Depression is the most common mental health disorder in community settings and is estimated to become the second largest cause of global disability by 2020. Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari and Erskine1 It is one of the most common reasons for consulting with a primary care physician and its associated personal and economic burden is considerable. Reference Layard2 Although antidepressants remain an important treatment option, many patients and healthcare professionals would like to access psychological therapy as an alternative or adjunct to drug therapy. Reference Roth and Fonagy3 Cognitive–behavioural therapy (CBT) has emerged as a leading evidence-supported form of brief psychological therapy for people with depression. 4 However, demand for CBT cannot be met from existing therapist resources. Reference Kaltenthaler, Brazier, De Nigris, Tumur, Ferriter and Beverley5 One promising alternative to therapist-delivered CBT is the use of self-help interventions including the provision of therapy via computer. Reference Andrews, Cuijpers, Craske, McEvoy and Titov6 In recent years a number of interactive programs have been developed that enable CBT to be delivered by computer (computerised CBT (cCBT)). If effective, such programs have the potential to expand the provision of psychological therapy in primary care and may represent an efficient and effective form of care for depression. Reference Christensen, Griffiths and Jorm7 In an earlier large-scale pragmatic trial (the first Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial) Reference Gilbody, Littlewood, Hewitt, Brierley, Tharmanathan and Araya8,Reference Littlewood, Duarte, Hewitt, Knowles, Palmer and Walker9 we compared two commonly used cCBT packages (MoodGYM or Beating the Blues) v. usual primary care under real-world conditions to test their effectiveness (rather than efficacy). Participants were proactively offered technical support and weekly encouragement to use the computer packages, but we purposely did not augment the content of psychological therapy over the telephone. The cCBT in the first REEACT trial was therefore a form of supported self-help, but was not one that was guided by a clinician. The first REEACT trial is, at the time of writing, the largest publicly funded independently conducted primary care trial of cCBT. The main finding of the trial was that for the primary outcome of depression severity at 4 months there was no significant benefit when participants were offered technically supported cCBT in addition to usual general practitioner (GP) care. The most likely explanatory mechanism of lack of effect was poor uptake and use of computer packages by trial participants under real-world conditions. Reference Littlewood, Duarte, Hewitt, Knowles, Palmer and Walker9
Systematic reviews have highlighted the potential for cCBT to be effective but have also further demonstrated variable effect sizes and substantial between-study heterogeneity. Reference Andersson and Cuijpers10,Reference Spek, Cuijpers, Nyklícek, Riper, Keyzer and Pop11 One important source of between-study heterogeneity is the level of support that is made available to people who are offered treatment with cCBT. cCBT requires a person with depression to engage with a self-help computer-based technology. Research has shown that people with depression often do not engage with cCBT, and only a minority actually complete all of the planned sessions of the computer package. Reference Waller and Gilbody12 This observation is consistent with a broader body of research into the uptake and effectiveness across the range of self-help interventions for depression such as bibliotherapy (self-treatment using written materials). Reference Cuijpers, Donker, Johansson, Mohr, van Straten and Andersson13 Research in the area of self-help treatments for depression has demonstrated that entirely self-guided materials (with no professional support) are likely to be less effective than self-help technologies where there is a level of guidance and professional support (‘guided self-help’). Unsupported self-help treatment (including unsupported computer-delivered self-help) has been shown in systematic reviews to have minimal or relatively small effect sizes. Reference Cuijpers, Donker, Johansson, Mohr, van Straten and Andersson13 In contrast, more intensively and professionally supported treatments have generally been found in efficacy trials to have moderate effect sizes claimed to be comparable with those achieved with face-to-face therapy. Reference Andersson, Cuijpers, Carlbring, Riper and Hedman14 To our knowledge the comparative effectiveness of minimally supported cCBT v. more intensively supported cCBT has not been directly tested in large-scale, independently-conducted, head-to-head, pragmatic trials (although there are some head-to-head comparisons in smaller-scale trials Reference Baumeister, Reichler, Munzinger and Lin15 ). We postulated on the basis of these findings, and on the basis of emerging trial-based evidence summarised in systematic reviews (for example Andersson & Cuijpers Reference Andersson and Cuijpers10 ), that people with depression might engage with cCBT and it might show an effect, but only if offered alongside a high level of facilitation and support. We designed the present study (the REEACT-2 trial, trial registration: ISRCTN55310481) to test this hypothesis and to generate trial-based evidence on the best means of delivering cCBT in primary care mental health services.
Method
Study design and patients
The REEACT-2 trial was designed to examine the additional benefits of telephone facilitation and structured guidance alongside a free-to-use computer-delivered CBT package (MoodGYM). The comparator was minimally supported cCBT. Participants in both arms were given access to MoodGYM, an accompanying booklet, a freephone number for technical support and continued with usual GP care. MoodGYM is a free-to-use, internet-based, interactive CBT program for depression developed and copyrighted at the Australian National University Centre for Mental Health Research. The online program is accompanied by a booklet with exercises and quizzes and consists of five interactive modules released sequentially and lasting approximately 30–45 min and a sixth session that is predominantly consolidation and revision. Study participants were asked to complete one session each week. The program provides patients with CBT techniques to overcome patterns of unhelpful thinking using cartoon characters to represent habits of thought.
Experimental arm
Participants in the telephone-facilitated cCBT (experimental) arm were allocated a telephone support worker (TSW) who provided a programme of weekly telephone calls. The background of the TSWs was that of a graduate-level support worker. The telephone facilitation programme comprised eight telephone calls to be completed alongside the cCBT program within 14 weeks of first contact from the TSW (and before the 4-month follow-up time point). The purpose of the first and longest session (30–40 min) was to introduce the participant to the principles of CBT and the MoodGYM program and booklet, explain the process and help the participant identify difficulties and goals, and feel confident about engaging with the intervention. The following six sessions were between 10 and 20 min long and were intended to provide motivation and to help participants identify any barriers to engagement with cCBT and to the achievement of their goal(s). The final session helped participants to consolidate what they had learned from cCBT and discuss their next steps and, if appropriate, how they might use the MoodGYM program in the future. The telephone facilitation programme was delivered according to a manual developed by co-investigator K.L. in conjunction with the REEACT-2 team. TSWs received 1 day of training in the delivery of the intervention. Clinical supervision was given to trial TSWs by investigators K.L., D.K. and S.G.
Comparator arm
All participants in the control group were registered as users of MoodGYM and given a unique password. As with the intervention group, they were supplied with a free helpline number to ring if they had technical problems or needed advice and a booklet explaining MoodGYM, but they did not receive regular phone calls. This comparator intervention replicated UK National Health Service care in most settings and represented what would happen if a patient were given the website of a cCBT package such as MoodGYM by their GP or primary care mental health worker without being offered proactive support.
The study population comprised patients selected from primary care with depression or low mood as determined by a score of ten or more on the Patient Health Questionnaire (PHQ)-9. Reference Kroenke and Spitzer16 This cut-off point is known to detect clinical depression (major depression) in primary care populations Reference Moriarty, Gilbody, McMillan and Manea17 with acceptable sensitivity and specificity. The REEACT-2 participants were recruited from a mix of rural and urban UK primary care practices in and around Bristol, Avon, Somerset, Gloucestershire, Manchester, Sheffield, Derbyshire, South Yorkshire, Humberside, East Yorkshire, Durham, Tyneside and Northumberland.
Participants meeting the following criteria were eligible to enter the study: (a) aged 18 or above; (b) not currently in receipt of cCBT or specialist psychological therapy; (c) a score of ⩾10 overall (indicating moderate, moderately severe or severe depression) and <3 for question 9 (measuring suicidal thoughts) on the PHQ-9 depression instrument. Reference Kroenke and Spitzer16 Both individuals with incident and prevalent primary care cases of depression were included. In line with the pragmatic nature of this trial, patients were eligible to participate whether or not they were in receipt of antidepressant medication or had comorbid physical illness or non-psychotic functional disorders. We excluded people currently in receipt of psychological therapy. We also excluded potential participants who: (a) were actively suicidal as identified by their GP or as reported by item 9 on the PHQ-9; (b) had been bereaved within the past year; (c) had given birth within the past year; (d) had a diagnosis of psychotic depression; (e) had a primary diagnosis of alcohol or drug misuse; (f) were not able to read and write in English.
Randomisation and masking
Simple randomisation was performed using a computer-generated random number sequence. At the end of the baseline appointment study researchers telephoned a secure randomisation line at the York Trials Unit and were given participant allocation and MoodGYM login details. Participants were informed immediately.
Outcome measures
The pre-specified primary outcome was depression severity and symptomatology as measured on a validated self-report continuous measure (PHQ-9) Reference Kroenke and Spitzer16 at 4 months. The secondary outcome measures were: PHQ-9 at 12 months (as a continuous measure); PHQ-9 at 4 and 12 months (dichotomous measure at cut-point PHQ-9 ⩾10); Reference Kroenke and Spitzer16 anxiety (Generalised Anxiety Disorder Questionnaire (GAD-7)); Reference Spitzer, Kroenke, Williams and Lowe18 somatoform complaints (PHQ-15); Reference Kroenke, Spitzer and Williams19 health-state utility (EuroQol – EQ5D); 20 and service use using the adapted Client Service Receipt Inventory (CSRI) Reference Chisholm, Knapp, Knudsen, Amaddeo, Gaite and Van Wijngaarden21 at 4 and 12 months.
The REEACT-2 trial was powered on the basis of an ability to detect a between-group difference in PHQ scores. We sought to recruit 350 patients with depression – 175 participants per arm. The REEACT-2 trial was designed to have sufficient power to detect a Cohen's d effect size of 0.30 with 80% power allowing for loss to follow-up of 20% in line with our empirically based estimates from the first REEACT trial. The final sample size for the two arms was 369 and we exceeded the pre-specified sample size.
Statistical analysis
All outcomes were summarised descriptively by intervention group and at each time point using mean, median, standard deviation, range and number of patients for continuous outcomes and number of patients and percentage for discrete outcomes. The primary outcome was the severity of depression as measured by the PHQ-9 as a continuous measure at 4 months. Statistical analyses were performed in SAS version 9.3.
The PHQ-9 score was summarised and analysed as a continuous outcome. This was summarised for each assessment time point (baseline, 4 and 12 months) using mean, standard deviation, median and range, and the number of missing values. Plots were presented showing the mean and 95% confidence interval at each time point. A repeated measures mixed regression model was used to analyse the change in PHQ-9 score over time. This included all randomised participants (intention to treat) and provides reliable estimates assuming the data are missing at random. The outcome was the PHQ-9 score at 4 and 12 months and the model included the baseline PHQ-9 score, treatment group, age, gender, baseline GAD-7 score and time. The treatment×time interaction was included to evaluate if the difference between treatments changed over time. The mean difference, 95% confidence interval and P-values are presented for all terms in the model. Effect sizes (Cohen's d) were calculated for the between-group differences in mean PHQ-9 score at 4 and 12 months using the difference between the means and corresponding standard errors from the mixed model. The standard errors were converted to standard deviations using the corresponding sample size in each treatment group.
The dichotomous analysis (not depressed (PHQ-9<10)/depressed (PHQ 9⩾10)) compared minimally supported cCBT with telephone-facilitated cCBT using a logistic regression model adjusting for the baseline PHQ-9 score, age, gender, baseline GAD-7 score and treatment. The dichotomous analysis was on a complete case basis (only including those with a 4-month assessment). A sensitivity analysis was performed using simple imputation and a worst case scenario. This assumed that all participants with a missing outcome were still clinically depressed with a PHQ-9 score ⩾10.
The GAD-7 and the PHQ-15 scores were analysed as continuous outcomes using the same repeated measures mixed models described for PHQ-9 above. Resource use data and health state utilities (derived from the EQ5D) formed the basis of a full economic evaluation and are described in the full study report. Reference Brabyn, Araya, Barkham, Bower, Cooper and Duarte22 Adherence by participants to the computer program was measured by requesting information from the website providing MoodGYM (hosted by the developers of MoodGYM at the Australian National University). We obtained computer usage data on the number of times each participant logged on to the MoodGYM program and whether each module was 25, 50, 75 or 100% complete. Adverse events were classified according to their seriousness and relationship to the intervention.
Results
A total of 369 participants were randomised to the two-armed comparison of minimally supported cCBT (n = 182) with telephone-facilitated cCBT (n = 187). The first participant was randomised on the 24 June 2011 and the last on the 25 April 2013. The flow of participants through the trial is shown in the CONSORT diagram (online Fig. DS1). The two groups were well balanced at baseline for gender, age, ethnicity and education. The mean age of participants was 40.6 years (s.d. = 13.8) (online Table DS1). The study population was mostly White British (94%) and 64.5% were women. The minimally supported cCBT and telephone-facilitated cCBT groups were balanced at baseline for employment. The majority (61.5%) of participants were employed and of these 23.6% were absent from work because of depression at the time of their baseline assessment. The severity of depression at baseline (as ascertained by the median PHQ-9 score) was 16 (range 10–25), which corresponds with a moderate to high level of severity.
PHQ-9 as a continuous outcome
At the 4-month primary outcome the between-group difference in PHQ-9 scores was 1.9 points (95% CI 0.5–3.3) in favour of telephone-facilitated cCBT, with a standardised effect size (Cohen's d) of 0.32 (P = 0.009) (online Table DS2). At 12 months, there was no longer evidence of a between-group difference in PHQ-9 scores (0.9, 95% CI −0.5 to 2.3). Using a repeated measures analysis over the whole trial period the between-group difference in PHQ-9 scores was 1.4 (95% CI 0.2–2.6) in favour of telephone-facilitated cCBT with a standardised effect size (Cohen's d) of 0.27 (P = 0.0253) (online Table DS2). Table 1 and Fig. 1 show the PHQ-9 scores at each assessment time point (baseline, 4 and 12 months) for both groups.
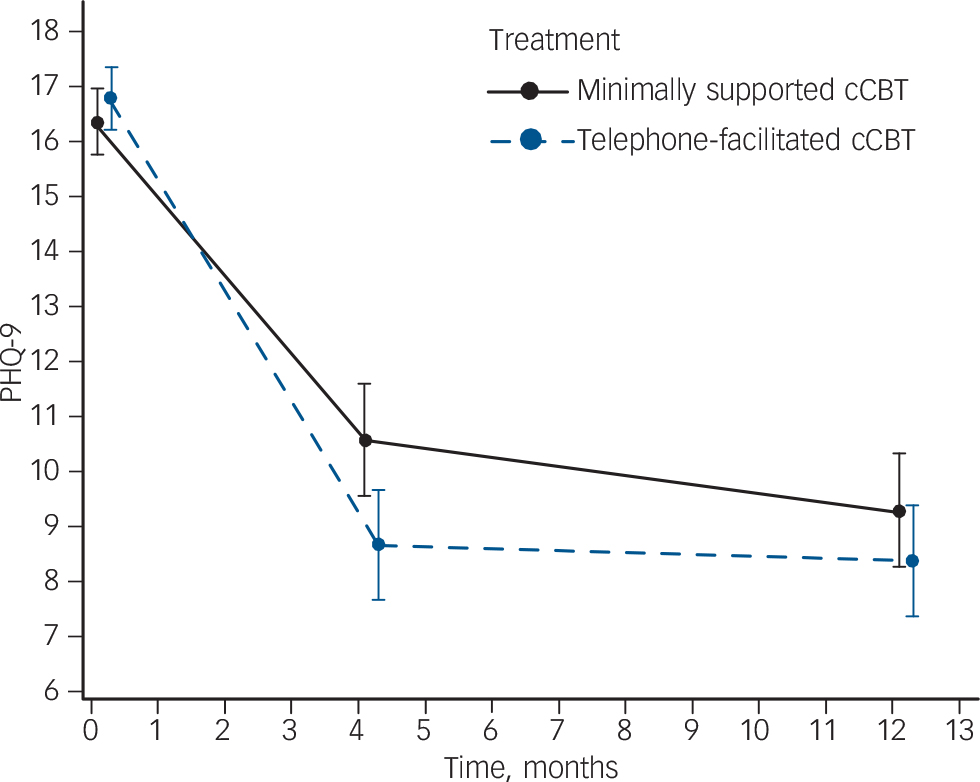
Fig. 1 Depression severity at each assessment.
Severity is mean and 95% confidence interval Patient Health Questionnaire (PHQ)-9 score. Results from a repeated measures, mixed model adjusting for baseline score, age, gender, baseline Generalized Anxiety Disorder Questionnaire (GAD)-7 score and time. cCBT, computerised cognitive–behavioural therapy.
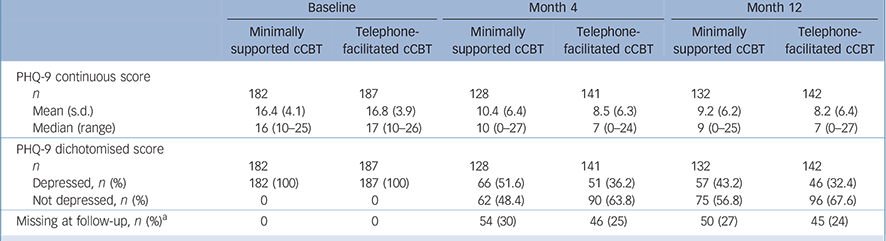
Table 1 Depression severity (Patient Health Questionnaire (PHQ-9) scores) at each time point
| Baseline | Month 4 | Month 12 | ||||
|---|---|---|---|---|---|---|
| Minimally supported cCBT |
Telephone- facilitated cCBT |
Minimally supported cCBT |
Telephone- facilitated cCBT |
Minimally supported cCBT |
Telephone- facilitated cCBT |
|
| PHQ-9 continuous score | ||||||
| n | 182 | 187 | 128 | 141 | 132 | 142 |
| Mean (s.d.) | 16.4 (4.1) | 16.8 (3.9) | 10.4 (6.4) | 8.5 (6.3) | 9.2 (6.2) | 8.2 (6.4) |
| Median (range) | 16 (10-25) | 17 (10-26) | 10 (0-27) | 7 (0-24) | 9 (0-25) | 7 (0-27) |
| PHQ-9 dichotomised score | ||||||
| n | 182 | 187 | 128 | 141 | 132 | 142 |
| Depressed, n (%) | 182 (100) | 187 (100) | 66 (51.6) | 51 (36.2) | 57 (43.2) | 46 (32.4) |
| Not depressed, n (%) | 0 | 0 | 62 (48.4) | 90 (63.8) | 75 (56.8) | 96 (67.6) |
| Missing at follow-up, n (%) a | 0 | 0 | 54 (30) | 46 (25) | 50 (27) | 45 (24) |
cCBT, computerised cognitive-behavioural therapy.
a. Percentage of those originally randomised (minimally supported cCBT n = 182 and telephone-facilitated cCBT n = 187) missing at follow-up.
PHQ-9 as a dichotomous outcome
After 4 months 66 (51.6%) of the 128 participants in the minimally supported cCBT group and 51 (36.2%) of the 141 in the telephone-facilitated cCBT group had a PHQ-9 score ⩾10 (Table 1). The odds of no longer being depressed (defined as PHQ-9 <10) at 4 months were increased twofold in the telephone-facilitated cCBT group compared with minimally supported cCBT (odds ratio (OR) = 2.05, 95% CI 1.23–3.42). The benefit of telephone-facilitated cCBTwas no longer significant at 12 months (OR = 1.63, 95% CI 0.98–2.71 P = 0.06).
Other secondary outcomes
For secondary outcomes there was a significant between-group difference in anxiety scores (GAD-7) in favour of telephone-facilitated cCBTwhen all time points were considered (between-group difference 1.2, 95% CI 0.1–2.3, P = 0.037) (Fig. 2 and online Table DS3). For somatic complaints there was a borderline significant difference in favour of telephone-facilitated cCBT when all time points were considered (between-group difference 1.1, 95% CI 0.0–1.8, P = 0.051) (Fig. 3 and online Table DS4).
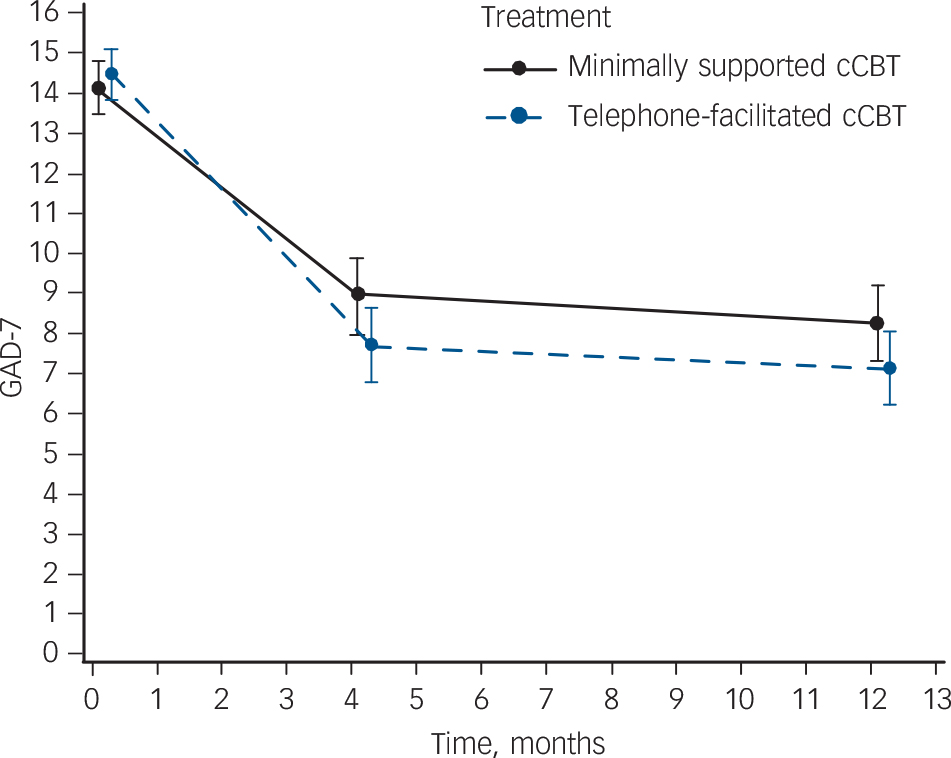
Fig. 2 Anxiety severity at each assessment.
Severity is mean and 95% confidence interval Generalized Anxiety Disorder Questionnaire (GAD)-7 score. Results from a repeated measures, mixed model adjusting for baseline score, age, gender and time. cCBT, computerised cognitive–behavioural therapy.
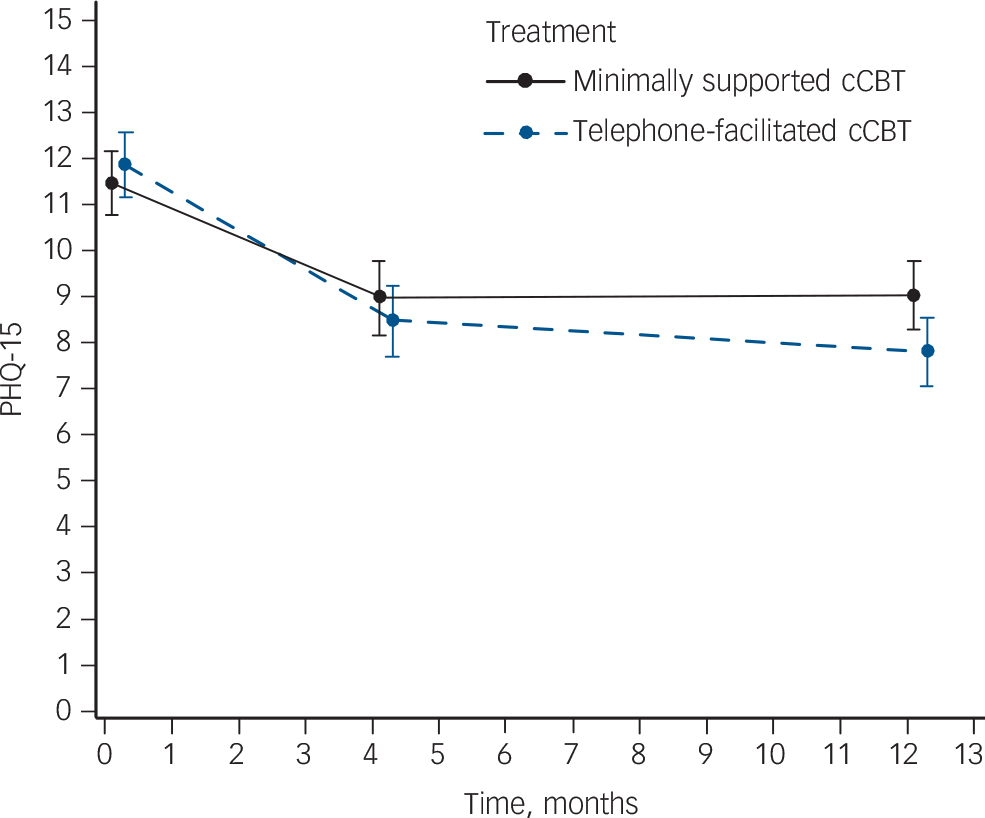
Fig. 3 Severity of somatoform complaints at each assessment.
Severity is mean and 95% confidence interval Patient Health Questionnaire (PHQ)-15 score. Results from a repeated measures, mixed model adjusting for baseline score, age, gender, baseline Generalized Anxiety Disorder Questionnaire (GAD)-7 score and time. cCBT, computerised cognitive–behavioural therapy.
Adherence and adverse events
When computer records were scrutinised there were few participants who completed all five sessions in either minimally supported (10.4%) or telephone-facilitated cCBT (19.4%) (online Table DS5). Usage was generally increased by a factor of between 1.5 and 2 when telephone facilitation was offered, with 46.2% of participants in receipt of telephone facilitation completing two or more sessions v. 29.1% of participants with minimal support (Fig. 4). There was a total of ten serious adverse events, none of which was thought to be related to the trial. All were reviewed by the Trial Steering Committee and the Data Monitoring and Ethics Committee.
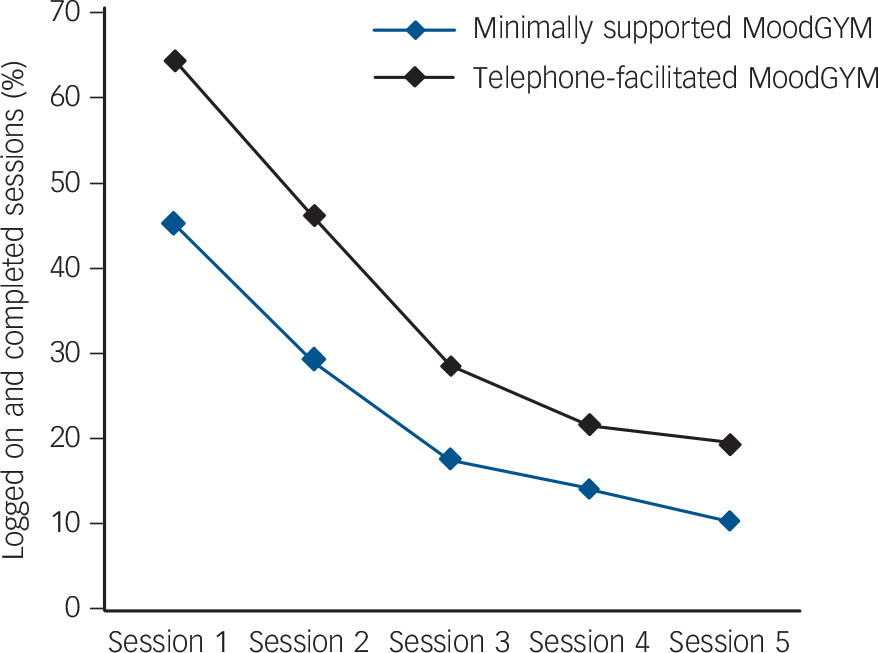
Fig. 4 MoodGYM usage, session by session, as ascertained by computer login records.
Discussion
Main findings
REEACT-2 is one of the largest trials of computer-delivered CBT to date. The trial tested whether the addition of structured telephone facilitation substantially increased engagement with computer-mediated CBT and resulted in improved outcomes. We purposely designed a pragmatic trial to test effectiveness under real-world conditions rather than efficacy under ideal but restrictive conditions in order to maximise the external validity of our results. Reference Thorpe, Zwarenstein, Oxman, Treweek, Furberg and Altman23 The main finding of the REEACT-2 trial is that the addition of structured telephone facilitation resulted in significant reductions in depression severity compared with cCBT with technical support alone. The effect size was moderate and was most evident in the short term (4 months) and had diminished by 12 months. Telephone facilitation of cCBT therefore expedited depression improvement, although the absence of benefit at 12 months is unsurprising given the average duration of an episode of depression is less than 12 months. When depression was considered as a binary outcome, the odds of no longer being depressed were twice as high in the telephone-facilitated group at 4 months. Benefits in terms of psychological outcomes were also observed using a validated anxiety scale and for somatoform complaints when outcomes were averaged over a 12-month period. Engagement with the technology was increased through the addition of telephone facilitation.
Comparison with findings from other trials
The REEACT-2 trial drew upon a manualised form of telephone support, which can readily be delivered after a relatively brief period of training. At present computerised CBT is offered by many healthcare systems as a minimally supported low-intensity psychological intervention and as part of a stepped care framework. The intervention trialled in the REEACT-2 study therefore represents an enhancement of care that can be readily delivered at scale in primary care settings. The results of this trial should be considered alongside other trials and systematic reviews of cCBT and low-intensity interventions for common mental disorders. Our earlier study (the REEACT trial) Reference Gilbody, Littlewood, Hewitt, Brierley, Tharmanathan and Araya8 was similarly a large-scale pragmatic trial of cCBT where one arm included the free-to-use cCBT package (MoodGYM). In that previous trial we offered a low-intensity form of technical telephone support and found that usage was low and there were no additional clinical benefits of cCBT when it was added to usual primary care. This led us to speculate that an enhancement in the level of support and guidance might increase uptake and effectiveness. Evidence that the addition of guidance to cCBT is associated with a greater level of effectiveness comes from systematic reviews, where pooled estimates of the effect size of trials with therapist guidance are larger than the pooled effect size obtained from unsupported cCBT. Reference Andersson and Cuijpers10 Evidence also comes from a systematic review of small-scale head-to-head comparisons of unsupported v. supported cCBT in a range of common mental disorders. Reference Baumeister, Reichler, Munzinger and Lin15 This hypothesis has now been directly tested in the present randomised controlled trial that, to our knowledge, is the first test of this in a large-scale (adequately powered) direct randomised head-to-head comparison. The results of the REEACT 2 trial are also comparable with other primary care based psychological treatments, Reference Cuijpers, van Straten, van Schaik and Andersson24 but the effect size observed in REEACT-2 is smaller compared with other developer-led trials of cCBT. Reference Andersson and Cuijpers10 The additional benefit of guided support is in line with the results of a systematic review of three small-scale studies in depression. Reference Baumeister, Reichler, Munzinger and Lin15
Strengths and limitations
The REEACT-2 trial has several strengths in its design. First, this trial was pragmatic in design and recruited from primary care, whereas most trials to date have recruited from online populations or by participant advertisement. This addresses a major short-coming of the literature identified by Andersson & Cuijpers in their 2009 review. Reference Andersson and Cuijpers10 The results of REEACT-2 are therefore more generalisable to clinical populations encountered in primary care. Second, the trial was significantly larger than other trials to date (see Baumeister et al Reference Baumeister, Reichler, Munzinger and Lin15 ) and had sufficient power to detect more modest effect sizes. Third, we conducted a pragmatic trial of effectiveness rather than efficacy by trialling a low-intensity enhancement to cCBT that could be delivered at distance to a range of people fulfilling very broad depression inclusion criteria (typical of those encountered in primary care). Fourth, the period of follow-up was 1 year and this allows some conclusions to be drawn about the durability of effect. Finally, we were able to study the actual use of computer technology by our trial participants with reference to computer records.
There were also limitations to the REEACT-2 study. First, in view of the pragmatic nature of the design there was loss to follow-up of around a quarter of the participants overall, and we know very little about the outcomes of these participants. Second, we did not measure outcome with a clinical interview to establish the presence of depression according to accepted classification systems. Instead we relied on self-report measures of depression severity; although these are well-validated against diagnostic systems. Reference Manea, Gilbody and McMillan25 Third, even with the provision of telephone facilitation, only a small proportion of participants in either arm completed all sessions of the cCBT program. There is possibly more still that can be offered to enhance the uptake of computer therapy. Finally, the level of depression severity at baseline was moderate to high, and this is at the upper range of severity recommended in some stepped care systems. 4 However, the positive results of the REEACT-2 trial provide supportive evidence that low-intensity interventions can be offered to this group and will result in improved outcomes. This finding is consistent with recent reviews of the effectiveness of low-intensity interventions across the range of severities of depression. Reference Bower, Kontopantelis, Sutton, Kendrick, Richards and Gilbody26
Implications
The implications for practice and policy that emerge from the first REEACT and REEACT-2 trials are twofold. The first is that minimally supported cCBT results in very low levels of uptake and confers little over usual care. We would therefore suggest that healthcare systems do not offer this form of unsupported treatment as part of stepped care. However, unsupported cCBT should still be offered as a form of direct access treatment to non-clinical populations, although the benefits that might be expected are likely to be small. The second implication is that the addition of structured telephone facilitation (such as that designed in REEACT-2 to work alongside MoodGYM) will result in greater levels of engagement with computer technology. In turn this will produce moderate clinical improvements and reductions in the proportion of people who continue to experience depression over a 4- to 12-month period. Telephone support is a low-intensity enhancement of care that can be offered at scale and could be readily implemented in most healthcare settings as part of a stepped care system.
Funding
This project was funded by the UK NIHR Health Technology Assessment programme (project number ). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. The funder played no role in the study design, in the collection, analysis or interpretation of the data, in the writing of the paper or in the decision to submit the article for publication. All authors were independent from the funders. Data sharing: reasonable requests for patient level data should be made to the corresponding author and will be considered by the REEACT publications management group. Consent for data sharing was not obtained but the presented data are anonymised and risk of identification is low.
Acknowledgements
We would like to thank especially the patients from primary care who agreed to be recruited to take part in this trial. Thanks also to members of the Primary Care Research Network (PCRN), GPs, research nurses, administrative and other staff at participating GP practices, the Mental Health Research Network (MHRN) and the site research teams. In addition, we would like to thank the Trial Steering Committee and Data Monitoring and Ethics Committee members for overseeing the study. We thank too Gwen Brierley who was the trial manager at the start of the trial and who co-wrote the trial protocol and REC applications; Anna Thake, D.T., S.K. and D.W. who were the trial coordinators at the sites; the many researchers, clinical studies officers and research nurses who recruited participants to the study and collected data; the York Trials Unit for providing the randomisation service, for managing the data and conducting the analysis of the clinical data; and the team of telephone support workers. We would also like to extend our gratitude to the developers of MoodGYM, in particular to Kylie Bennett and Ada Tam, for providing us with participant logins and usage data.
The REEACT-2 trial is dedicated to the memory of Professor Helen Lester (1961–2013) who contributed time and wisdom at every stage of the REEACT trial programme.








eLetters
No eLetters have been published for this article.