genera, was observed after just 24 hours with an intervention of reduced carbohydrate and fibre diet in participants with obesity and metabolic dysfunction-associated steatotic liver disease. Moreover, the carbohydrate reduction expectedly decreased the abundance of fibre-degrading bacteria (Lactococcus, Eggerrthella and Streptococcus), which resulted in decreased levels of SCFAs(Reference Mardinoglu, Wu and Bjornson30). On the other hand, observational data showing a prolonged loss of fibre intake linked to the Western Diet was associated with reduced fibre-fermenting Prevotella strains in Asians who emigrated to the United States. Further losses in the functional capacity to degrade complex fibres were observed, alongside reductions in microbial diversity that decreased with each generation(Reference Vangay, Johnson and Ward31). This data is consistent with observational comparisons of individuals from cultures who consistently consume higher quantities of fibre. For example, children from rural Burkina Faso whose staple diet includes larger quantities of cereals, legumes and vegetables displayed a greater abundance of bacteria from Prevotella and Xylanibacter genera and increased levels of SCFAs than European children(Reference De Filippo, Cavalieri and Di Paola32). An overall increased microbial diversity has also been detected in observational studies examining regular fibre consumers compared to non-consumers(Reference Schnorr, Candela and Rampelli33,Reference Martínez, Stegen and Maldonado-Gómez34) . Fibre intake has further been shown to manipulate gut microbiota functionality.
Fermented foods
Fermented foods are defined as ‘foods made through desirable microbial growth and enzymatic conversions of food components’(Reference Marco, Sanders and Ganzle35). Fermented foods have been culturally embedded as staple foods in nearly all societies since the beginning of human civilisation. Given this rich history, it is unsurprising that the process of fermentation is often relatively simple and requires few ingredients and minimal preparation/processing to achieve an end-product that (1) naturally prolongs shelf-life, (2) reduces toxicity of raw materials and increases digestibility, and (3) alters the flavour profile(Reference Balasubramanian, Schneider, Gunnigle and Cotter20,Reference Marco, Heeney and Binda36) . As such, fermented foods are an affordable and convenient food option that are already available and frequently consumed in most societies.
The primary microbes involved in fermented foods include yeast, acetic acid bacteria, lactic acid bacteria (such as Leuconostoc, members of the former genus Lactobacillus, and Streptococcus), Propionbacterium freudenreichii, Bacillus, and moulds(Reference Marco, Heeney and Binda36). There is an assumption that the bacteria in fermented foods are probiotic, but this is not necessarily so. Probiotics are defined as ‘live microorganisms, when administered in adequate amounts confer a health benefit on the host’(Reference Hill, Guarner and Reid37). Fermented foods house a rich repertoire of microorganisms (some of which have the potential to be probiotics), bioactive peptides, phytochemicals and peptides that can influence human health(Reference Balasubramanian, Schneider, Gunnigle and Cotter20). Predominantly by manipulating the composition and enhancing microbial diversity, fermented foods can also modulate the production of several metabolites with neuroactive potential including SCFAs, polyphenolic, tryptophan and bile metabolites(Reference Marco, Heeney and Binda36,Reference Mukherjee, Breselge and Dimidi38,Reference Valentino, Magliulo and Farsi39) .
Because the ingestion of live microbes may be an important aspect underlying their health effects, for the purpose of this review, we focus on fermented foods with viable microorganisms at the time of consumption (e.g. sauerkraut, kombucha, yogurt). Foods where the microorganisms have been deactivated through heat (tea, coffee, bread, cocoa) or through the addition of vinegar (pickles) are also interesting but outside of the scope of the current narrative. Due to the known direct behavioural properties resulting from acute consumption of alcoholic products (e.g. memory lapse, reduced alertness, changes in affect), alcohol is also not relevant to the current discussion.
Fermented foods and the gut microbiota
Observational reports demonstrate increased microbial diversity with fermented food consumption(Reference Noh, Jang and Kim40,Reference Taylor, Lejzerowicz and Poirel41) . For example, a higher alpha diversity and enrichment of Lactobacillus, Ruminococcus and Eubacterium was reported within an observational study comparing Koreans who consume large amounts of fermented legumes and high-fibre foods, including vegetables and nuts/seeds, showing potential synergistic effects from fermented food and fibre intake combinations(Reference Noh, Jang and Kim40). Differences in beta diversity were also observed in another observational study comparing regular fermented food consumers to occasional consumers, alongside increased abundances of Faecalibacterium prausnitzii, Prevotella spp, Pseudomonas spp, Clostridiales, Enterobacteriaceae, Lachnospiraceae and Bacteroides spp.(Reference Taylor, Lejzerowicz and Poirel41). Conversely, interventions with fermented foods largely fail to alter microbial diversity(Reference Berding, Bastiaanssen and Moloney8,Reference Le Roy, Kurilshikov and Leeming14,Reference Alvarez, Tap and Chambaud42,Reference Walsh, Walsh and Garcia-Perez43) . This discrepancy could be due to a number of factors that include differences in the food matrix of the fermented foods, minimal frequency/quantity of fermented foods consumed, differences in baseline microbial composition and provenance of raw materials, heterogeneity in analysis techniques with 16S rRNA sequencing being unable to capture changes at the strain level, and, importantly, diversity of the fermented foods selected for intervention. Moreover, the duration of the intervention may be insufficient to significantly shift the composition of gut microbiota, potentially masking effects detected in observational studies that reflect regular eating habits.
Cognition
Much of the evidence linking fibre intake to cognitive performance comes from observational data investigating whole dietary patterns such as fruit and vegetable intake, often in older adults. Greater fibre intake was associated with protection against age-related cognitive decline(Reference Ortega, Requejo and Andrés44–Reference Fortune, Harville and Guralnik49). Similar beneficial effects of fibre on cognitive inhibition(Reference Khan, Raine and Drollette50) and reasoning(Reference Naveed, Venäläinen, Eloranta and Erkkilä51) have been observed in children aged 6–9 years. Observational studies linking fibre to cognitive performance in young-middle adulthood is lacking, but the fact that higher intake of fruits and vegetables at 18–30 years was associated with better executive functioning, attention and verbal memory in middle adulthood(Reference Mao, Chen and Pengcheng52) provides tentative support for a potential relationship.
Evidence from randomised-controlled trials (RCTs) with fibre intervention is mixed, both in terms of study quality and results obtained. A mixed-grain dietary intervention for 9 weeks to high school students improved sustained attention and inhibition alongside increased plasma brain-derived neurotrophic factor (BDNF) levels(Reference Chung, Park and Kwon53). In undergraduates, increased fibre intake from various sources improved memory performance and microbial richness(Reference Zhu, Ming and Wu54). In adults aged 50–70, a berry beverage containing 11 g of fibre and 795 mg of polyphenols improved working memory performance(Reference Nilsson, Salo and Plaza55). However, administration of a whole grain rye bread diet supplemented with resistant starch for 3 days to adults aged 52–70 improved mood but failed to improve cognitive performance despite increasing plasma SCFAs(Reference Sandberg, Björck and Nilsson56). The discrepant RCT data suggests that habitual intake/longer intervention may be needed to capture/facilitate the effects of fibre on cognition, and that elevated BDNF levels may mediate these effects. Supporting evidence can be seen in the fact that 3 ounces of almonds consumed daily for 6 months improved learning, planning, working memory, and visual memory in adults 50–75 years old, but no effects were seen after 3 months(Reference Mustra Rakic, Tanprasertsuk and Scott57). However, this delayed effect could be due to the accumulation of tocopherol, which is also related to cognitive functioning(Reference Ashley, Bradburn and Murgatroyd58,Reference Dorey, Gierhart and Fitch59) . The combination of fibre with other bioactives that are known to be associated with healthier eating patterns, including fatty acids and polyphenols(Reference Caruso, Torrisi and Mogavero60–Reference Ross, Mayer and Horn62), and also to influence cognitive function is problematic and requires unravelling to parse specific mechanisms of action of fibre per se.
The majority of RCTs with fermented food measure age-related cognitive decline in older adults to which fermented foods show a protective effect(Reference Hwang, Park and Paik63–Reference Ohsawa, Nakamura and Uchida68), which is also reported in the observational literature(Reference Hogervorst, Sadjimim and Yesufu69,Reference Tessier, Presse and Rahme70) . The RCTs conducted in older adults largely lack biological sampling with the exception of a small number of studies. In older adults, elevated serum BDNF levels from consumption of fermented soybean for 12 weeks positively correlated with improved attentional and memory performance(Reference Hwang, Park and Paik63), but camembert consumption for 12 weeks failed to improve memory despite an increase in serum BDNF levels(Reference Suzuki, Kojima and Osuka71), suggesting divergent results from different food substrates. Such heterogenous results, in general, are unsurprising given the broad definition of fibre and fermented foods.
While studies that examine the effects of fermented foods on cognitive processes in younger/middle-aged adults are sparse, the fact that a fermented milk drink altered brain activity in the frontal cortex of healthy women with a mean age of 30(Reference Tillisch, Labus and Kilpatrick72) suggests potential effects on executive functioning in addition to the observed hippocampal effects. Moreover, in younger adults aged 25–45, 4-week kefir consumption improved performance on hippocampal-dependent relational memory-associated tasks, but memory improvements were not correlated with the increased Lactobacillus levels(Reference Cannavale, Mysonhimer and Bailey73), suggesting indirect effects of microbial signalling to the brain that were not measured in that study.
Taken together, fibre intake appears to alter attention, memory, and executive functioning when consumed habitually for longer periods across the lifespan, although more research in younger/middle-aged adults is still warranted. For older adults, in which much of the data is derived, fermented foods exhibit a protective effect against global age-related cognitive decline, both over time and following briefer interventions. Nevertheless, additional effects of fermented foods on executive functioning in younger/middle-aged adults cannot be ruled out given the lack of studies that directly assess these cognitive domains in younger populations. The effects of fermented foods on cognitive processes in children and adolescents also remain largely unknown. Moreover, the lack of functional imaging data to determine a psychophysiological interaction for each food type precludes neurological insights. Increased BDNF could be a mediating signalling pathway, but formal exploration within a statistical mediation model is needed wherein BDNF is entered as a predictor to determine if it partially or fully explains the relationship between fibre/fermented foods on cognition. By determining the mechanism or pathway through which an independent variable influences a dependent variable, mediation models are useful analyses for statistically disentangling causal factors(Reference Pearl74). Moreover, the relationship between peripheral and central BDNF is disputed(Reference Klein, Williamson and Santini75), thus warranting some caution in interpreting effects on the brain. More attention is required to parse the mechanisms from the gut to the brain that are mediating such effects(Reference Ribeiro, Ferri and Clarke76).
Emotion
Fibre consumption alone and as part of a healthy dietary pattern has been consistently associated with better mood in clinical and non-clinical populations(Reference Kim, Byeon and Shin77–Reference Jaatinen, Korpela and Poussa81), but a systematic review and meta-analysis concluded that the extant RCT data is inconsistent with observational findings(Reference Aslam, Lotfaliany and So18). Recently, it was shown that an 8-week diet with a high potential prebiotic content improved anxiety, stress, and sleep in adults compared to a probiotic alone, a combination of the pre- and probiotic (synbiotic), or placebo and diet as usual. The prebiotic diet comprised a minimum of 5 g/d of asparagus, garlic, onion, oats, whole wheat, chickpeas, or watermelon. The probiotic contained Bifidobacterium bifidum (Bb-06), Bifidobacterium animalis subsp. lactis (HNO19), Bifidobacterium longum (R0175), Lactobacillus acidophilus (La-14), Lacticaseibacillus helveticus (R0052), Lacticaseibacillus casei (Lc-11), Lactiplantibacillus plantarum (Lp-115), Lacticaseibacillus rhamnosus (HN001)(Reference Freijy, Cribb and Oliver82). The beneficial effects observed in this study may be attributed to the high dosage of prebiotic-specific foods, meriting further attention. On the other hand, very few studies have explored the link between fermented foods and emotion, indicating a gap in understanding their chronic effects on mood. In young adults, higher frequency of fermented food consumption from sources that included live and inactive fermented foods in addition to non-fermented foods (i.e. yogurt, kefir, soy beverages and foods, miso soup, sauerkraut, dark chocolate, juices that contain microalgae, pickles, tempeh, kimchi) over the previous 30 days was linked to reduced social anxiety symptoms(Reference Hilimire, DeVylder and Forestell83). Conversely, Karbownik et al. discovered that higher total fermented food consumption from a variety of sources (cheese, yogurt, kefir, soured milk, kvass, unpasteurised beer, fermented and pickled vegetables with brine) over the previous 7 days was associated with increased depressive and anxiety symptoms in healthy medical students, but the inverse was found for medical students who reported an ongoing psychiatric disorder(Reference Karbownik, Mokros and Kowalczyk84). The difference in the timeframe of the assessment of fermented foods intake and the discrepancy in criteria for fermented foods might explain the inconsistent results.
RCTs with fermented food intervention show inconsistencies in effects, which could be due to differences in participant demographics. For example, fermented dairy products for 2 weeks(Reference Cannavale, Mysonhimer and Bailey73), 8 weeks(Reference Ohsawa, Nakamura and Uchida68), or 12 weeks(Reference Chung, Jin and Cui66) failed to improve mood in healthy adults. However, yogurt and the fermented dairy product, quark, improved mood only in participants with poor health status (i.e. immune depressed or chronic disease) and not for healthy participants(Reference Baars, Berge and Garssen85). Similarly, fermented kefir improved mood in participants with overweight(Reference Pražnikar, Kenig and Vardjan86) and fermented bonita fish broth improved fatigue and mood disturbances in adults with chronic fatigue(Reference Kuroda, Ishizaki and Maruyama87). Despite reports of improved mood in healthy women after consuming bonita broth for 2 weeks(Reference Nozawa, Ishizaki and Kuroda88), the results collectively suggest that fermented foods have larger effects on mood for individuals with compromised health status.
Similar to the cognitive data, prolonged fibre intake is likely to improve mood more than briefer periods of intake. Moreover, research that examines the impact of fibre and fermented foods on emotion in adolescence or middle adulthood is currently lacking. Considering this is a period with greater mood disturbances(Reference Pedrelli, Shapero and Archibald89,Reference Black and Rofey90) , more research is urgently needed in this population. The effects of fermented foods on mood in clinical and non-clinical populations, especially over longer time periods, is still largely unknown(Reference Ribera, Sánchez-Ortí and Clarke91). However, it appears that fermented foods may have preferential effects on individuals with comorbid health conditions.
Signalling pathways
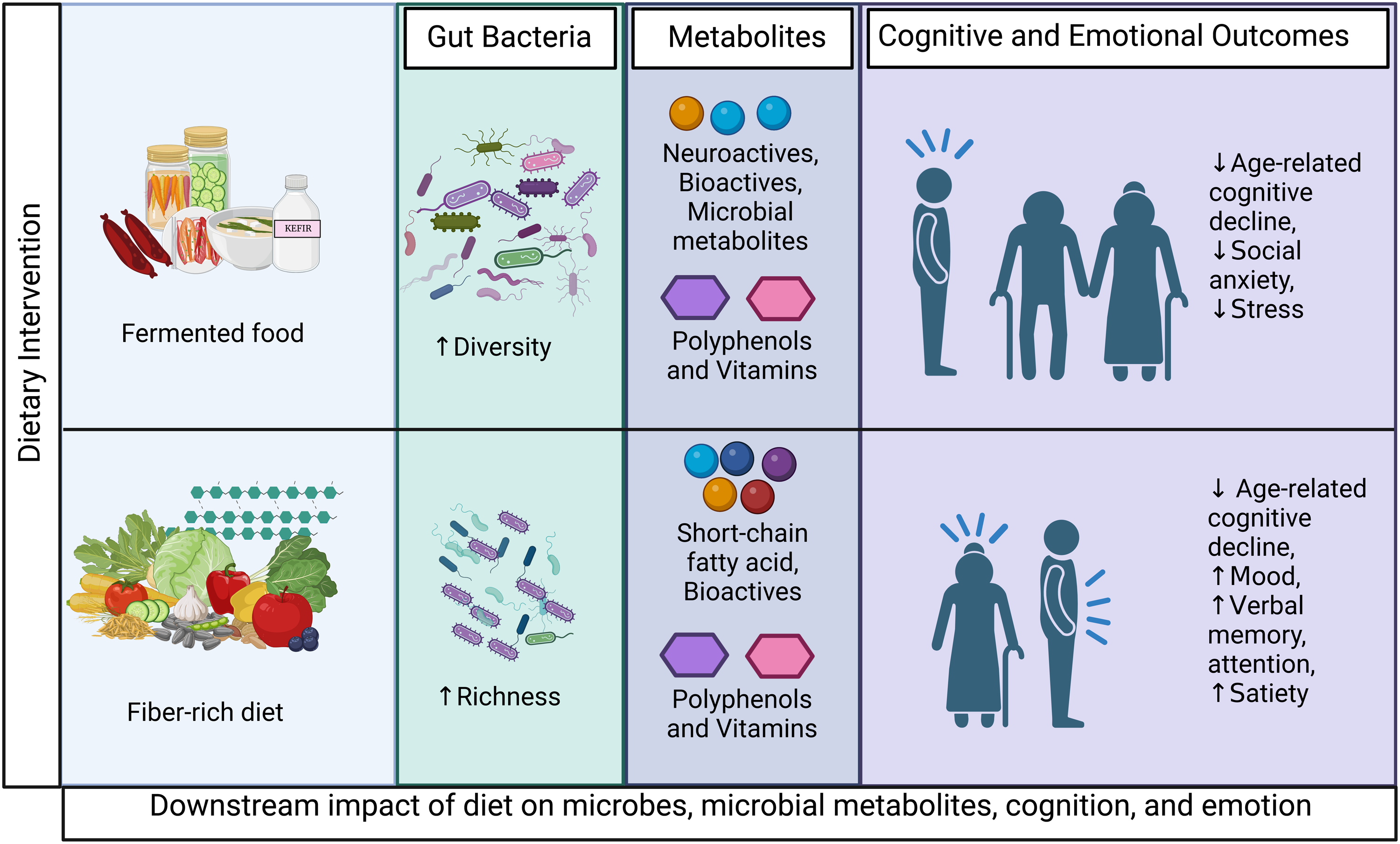
Few studies incorporate the assessment of potential biological mechanisms to support behavioural findings, which precludes insight into mediating pathways. Given the demonstrated capacity for fibre and fermented foods to concurrently modulate gut microbiota and the brain, each food likely alters signalling pathways from the gut to the brain. It is now accepted that the gut microbiota communicates with the brain along several pathways including immune, hypothalamic-pituitary-adrenal (HPA) axis, serotonin/tryptophan/kynurenine, vagal, neuroendocrine, and metabolome signalling(Reference Cryan, O’Riordan and Cowan92) (see Fig. 1). In the next sections we outline the effects of fibre and fermented foods on each communication pathway, prioritising evidence from humans where possible and available, to determine potential mechanisms of action underlying cognitive and emotional effects.

Fig. 1. Fermented foods are rich in microorganisms, bioactive peptides, phytochemicals, and peptides that can modulate brain function through enhancing microbial diversity leading to an enrichment of diverse microbial metabolites. Dietary fibre increases microbial richness, and neuroactive SCFAs are produced as a result of microbial fermentation of dietary fibre. Both fermented foods and fibre support intestinal and BBB integrity to prevent peripheral and central inflammation for optimal cognitive and emotional functioning. BBB: blood-brain barrier. GLP-1: glucagon-like peptide 1. IL: interleukin. PP: polyphenol. PYY: peptide YY. SCFA: short-chain fatty acid. Vit K, B: Vitamin K and B. Created with BioRender.com.
Immune system
The immune system is one of the most direct signalling pathways along the microbiota-gut-brain axis and has been shown to be fundamental in modifying behaviour across the lifespan(Reference Ratsika, Cruz Pereira and Lynch93–Reference Macpherson, Pachnis and Prinz95). The highest concentration of immune cells is located within the gastrointestinal tract, and these cells are in constant communication with the gut microbiota for the identification of potentially harmful pathogens. In mice, short-term exposure to a Western Diet deprived of fibre was sufficient to impair mucosal and systemic immunity, which created a window for opportunistic pathogens to invade intestinal tissue(Reference Siracusa, Schaltenberg and Kumar96). Re-introduction of dietary fibre re-programmed T cell metabolism and restored mucosal and systemic immunity. In this same study, healthy adults received a high-fibre diet comprised of a variety of food sources for five days before receiving a low-fibre diet for an additional five days. Not only did fibre deprivation reduce the abundance of fibre-fermenting bacteria (Eubacterium and bacteria from Lachnospiraceae family), but the main butyrate producer in the human microbiota (Agathobaculum butyriciproducens and Faecalibacterium prausnitzii) was decreased. These results were mirrored by a significant peripheral reduction of systemic TH17 cells co-expressing IL-17A and TNF-α and TH1 cells(Reference Siracusa, Schaltenberg and Kumar96). However, the anti-inflammatory effects of fibre have been shown to be moderated by baseline conditions of the individual. For example, Wastyk et al. found differential effects of fibre on inflammation that was dependent on baseline inflammation levels(Reference Wastyk, Fragiadakis and Perelman15). Similarly, the efficacy of soluble fibre intake on inflammation markers was reduced in individuals with lower microbiota richness(Reference Cotillard, Kennedy and Kong97).
In a recent RCT that compared administration of fermented yogurt against heat-treated yogurt with inactivated bacteria and two unfermented controls of whole milk and chemically acidified milk for 16 weeks to men with obesity, there were no differences between fermented yogurt and controls on c-reactive protein (CRP), IL-6, and TNF alpha levels(Reference Sandby, Magkos and Chabanova98). Despite these null effects, it is worth noting the sophisticated design of this study which incorporated several different fermented controls in line with our previous recommendations to enhance the scientific quality of future research(Reference Balasubramanian, Schneider, Gunnigle and Cotter20). These findings are also accordant with the results of a meta-analysis on the effects of fermented food intervention on inflammation in heterogeneous cohorts, which revealed that there was no dose-dependent association between fermented food consumption and inflammatory cytokine profile (IL-6, TNF-α, CRP)(Reference SaeidiFard, Djafarian and Shab-Bidar99). However, similar to dietary fibre, these effects interact with other factors such as age and food substrate. Differential results were shown when the analysis was split between participants below or above 50 years of age, such that fermented foods enhanced CRP and IL-6 levels in participants under 50 but significantly reduced IL-6 levels in participants over 50. Fermented foods decreased TNF-α levels in both age groups. The authors suggested the moderating effects of age are likely due to increased inflammation associated with aging. Furthermore, subgroup analyses of fermented dairy products demonstrated a reduction in CRP and elevation in IFN-γ, and no effect on other inflammatory markers (IL-10, IL-6 and Il-12)(Reference SaeidiFard, Djafarian and Shab-Bidar99). This may explain the anti-inflammatory effects observed following a varied, but predominantly yogurt, 10-week dietary intervention in healthy adults(Reference Wastyk, Fragiadakis and Perelman15). Pooling fermented foods for a meta-analysis likely obscures physiological effects of each food individually given their diverse substrate nature, bioactive profile, and microbial consortia that they host.
Hypothalamic-pituitary-adrenal (HPA) axis and stress
The HPA axis and its hormone, cortisol, is activated in response to acute and chronic stress and has been shown to be crucially regulated by the microbiota-gut-brain axis(Reference Leigh, Uhlig and Wilmes100–Reference Nagpal and Cryan102). Germ-free rodents, deprived of exposure to microorganisms at birth and reared in sterile environments, display exaggerated HPA axis responsiveness to stressors compared to colonised controls(Reference Sudo, Chida and Aiba103–Reference Lyte, Gheorghe and Goodson106), and Bifidobacterium infantis administration reverses this response in a time-window dependent manner(Reference Sudo, Chida and Aiba103). Additionally, administration of strains of Lactobacillus and Bifidobacterium has been shown to attenuate anxiety and depressive-like behaviour brought about by early-life stress(Reference Eutamene, Lamine, Chabo and Theodorou107,Reference Desbonnet, Garrett and Clarke108) , indicating a key signalling pathway from the gut to the brain(Reference Cryan, O’Riordan and Cowan92).
In humans, responses to stressors can be evaluated from naturally occurring acute and chronic stressors to provide greater generalisability. Conversely, experimental induction of acute stress in a laboratory setting has the advantage of reducing extraneous factors, but insights into chronic stress cannot be gained(Reference Allen, Kennedy and Cryan109). Reductions in salivary cortisol levels from fermented milk with Lacticaseibacillus casei strain Shirota(Reference Takada, Nishida and Kataoka-Kato110,Reference Kato-Kataoka, Nishida and Takada111) , but not from Lactobacillus casei DN-114001 fermented milk(Reference Marcos, Wärnberg and Nova112), have been observed in students undergoing academic exams and in school-aged students consuming a West African fermented milk product containing sugar and millet(Reference Brett, Koko and Doumbia113). Despite this encouraging evidence, more studies are needed that assess the impact of non-dairy fermented foods on acute and chronic stress in heterogeneous populations including older aged adults and patients with clinical stress disorders.
Much of the evidence for a link between dietary fibre and cortisol comes from observational or RCT studies that examine dietary patterns as opposed to fibre alone. For example, acute intake of low-fibre/high-sugar meal to ethnic minority adolescents with obesity was related to elevated cortisol levels compared to a low-sugar/high-fibre meal(Reference Wen, Chou and Belcher114). Chronic adherence to a healthy diet rich in fibrous foods and polyunsaturated fats decreased cortisol levels in response to an acute laboratory stressor in women with overweight/obesity(Reference Soltani, Keim and Laugero115). Intriguingly, sweet, fatty and snack food consumption frequency was positively linked to cortisol levels in a large sample of children aged 5–10, but fruit and vegetable intake had no effect(Reference Michels, Sioen, Braet and Huybrechts116). Combined, these results suggest that the negative effects of high-fat/sugar foods may have a greater impact on cortisol levels than from fibre alone(Reference Pearlmutter, DeRose and Samson117). While dietary patterns more closely resemble actual eating patterns, specificity in determining the mechanism of action of the efficacious compound is needed to determine if the effects observed are due to fibre or polyunsaturated fat intake.
Vagus nerve
The vagus nerve is the most direct pathway linking the gut to the brain(Reference Fülling, Dinan and Cryan118). Interestingly, specific probiotic bacteria, such as Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus, that are present in some fermented foods have demonstrated dependency on vagal signalling to exert effects on the brain(Reference Sgritta, Dooling and Buffington119–Reference Bercik, Park and Sinclair121). Similarly, SCFAs have been shown to stimulate vagal signalling(Reference Bruning, Chapp and Kaurala122,Reference Goswami, Iwasaki and Yada123) , suggesting another pathway through which fibre influences cognition. In rodents, a high-fat, high-sugar carbohydrate diet impaired vagus nerve signalling of satiety(Reference Loper, Leinen and Bassoff124), and administration of potato-derived resistant starch inhibits remodelling of vagal satiety signalling from a high-fat diet(Reference Klingbeil, Cawthon and Kirkland125).
Short-chain fatty acids
Microbial fermentation of undigested dietary fibre stimulates the formation of organic acids (lactic, succinic acid) and SCFAs (acetate, propionate, and butyrate). There are several pathways through which SCFAs influence brain activity, indicating the prominence of this pathway between fibre intake and the brain. First, SCFAs can modulate concentrations of neurotransmitters (e.g. GABA, serotonin, glutamate) and neurotropic factors(Reference Silva, Bernardi and Frozza126) that are directly involved in cognitive and emotional processes. Through supporting the intestinal and blood-brain barrier (BBB), SCFAs protect against neuroinflammation(Reference O’Riordan, Collins and Moloney11,Reference Khoshbin and Camilleri127) . Finally, a combination of SCFAs (acetate, propionate, and butyrate), but not butyrate alone(Reference Dalile, Fuchs and La Torre128), alters the HPA axis to attenuate stress-induced cortisol increases(Reference Dalile, Vervliet and Bergonzelli129), suggesting synergistic effects of different SCFAs on stress. Fermented foods have also been shown to alter SCFAs. For example, Spanish residents who consumed greater amounts of cheese had higher levels of all SCFAs(Reference González, Fernández-Navarro and Arboleya130). However, given that SCFAs are produced as a result of fibre fermentation, fibre is likely to have stronger effect on concentrations than fermented foods.
Other signalling pathways
Enteroendocrine
Enteroendocrine L cells within the intestinal epithelial cells secrete glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in the postprandial state. Beyond their effects on satiety, gut-derived peptides GLP-1 and PYY have be linked to cognitive and emotional processing(Reference McIntyre, Powell and Kaidanovich-Beilin131,Reference Stadlbauer, Woods and Langhans132) . Short- and long-term dietary fibre intake concurrently increases self-report feelings of satiety and the release of gut peptides GLP-1 and PYY(Reference Wanders, van den Borne and de Graaf133–Reference Al-Mana and Robertson135). Moreover, SCFAs can alter the production of GLP-1 and PYY, indicating indirect pathways(Reference Zhao, Zheng and Ding136). GLP-1 can also be stimulated by certain Lactobacillus strains present in fermented foods(Reference Simon, Strassburger and Nowotny137), but further research is needed that assesses GLP-1 and PYY release from whole foods in human participants.
Bile acids
Primary bile acids that are formed in the liver from cholesterol breakdown are further transformed into secondary bile acids by gut microbiota(Reference Rowland, Gibson and Heinken138). Bile acids can cross the blood-brain barrier to influence brain function. Indeed, altered bile acid profiles have been shown in mild cognitive impairment and Alzheimer’s Disease(Reference Mahmoudian Dehkordi, Arnold and Nho139,Reference Varma, Wang and An140) , but bile acids have also shown neuroprotective effects on the brain in preclinical models(Reference Theofilopoulos, Wang and Kitambi141). The binding of dietary fibre to conjugated bile acids prior to metabolism by the gut microbiota hints at the connection between dietary intake and microbial metabolites(Reference Singh, Metrani and Shivanagoudra142). Indeed, increased secondary bile acid, taurolithocholic acid, was observed following a four-week diet high in whole grains, legumes, fruits, and vegetables in healthy adults(Reference Ginos, Navarro and Schwarz143). Although the relationship between fermented foods and bile acids is less established, fermented milk kefir increased secondary bile acids that were decreased from high-fat diet in rats(Reference Gao, Mao and Wang144).
Other metabolites
Polyphenols and polyamines
Fibrous and fermented foods are rich sources of polyphenols, and fermentation of plant and vegetables through lactic acid bacteria enhances the conversion of phenolic compounds to biologically active metabolites leading to an increase the production of phenolic metabolites(Reference Johnson and de Mejia145,Reference Filannino, Bai and Di Cagno146) . Moreover, polyamines (spermine, spermidine and putrescine), which are also produced by the gut microbiota and affect the brain(Reference Makletsova, Rikhireva and Kirichenko147), are found in plant-derived foods linked to phenolic compounds(Reference Di Martino, Campilongo and Casalino148,Reference Muñoz-Esparza, Latorre-Moratalla and Comas-Basté149) . Gut microbiota utilisation of polyphenols results in phenolic compounds, which has shown associations with cognitive resilience in rats(Reference Frolinger, Sims and Smith150). As such, it is still largely unknown if polyphenols in fibrous and fermented foods are driving their beneficial impact on health.
Neurotransmitters
The gut microbiota indirectly and directly stimulates the production of neurotransmitters to influence central nervous system activity. There is a growing appreciation that tryptophan and its metabolic pathways to serotonin or kynurenine can be key signalling pathways within the microbiota-gut-brain axis(Reference Gheorghe, Martin and Manriquez151–Reference Rothhammer, Mascanfroni and Bunse153). Although the gut produces the majority of serotonin(Reference Appleton154), it is unable to cross the BBB directly. However, dietary-derived tryptophan is absorbed by the gut and then transported, along with its metabolite kynurenine, to the brain to influence behaviour(Reference O’Mahony, Clarke and Borre155). A strain of Bifidobacterium infantis, a bacterium present in fermented foods, has been shown to elevate plasma tryptophan levels to increase supply to the central nervous system for serotonin production(Reference Desbonnet, Garrett and Clarke108). Evidence for neuromodulatory capacity of dietary fibre and fermented foods in humans is sparse. One study showed that Lacticaseibacillus casei fermented milk product consumption for 8 weeks increased faecal serotonin and decreased gastrointestinal distress without altering serum tryptophan or kynurenine levels(Reference Kato-Kataoka, Nishida and Takada111). High dietary fibre intake positively correlates with indolepropionic acid, a microbial metabolite of tryptophan(Reference Tuomainen, Lindström and Lehtonen156), and administration of whole grain rye lowered plasma serotonin concentrations in healthy adults(Reference Keski-Rahkonen, Kolehmainen and Lappi157).
Like serotonin, dopamine produced within the gut(Reference Asano, Hiramoto and Nishino158) cannot cross the BBB, but its precursor L-3,4-dihydroxyphenylalanine (L-DOPA) can via large neutral amino acid transporters(Reference Kageyama, Nakamura and Yamasaki159). Given the known relationship between dopamine and cognitive and emotional processing(Reference Alexander, Aragon and Bookwala160), and the fact that hyper-palatable foods deprived of fibre consumed chronically blunts central dopaminergic activity(Reference Volkow, Wang and Baler161), it is necessary to understand how the gut microbiota and brain communicate through food-induced/microbiota mediated alteration of peripheral and central dopamine.
The primary inhibitory neurotransmitter, GABA, plays a key role in affective and cognitive processing(Reference Koh, Kwak and Cheong162,Reference Luscher, Maguire and Rudolph163) . GABA is synthesised by bacterial strains found in fermented foods(Reference Richard and Foster164,Reference Siragusa, De Angelis and Di Cagno165) , and fermented foods that contain high levels of lactobacilli frequently also contain millimolar levels of GABA(Reference Li, Gao, Cao and Xu166). Intriguingly, fermentation by Lactiplantibacillus plantarum VTT E-133328 of faba bean flour enhanced GABA levels of the faba bean flour(Reference Coda, Melama and Rizzello167), indicating additive effects from fermenting high-fibre foods. Notably, the ability of GABA to cross the BBB in appreciable quantities is disputed and further research in warranted to understand the contribution of peripheral v. central mechanisms(Reference Boonstra, de Kleijn and Colzato168).
The cholinergic neurotransmitter, acetylcholine, is found in both the central and peripheral nervous system. Acetylcholine is highly implicated in shaping emotional and cognitive processes(Reference Picciotto, Higley and Mineur169). Several bacteria commonly found in fermented foods produce acetylcholine(Reference Horiuchi, Kimura and Kato170), demonstrating the potential for fermented food interventions to modulate peripheral acetylcholine and possibly its signalling to the brain. It was recently shown that a cafeteria diet deficient in dietary fibre impaired hippocampal-dependent memory and hippocampal acetylcholine signalling in rats(Reference Hayes, Tierno Lauer and Kao171). It will be interesting to test whether fibrous or fermented foods can rescue deficits in acetylcholinergic signalling induced by the Western Diet.
Vitamins and minerals
The effects of vitamin and mineral deficiencies on cognitive and emotional health are well-defined(Reference Muscaritoli172). Vitamin K and several B vitamins are produced by gut microbiota and host intake of these vitamins increases microbial diversity and richness, alongside an increase in the production of SCFAs(Reference Pham, Dold and Rehman173). Dietary fibre and substrates used for fermentation are rich in vitamins, and fermentation further increases the abundance of vitamins(Reference Septembre-Malaterre, Remize and Poucheret174). Similarly, minerals, such as magnesium and zinc found in both fibrous and fermented foods, have limited bioavailability when obtained from vegetable sources due to the presence of phytates and oxalates(Reference Gibson, Raboy and King175), which form complexes with minerals and limit their absorption. Fermentation through different microorganisms improves their bioavailability and absorption by breaking down phytate and oxalate complexes with minerals(Reference Ahmed, Xu and Sulieman176,Reference Wadamori, Vanhanen and Savage177) . Like polyphenols, more granularity is needed to understand mechanisms of action specific to the vitamin and mineral content present in fibrous and fermented foods.
Future directions
In addition to evaluating the unique effects of fermented foods or fibre on the microbiota-gut-brain axis, we have highlighted several gaps in the literature that are summarised in Fig. 2 and described herein.

Fig. 2. Potential approaches to address challenges in nutrition studies for the microbiota-gut-brain axis. Created with BioRender.com.
A review of the cognitive and emotional literature has emphasised the need for multimodal outcomes that assess behavioural changes on a full battery of cognitive tasks and self-report measures of affect in addition to biological samples within the same study design. To the best of our knowledge, only two studies directly assess the impact of fermented foods on brain functioning, and no studies used imaging techniques to study the neurological effects of fibre in humans. Combined methodology will shed light on the biopsychological mechanisms necessary to inform the development and selection of potential psychobiotic treatments.
We continue to know very little about how genetic backgrounds moderate the effects of each food type on gut-brain signalling. For example, some individuals exhibit differences in the metabolism of fermentation byproducts including biogenic amines such as histamine, which can indirectly affect cognitive and emotional processing through physical side effects and alterations of arousal(Reference Maintz and Novak178). Alternatively, fermented foods can also indirectly improve brain activity through enhancing digestibility in individuals with certain food intolerances by, for example, converting lactose into lactic acid. Future studies should investigate how individuals from different populations and genetic backgrounds respond to interventions with fibre or fermented foods. Baseline gut microbiota composition and functionality is also highly individual, and baseline differences in the fermentation and/or colonic absorption of fibre can impact butyrate production(Reference So, Whelan, Rossi and Morrison26,Reference Holmes, Villa and Durand179) . Employment of a crossover design, where each participant serves as their own control, is a possible solution to this confound. It is imperative, however, to counterbalance experimental arms in a crossover design and include a washout period to ensure the absence of prolonged effects on the gut microbiota that can spillover into the next experimental arm. The duration of the intervention should also be prolonged, because effects of fibre on cognition were observed at six months, but not three months, which may explain the discrepancies between observational and interventional data(Reference Mustra Rakic, Tanprasertsuk and Scott57). It is likely that longer interventions are needed in healthy populations for which changes may be small in effect size. Longer interventions are also useful to allow for tolerance to a high-fibre and/or fermented food diet that may initially cause gastric distress and therefore affect cognitive/emotional measures. Testing in healthy populations may further necessitate selection of behavioural measures that can capture small changes alongside utilisation of next generation sequencing of the microbiota and metabolome (e.g. shotgun metagenomics) to detect resolution at the strain level alongside functional changes(Reference Petrone, Aqeel and Jiang180). Moreover, biomarkers are needed to identify food consumption patterns and their potential downstream metabolites that are produced as a consequence of intake and can affect the overall health of the consumer(Reference Li, Burton-Pimentel and Brouwer-Brolsma181). Throughout the intervention, longitudinal captures of the gut microbiota via repeat faecal sampling can provide valuable information about the time course of effects.
A granular understanding of specific fibre subtypes on health outcomes is needed for personalised/precision approaches. However, the fact that fibrous foods can simultaneously comprise fermentable soluble and insoluble fibre provides a challenge. Collaboration with bioinformaticians to establish an open-source database that catalogues the constituents of each fibre is needed to advance mechanistic insight. More specificity is similarly required for fermented foods. There are several studies that evaluate the effects of singular fermented foods, but heterogeneity in the participants, length of the intervention, and outcomes limits insights gained from aggregating data(Reference Marx, Scholey and Firth21). In an RCT where participants consumed a variety of fermented foods, a stronger correlation to alpha diversity from yogurt and vegetable brine drink consumption was observed, but this could be due to the higher consumptions rates of these items relative to the other fermented food types (kefir, cottage cheese, kombucha, and fermented vegetables)(Reference Wastyk, Fragiadakis and Perelman15). Exploitation of large datasets from individuals who consume a variety of fermented foods is needed to avoid floor effects and to achieve adequate statistical power required to determine which fermented foods are superior in modulating brain activity. It is worth noting that metagenomics revealed that, of those studied, water kefirs, sauerkrauts, and kvasses contained the greatest concentration of potential health-associated gene clusters(Reference Leech, Cabrera-Rubio and Walsh182). Combining in silico methods with functional behavioural readouts is a promising approach to identify top-performing fermented foods to improve neurobehaviour. Another limitation in the isolation of specific fermented foods is the lack of validated, culturally sensitive and/or feasible tools to measure fermented food intake(Reference Li, Brouwer-Brolsma and Burton183,Reference Campbell, Hauptmann and Campbell184) . Specifically, fermented foods are currently assessed via unvalidated self-report measures of frequency or through self- or dietitian-led food logging or food recall, which is onerous for the participant and/or the researcher.
Towards new psychobiotic therapies
To date, only one study has directly compared the differential effects of fibre and fermented foods in humans within the same experimental design(Reference Wastyk, Fragiadakis and Perelman15). In this study, healthy adults were randomly allocated to consume a high quantity of a variety of fibrous foods (n=18) or fermented foods (n=18) for 10 weeks with no control group. Distinct effects on the gut microbiome were found such that fibre altered microbial functionality by decreasing the production of branched-chain fatty acids (isobutyric, isovaleric, valeric acid), while fermented foods increased alpha diversity. Fermented foods further reduced inflammatory markers, while fibre inflammatory effects were person-dependent. Unexpectedly, no differences were observed on self-reported perceived stress, wellbeing, fatigue, or cognition(Reference Wastyk, Fragiadakis and Perelman15), but this could be due to a reliance on self-report measures of cognitive performance and the small sample size that obscured effects.
The results from Wastyk et al. suggested potential additive effects from combining fermented and fibrous foods on the microbiota-gut-brain axis(Reference Wastyk, Fragiadakis and Perelman15). When combined with fermented foods, fibre can enhance the colonisation, survival and function of the microbes within fermented foods and from host microbes(Reference Preidis and Versalovic185). Recently, we have shown in our own lab that a combined diet rich in fibre and fermented foods from a variety of food sources for four weeks improved perceived stress in a dose-dependent manner in healthy adults compared to a healthy diet in line with Irish dietary guidelines(Reference Berding, Bastiaanssen and Moloney8). However, the combined diet had only subtle effects on gut microbiota composition and local gastrointestinal functional outputs, but this could be due the short duration of the intervention. Similarly, an inulin-enriched fermented yogurt decreased self-report scores of anxiety and depression in menopausal women compared to yogurt alone(Reference Shafie, Homayouni Rad and Mirghafourvand186). Future work should investigate whether consuming fermented and fibrous foods within the same meal elicits additive benefits, both acutely and chronically. The collective data provides support for the use of psychobiotics(Reference Sarkar, Lehto and Harty187), as a combination of pre-, pro-, post-, and/or synbiotics, to treat disorders related to dysfunction in cognitive and/or emotional processes. Psychobiotics have already demonstrated efficacy in attenuating depressive symptoms(Reference Zhang, Chen and Zhang188) and improving cognition in healthy populations(Reference Nieto-Ruiz, Garcia-Santos and Verdjo-Roman189) and populations with cognitive impairments(Reference Louzada and Ribeiro190), but more research with whole-food interventions in healthy and clinical populations is needed.
Conclusion
Taken together, both fibre and fermented foods exert protective effects on cognitive and emotional processes. Although only one study has investigated the differential mechanisms of fibre and fermented foods on the microbiota-gut-brain axis(Reference Wastyk, Fragiadakis and Perelman15), there is a growing interest in understanding the role of the gut microbiota underlying dietary patterns that have been shown to alter central nervous system processes(Reference Link, Subramanian and Cheung191). Such advancements are essential for development of future therapeutics to treat brain dysfunction.
In considering future therapeutics, the challenge in consuming high-fibre foods is reflected by reports of inadequate consumption of fibre in nearly all Western societies(Reference Burley, Champ and Cloran192,Reference Clemens, Kranz and Mobley193) . For some, fermented foods could be an alternative solution for individuals who struggle to consume dietary fibre due to issues with taste and/or tolerance, for example. It is worth highlighting the encouraging work currently underway by Jeff Gordon and colleagues. In this pioneering work, Gordon et al. aims to treat cognitive dysfunction in children brought upon by malnutrition to the gut microbiota in early life by fibrous food formulations that are culturally appropriate, environmentally sustainable, and affordable to produce(Reference Goyal, Venkatesh and Milbrandt194–Reference Gehrig, Venkatesh and Chang196). This work suggests that the inexpensive and ubiquitous nature of fibre and fermented foods is a viable means of targeting brain health, at least partially via the gut microbiota, to ensure optimal functioning across all stages of life.
Acknowledgements
None.
Financial support
This work was supported by a Science Foundation Ireland (SFI) grant to APC Microbiome Ireland through the Irish Government’s National Development Plan [grant SFI/12/RC/2273_P2]. ES is funded by an Irish Research Council Postdoctoral Fellowship [GOIPD/2024/273]. ES and AF are supported by a private philanthropy donation awarded to JFC. The funding sources had no involvement in the decision to submit this manuscript for publication.
Author contributions
Authors ES and JFC were responsible for the design, and ES drafted the article. Authors ES, RB, AF, PDC, GC and JFC assisted in the interpretation and revision of the content.
Competing interests
Author ES has received honorarium from Janssen Sciences Ireland UC. PC is a co-founder and is the CTO of SeqBiome Ltd. and also serves as the Field Chief Editor for Frontiers in Microbiology. He has also been occasionally paid, or received hospitality, to deliver talks on his research. Research in the Cotter laboratory has been funded by Danone, PepsiCo, Friesland Campina and the PrecisionBiotics Group. GC has received honoraria from Janssen, Probi and Apsen as an invited speaker, is in receipt of research funding from Pharmavite, Reckitt, Tate and Lyle, Nestle and Fonterra, and has received payments as a consultant from Yakult, Zentiva and Heel Pharmaceuticals. JFC has received research funding from 4D Pharma, Cremo, Dupont, Mead Johnson, Nutricia and Pharmavite; has been an invited speaker at meetings organised by Alimentary Health, Alkermes, Ordesa, and Yakult; and has served as a consultant for Alkermes and Nestle.