INTRODUCTION
Gelatinous macrozooplankton such as jellyfishes have been reported to bloom in response to anthropogenic eutrophication, climate change and/or human activities such as overfishing (Purcell, Reference Purcell2012). Recent research using submersibles and remotely operated vehicles (ROVs) has revealed that gelatinous macrozooplankton are also ubiquitous and predominant taxa in the deep sea (e.g. Lindsay & Hunt, Reference Lindsay and Hunt2005; Robison et al., Reference Robison, Sherlock and Reisenbichler2010).
Recently in the deep sea, a new anthropogenic risk to the marine environment through seafloor mining has arisen and it has been predicted that impacts will extend not just to the seafloor but to all marine environments including the water column (Boschen et al., Reference Boschen, Rowden, Clark and Gardner2013). Pre-emptively, proposed requirements for Environmental Impact Assessments (EIAs) related to these activities have been investigated (Boschen et al., Reference Boschen, Rowden, Clark and Gardner2013). Because mineral resource-rich areas, such as seafloor massive sulphide (SMS)-rich areas, include rare and unique benthic communities (e.g. chemosynthetic communities supported by hydrothermal vents), the possible impacts on these environments have attracted more study (e.g. Van Dover, Reference Van Dover2014; Boschen et al., Reference Boschen, Rowden, Clark, Barton, Pallentin and Gardner2015; Nakajima et al., Reference Nakajima, Yamamoto, Kawagucci, Takaya, Nozaki, Chen, Fujikura, Miwa and Takai2015). In contrast, the effects on the macrozooplankton fauna through anthropogenic disturbances have barely attracted attention, even though gelatinous macrozooplankton could well be important predators of larvae and other plankton around such communities (Lindsay et al., Reference Lindsay, Umetsu, Grossmann, Miyake, Yamamoto, Ishibashi, Okino and Sunamura2015; Phillips, Reference Phillips2017). Scyphomedusae, hydrozoans (hydromedusae, siphonophores) and ctenophores are all known to exponentially increase their numbers when environmental conditions become favourable (e.g. Purcell, Reference Purcell2005; Uye, Reference Uye2008; Abe et al., Reference Abe, Yamaguchi, Matsuno, Kono and Imai2014). The hydrozoan Hydra has been used for biological toxicity testing in freshwater ecosystems (e.g. Arkhipchuk et al., Reference Arkhipchuk, Blaise and Malinovskaya2006; Quinn et al., Reference Quinn, Gagné and Blaise2012), and hydrozoan medusae might also be useful for bioassays in the ocean. Compared with micro- and meso-zooplankton, gelatinous macrozooplankton are easier to survey visually, in a non-destructive, environmentally friendly way. To investigate the possibility of using non-destructive technology like image-based observations (e.g. ROV surveys) and using gelatinous macrozooplankton as indicator organisms, we investigated their distribution and diversity in an area that may be targeted for deep sea mineral mining.
The Izu-Bonin Arc, within the southern part of Japan's exclusive economic zone (EEZ), is known to contain large SMS deposits. A semi-closed caldera within this arc was selected for the present study. This caldera, the Kurose Hole, is about 5–7 km diameter, with the depth of its rim at around 250 m at its deepest point and 114 m at its shallowest point, and with the maximum depth of the caldera floor being just over 790 m depth (Iwabuchi et al., Reference Iwabuchi, Ashi and Fujioka1989; Yuasa et al., Reference Yuasa, Murakami, Saito and Watanabe1991). Water temperatures inside the caldera are several degrees warmer than those outside (Iwabuchi et al., Reference Iwabuchi, Ashi and Fujioka1989). This makes it an ideal site to study how the vertical distributions of gelatinous macrozooplankton are related to the physico-chemical characteristics of their aqueous environment. Video data from ROV dives inside and outside the Kurose Hole, along with supplementary data from a crewed submersible dive within the caldera, were analysed for the present study.
MATERIALS AND METHODS
Data collection
Data were collected using the ROV ‘Dolphin-3K’ and the human-occupied vehicle (HOV) ‘Shinkai 2000’ in the Kurose Hole (33°24′N 139°41′E, Figure 1), which is located in the Izu-Bonin Arc, due south of Tokyo (Yuasa et al., Reference Yuasa, Murakami, Saito and Watanabe1991). Two ‘Dolphin-3K’ dives (3K488, 3K489) were performed inside and outside the Kurose Hole, respectively, on 24 September 2000 during the RV ‘Natsushima’ cruise NT00-10, as pre-dive safety surveys for subsequent ‘Shinkai 2000’ dives. Although one dive inside and one dive outside the Kurose Hole caldera were planned for cruise NT00-11 of the RV ‘Natsushima’, due to bad weather conditions only one dive was carried out. This was ‘Shinkai 2000’ Dive 1227, inside the Kurose Hole on 17 October 2000. Observations were made and video recorded from the surface to the seafloor in order to analyse the community composition of the midwater gelatinous macrozooplankton.
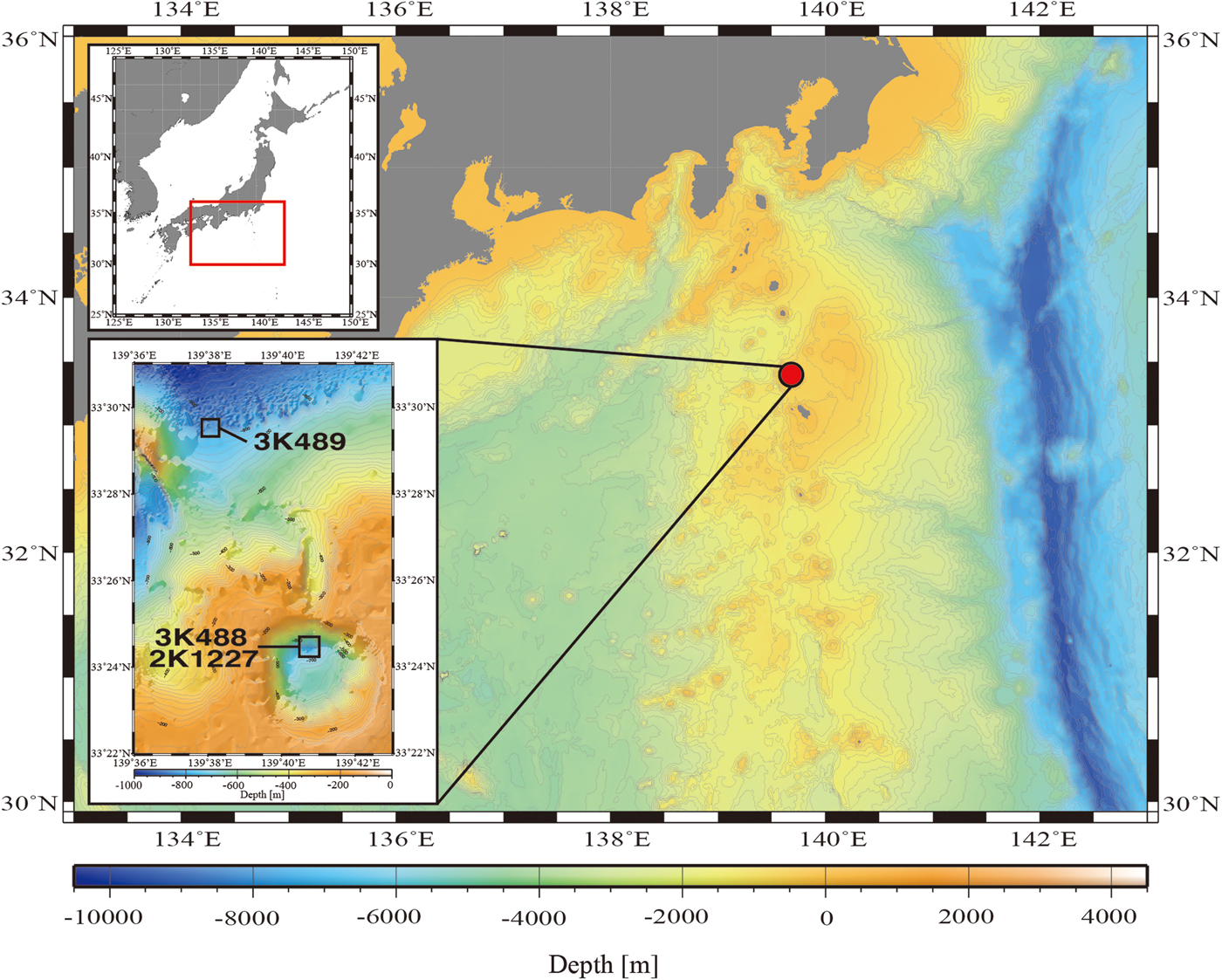
Fig. 1. Map showing the dive locations.
Details of survey platforms
DOLPHIN-3K
The ‘Dolphin-3K’ was equipped with a Victor/JVC KY-F32 three chip CCD camera and six lights: three 400-SeaArc HMI/MSR forward-facing metal halide lamps and three 250 W SeaLine SL-120/250 halogen lamps (one in the rear, two in the front). Video footage was recorded on BCT-D124L Digital Betacam tapes, which were reviewed in their entirety, animals being identified wherever possible. Specimens were collected for positive identification using a single canister suction sampler (3K488) or an 18-canister suction sampler (3K489) and were transferred to shipboard aquaria for positive identification. Environmental parameters (pressure, temperature, conductivity, dissolved oxygen concentration) were measured using a SeaBird SBE19 CTD with an SBE13 oxygen sensor attached to the vehicles, and depth, salinity and water density were calculated from these parameters. Physico-chemical parameters were correlated to the presence of a given animal by matching the time on the CTD dataset to the time on the video.
SHINKAI 2000
The ‘Shinkai 2000’ was equipped with a Victor GF-S1000 HU three chip, CCD camera specially modified for the vehicle. There were eight lights: five 250 W SeaLine SL-120/250 halogen lamps and three 400 W SeaArc HM1/MSR metal halide lamps. Video footage was recorded on BCT-D124L Digital Betacam tapes. Specimens were collected for positive identification using a 6-canister suction sampler and a gate valve sampler (see Hunt et al., Reference Hunt, Hashimoto, Fujiwara, Lindsay, Fujikura, Tsuchida and Yamamoto1997). Physico-chemical data were collected and correlated with observations as above. During the midwater dive, comments by the observer (DJL) were recorded on the audio track of the tapes and the observational database that resulted from this dive therefore consisted of both video-recorded data and live observations made through the observation port of the vehicle.
Observational analysis
DISTRIBUTION AND COMMUNITY COMPOSITION
To compare the distribution and community composition of gelatinous macrozooplankton inside and outside the Kurose Hole, both ‘Dolphin-3K’ dives 488 (3K488: inside, launched at 33°24.500′N 139°40.500′E) and 489 (3K489: outside, launched at 33°29.500′N 139°38.000′E) were analysed in their entirety from the surface to the seafloor. There was too much ambient sunlight to reliably observe translucent animals using the ROV video footage in the shallow strata and the upper 100 m were therefore excluded from the analyses. In addition to the ROV dives, ‘Shinkai 2000’ Dive 1227 (2K1227: inside, launched at 33°24.500′N 139°41.000′E) was reanalysed and species reidentified by the original observer (DJL in October 2016). Animals were identified to the lowest taxonomic level possible using the most recent taxonomic and field guides to each group (Bouillon et al., Reference Bouillon, Gravili, Pagès, Gili and Boero2006; Kitamura, Reference Kitamura, Fujikura, Okutani and Maruyama2008; Kitamura et al., Reference Kitamura, Miyake, Lindsay, Fujikura, Okutani and Maruyama2008a, Reference Kitamura, Miyake, Lindsay, Horita, Fujikura, Okutani and Maruyamab; Widmer et al., Reference Widmer, Cailliet and Geller2010; Lindsay et al., Reference Lindsay, Umetsu, Grossmann, Miyake, Yamamoto, Ishibashi, Okino and Sunamura2015; Minemizu et al., Reference Minemizu, Kubota, Hirano and Lindsay2015). However, mesozooplankton such as copepods and larvaceans were unable to be analysed quantitatively. For the ROV dives, where descent speed was relatively constant, the amount of time spent observing in each 20 m depth strata was calculated and animal abundances normalized (Figure 2). The average observation time in each 20 m depth strata was 1 min 15 s during dive 3K488 (range: 53–118 s, total observing time 42.75 min) and 1 min 13 s during dive 3K489 (range: 51–102 s, total observing time 42.58 min). Data were graphed using the software packages ‘Aabel 3’ (build 3.0.6, Gigawiz Ltd Co.) and ‘ArcGIS’ (Environmental Systems Research Institute, Inc.). Vertical profiles of environmental physicochemical parameters were made using the software package ‘R’ (build 3.3.2, R Development Core Team, 2008) (Figure 3).
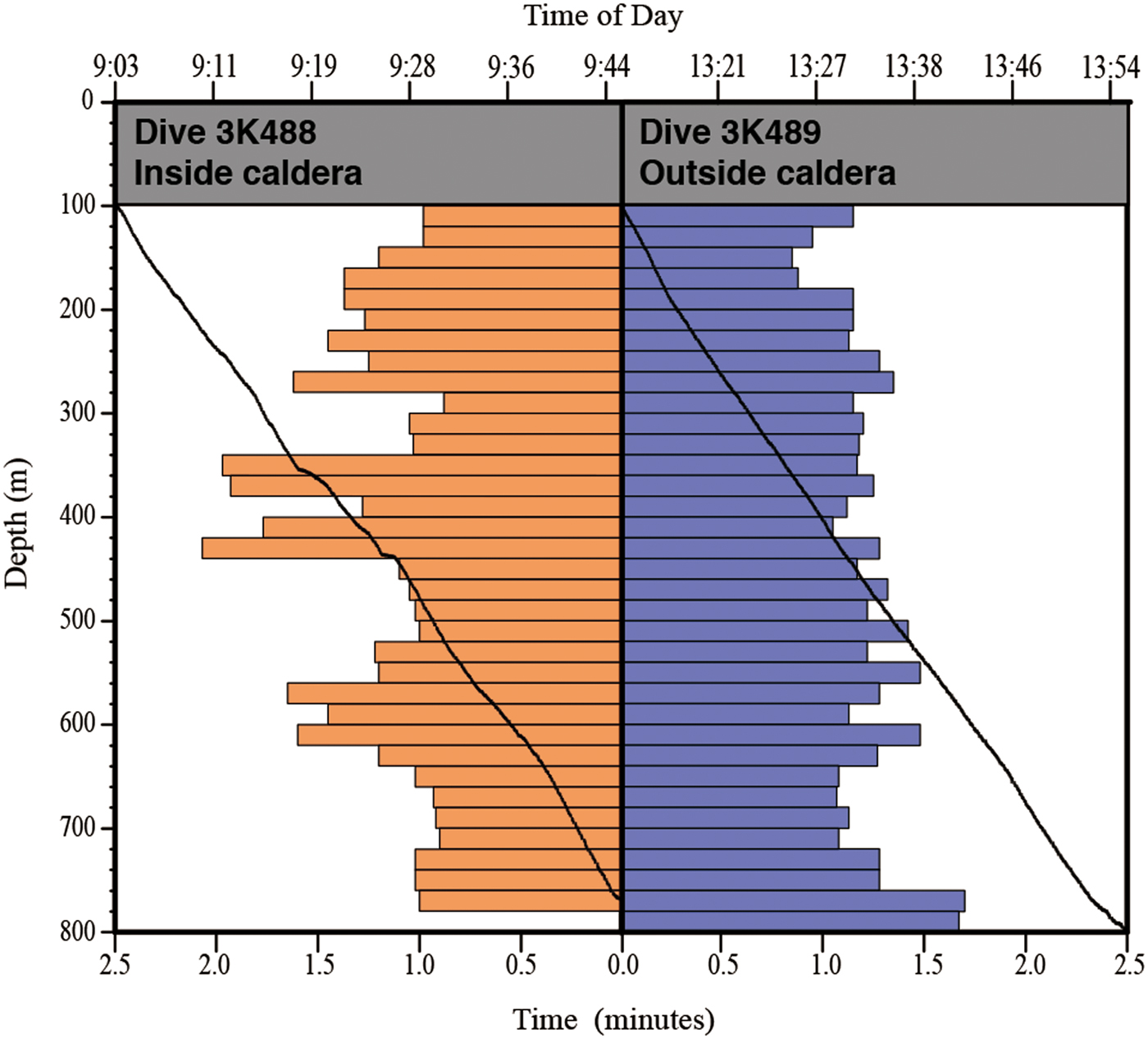
Fig. 2. Time spent observing inside and outside the Kurose Hole. Black lines show Dolphin-3K time vs depth dive profiles. Bar graphs show time spent observing for every 20 m depth stratum.

Fig. 3. Vertical profiles of temperature, salinity, oxygen and density vs depth. (A) 3K488; (B) 2K1227; (C) 3K489.
MORPHOLOGICAL OBSERVATIONS OF EARLERIA BRUUNI (NAVAS, 1969)
Two specimens of E. bruuni were collected during ‘Shinkai 2000’ Dive 1227 using the 6-canister suction sampler (samples 2K1227SS5 and 2K1227SS6).
The specimens were transferred to a Petri dish for taxonomic observations under a shipboard dissecting microscope (Nikon SMZ-U, 0.75–7.5×) outfitted with a video camera mounted on a C-0.45× Nikon TV lens and recorded in HDV format. Whenever magnification was changed, a ruler was placed beneath the Petri dish and recorded for information on scale. Still images of 720 × 480 pixels were captured in TIFF format (.tif). All images were processed in Adobe Photoshop CC (Ver. 2015) using the noise reduction filter, auto tone, auto contrast and auto colour on the default values. Size measurements in this study are based on the footage of living specimens. After observation, one specimen was preserved by freezing (2K1227SS5b) and the other one was preserved in 5% buffered formalin-seawater solution (2K1227SS6a). Specimen 2K1227SS6a was re-observed for this study under two types of microscopes (Leica MZ16F with 2.0× lens, and OLYMPUS IX71 with 40.0× and 100.0× lens). The frozen sample was sequenced according to the method of Collins et al. (Reference Collins, Bentlage, Lindner, Lindsay, Haddock, Jarms, Norenburg, Jankowski and Cartwright2008).
RESULTS
Environmental profiles
The caldera of the Kurose Hole was filled with warm water, with a minimum temperature of around 10°C, during both the ‘Dolphin-3K’ and ‘Shinkai 2000’ dives. Higher water temperatures were observed in the surface layers during the ‘Dolphin-3K’ dives when the main axis of the Kuroshio Current lay above the dive sites (Figure 3). Extremely different vertical profiles of environmental parameters were apparent below 200 m depth between the inside (3K488, 2K1227) and outside (3K489) of the Kurose Hole. Water density Sigma-T values were lower, while temperature, salinity and oxygen concentrations were higher below the edge of the rim inside the caldera. A strong signal (low salinity, high oxygen) indicating the presence of North Pacific Intermediate Water was observed in the 400–500 m depth strata outside the Kurose Hole.
Comparison of macrozooplankton community inside and outside the Kurose Hole
Comparative taxon richness and abundances of gelatinous macrozooplankton are shown in Figure 4, based on observations made during descent for Dolphin-3K dives 3K488 and 3K489. Inside the Kurose Hole, the total number of individuals observed was about two times higher than outside, although taxon richness was much lower and scyphomedusae were not observed. Siphonophores and ctenophores were dominant taxa outside but not inside the Kurose Hole. Even though total observation times were the same during both dives (43 min total each), the number of taxa divided by the number of individuals observed inside and outside the Kurose Hole were 0.14 and 0.60, respectively.

Fig. 4. Pie graphs comparing taxon richness and eveness of macrozooplankton between dives inside (3K488) and outside (3K489) the Kurose Hole.
All gelatinous macrozooplankton occurrences are graphed in Figure 5, along with data from Dive 2K1227 inside the caldera. A total of 38 taxa were identified in the three dives: 10 ctenophores, 12 siphonophores, 10 hydromeduse, two scyphomedusae, four others. Most individuals were distributed below 300 m depth. Some taxa seemed to be associated with warm waters, some with cold waters, and others were indeterminate. Hydromedusae were the most dominant ‘warm-taxon’, with abundances of a leptomedusa with four round gonads and a dark red manubrium being extremely high between 519–777 m depth. Two of the medusae were captured and identified under the microscope as Earleria bruuni (Navas, Reference Navas1969), which has been redescribed below. These medusae were limited to the near-bottom layer, inside the isothermic water mass in the Kurose Hole (Figure 6). Nanomia bijuga (Delle Chiaje, 1844) was only observed inside the caldera (Figure 5). Colobonema sericeum Vanhöffen, 1902 occurred in every dive, always between 483 to 693 m depth. Solmissus incisa sensu lato and scyphomedusae species occurred only outside of the caldera. Bathocyroe spp. were only observed below 650 m depth outside the caldera. Two individuals of an undescribed Lobata taxon that lacks auricles were observed – one inside and the other outside the caldera, in different water masses. Salps were common in the warm water masses.
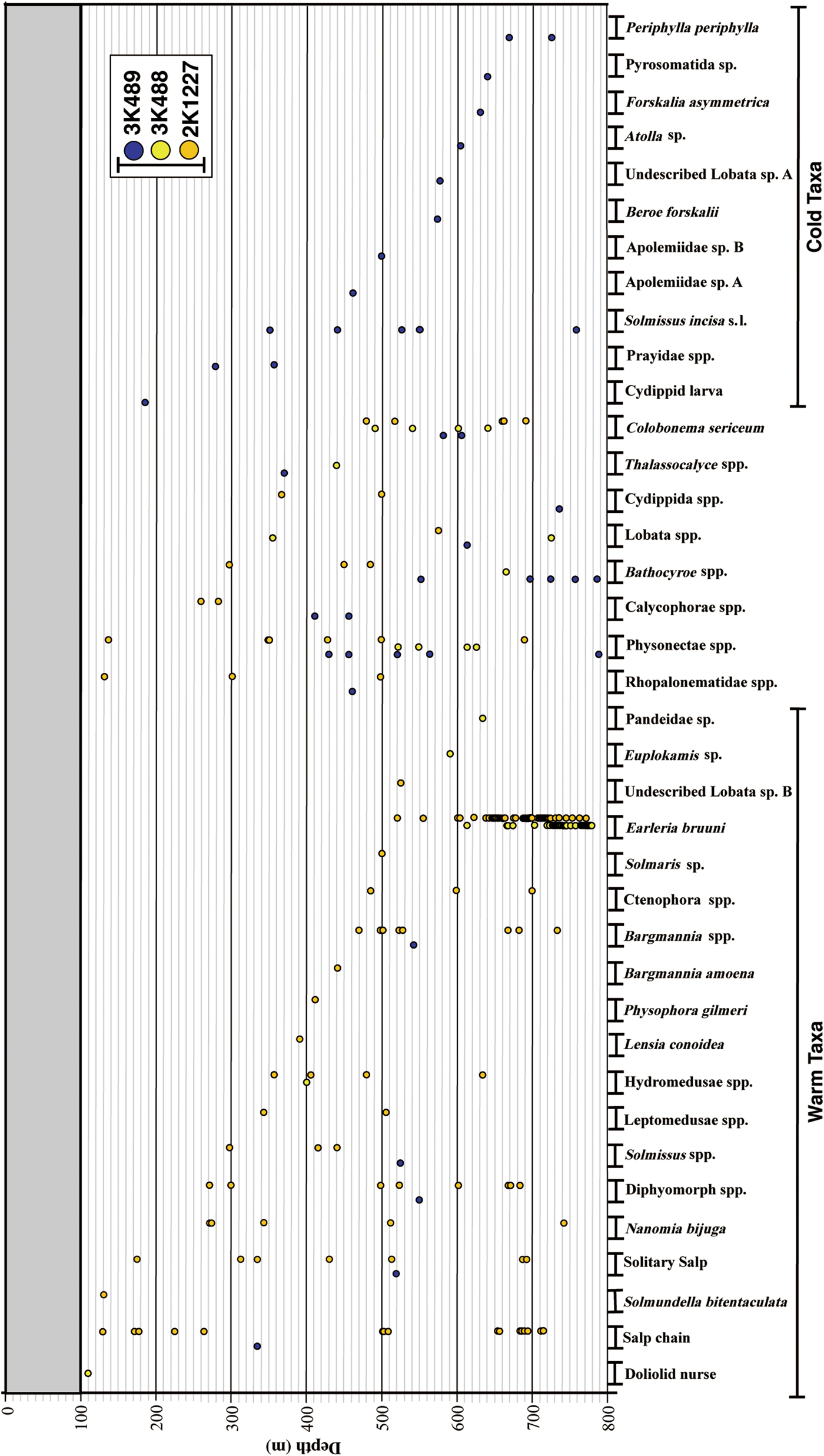
Fig. 5. Taxon occurrence records vs depth for the two ‘Dolphin-3K’ dives (3K488, 3K489) and the ‘Shinkai 2000’ dive (2K1227).
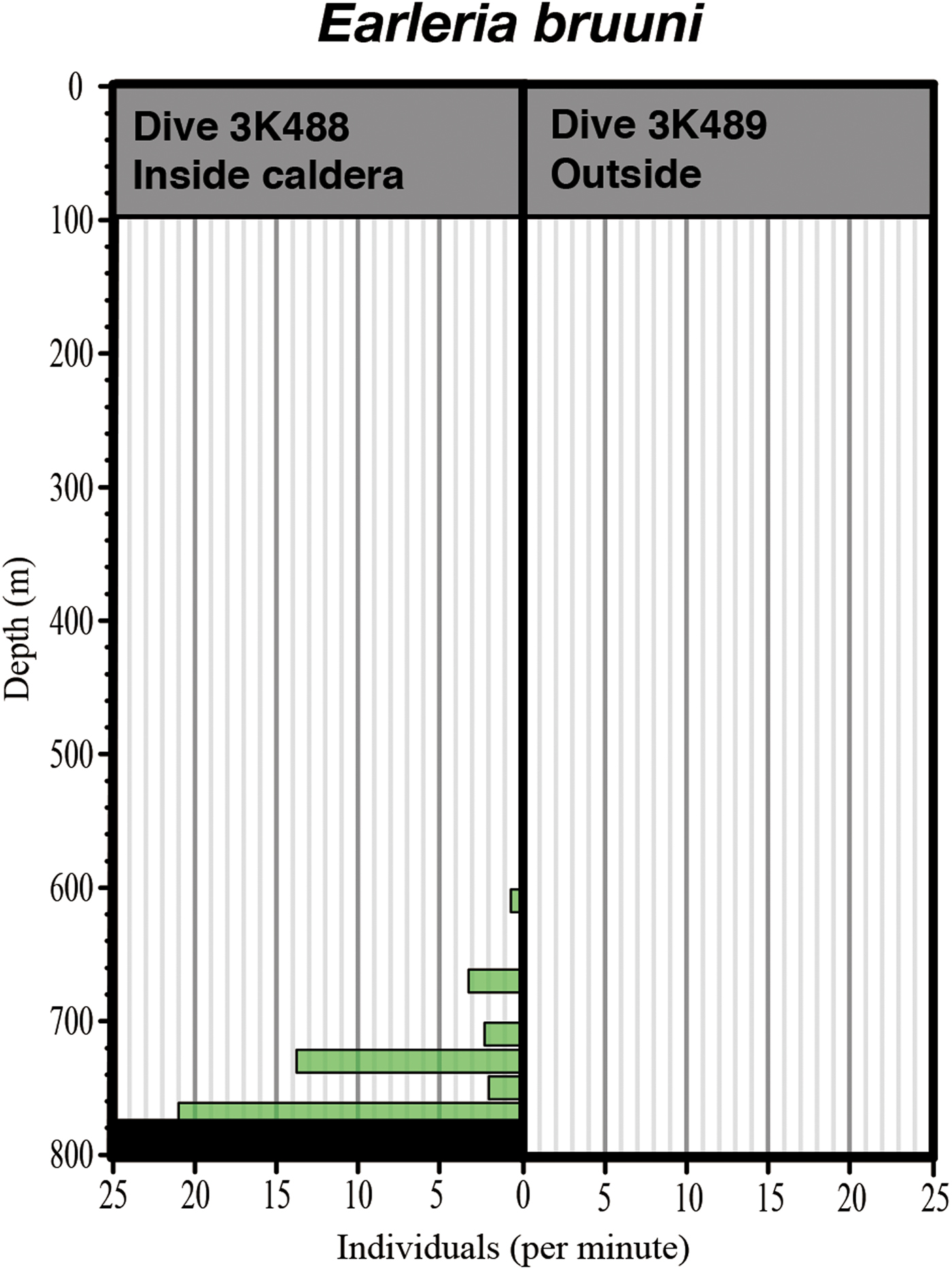
Fig. 6. Comparative vertical distribution of Earleria bruuni inside and outside the Kurose Hole.
SYSTEMATICS
Order LEPTOTHECATA Cornelius, 1992 Family MITROCOMIDAE Haeckel, 1879 (part); Torrey, 1909
Leptomedusae with basis of manubrium attached to subumbrella along continuation of radial canals, four or more simple radial canals, marginal tentacles hollow, marginal cirri present in some genera, gonads oval or linear, only radial canals, open statocysts, no ocelli.
Genus Earleria Collins, Ross, Genzano & Mianzan, Reference Collins, Ross, Genzano and Mianzan2006.
Earleria Collins et al., Reference Collins, Ross, Genzano and Mianzan2006: 125. Type species: Earleria bruuni (Navas, Reference Navas1969).
Mitrocomidae with four radial canals, with numerous open marginal statocysts, without ocelli and without marginal cirri.
Earleria bruuni (Navas, Reference Navas1969) (Figure 7)
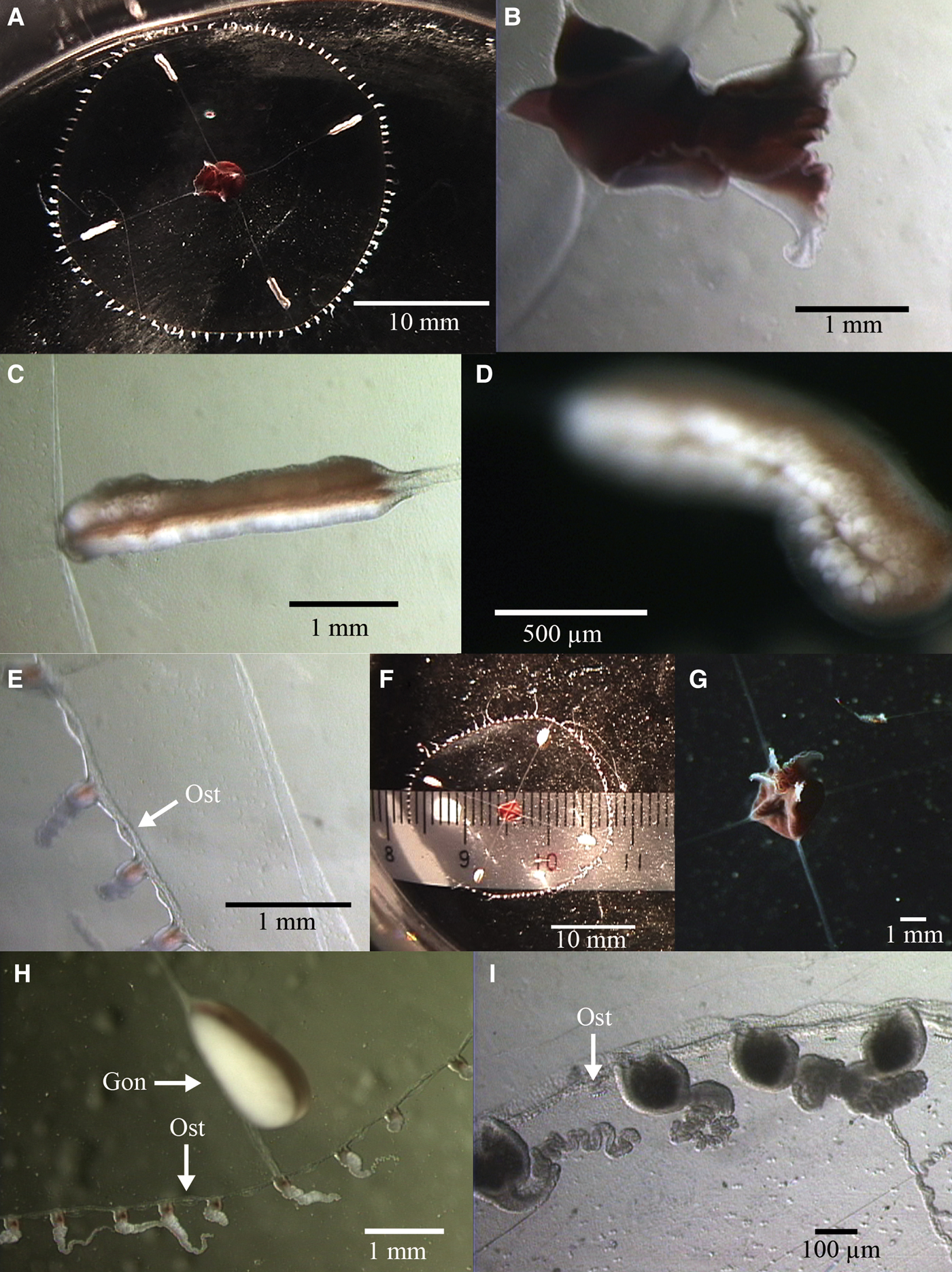
Fig. 7. Earleria bruuni (Navas, Reference Navas1969). A-E: 2K1227SS5b. (A) aboral view of mature female; (B) close-up of manubrium; (C–D) close-up of gonads; (E) close-up of umbrella margin. F–I: 2K1227SS6a; (F) aboral view of mature male; (G) oral view of manubrium; (H) close-up of gonad and umbrella margin; (I) close-up of umbrella margin and tentacles. Ost, Open statocyst. Gon, Gonad.
SYNONYMY
Halistaura bruuni. Navas, Reference Navas1969: 307–310.
Foersteria bruuni. Gili et al., Reference Gili, Bouillon, Pagès, Palanques, Puig and Heussner1998: 113–134.
Foersteria bruuni. Gili et al., Reference Gili, Bouillon, Pagès, Palanques and Puig1999: 313–329.
Earleria bruuni. Collins et al., Reference Collins, Ross, Genzano and Mianzan2006: 125.
Foersteria bruuni. Lindsay & Miyake, Reference Lindsay and Miyake2009: 424 [2K1227SS5b].
Earleria bruuni. Widmer et al., Reference Widmer, Cailliet and Geller2010: 56.
MATERIAL EXAMINED
[2K1227SS5b: 032345] 2.5 cm diameter, mature female, inside Kurose Hole, Izu-Bonin Arc, North-western Pacific, 33°24.708′N 139°40.482′E, 615 m depth, 12:27, 11.11°C, 33.41, 7.67 ml l−1, 5% formalin. [2K1227SS6a: 032359] 2.4 cm diameter, mature male, inside Kurose Hole, Izu-Bonin Arc, North-western Pacific, 33°24.708′N 139°40.482′E, 532 m depth, 15:45, 11.16°C, 33.41, 7.66 ml l−1, −80°C frozen.
DIAGNOSIS (EMENDED)
No peduncle, number of tentacles 40–89, with large cnidocysts in tentacle bulbs, gonads oval, laterally flattened, on distal half of radial canals, marginal statocysts open, one between each 2 successive tentacles.
DESCRIPTION
Umbrella flattened, mesoglea relatively thin, ex-umbrella transparent, sub-umbrella sub-hemispherical and relatively flat, transparent. No gastric peduncle. Base of manubrium quadratic. Short, small, quadratic manubrium dark red or brownish-coloured. Quadrangular mouth with four flared lips. 4 radial canals, whitish, translucent, straight, of uniform width. Four gonads on distal half of each radial canal, not extending onto upper half and cream-coloured, mature male (2K1227SS6a) gonads ovoid and laterally flattened, mature female (2K1227SS5b) gonads sausage-shaped, longer than mature male gonads, split by a median groove. Number of tentacles were 85 and 89, respectively. Nematocysts of two types – spherical nematocysts of mean diameter 2–3 µm (N = 10) and oblong nematocysts of approximate dimensions 2 × 4 µm (N = 10). Without marginal cirri. Numerous open marginal statocysts, 1 between each 2 marginal tentacle bases.
COLOUR
Manubrium dark red, tentacle bulbs red pigmented and tentacles white.
SIZE
Maximum size to at least 2.5 cm diameter.
COMPARISONS
Earleria bruuni can be distinguished from other members of the genus by the combination of the following characters: flat umbrella, no peduncle, tentacle bulbs with cnidocysts and more than 40 tentacles.
DISTRIBUTION
Bay of Bengal (Navas, Reference Navas1969), Kurose Hole (present material).
REMARKS
Earleria bruuni was described from the Bay of Bengal by Navas (Reference Navas1969) as Halistaura bruuni Navas, Reference Navas1969. The only other record in the literature occurred some 50 years later off Japan as Foersteria bruuni (Navas, Reference Navas1969), but was written in Japanese and gave no morphological description (Lindsay & Miyake, Reference Lindsay and Miyake2009). The authors gave the species a Japanese name, ‘Kurose kurage’, meaning ‘jellyfish from Kurose’, based on one of the specimens from Shinkai 2000 dive 1227 in their checklist of mid-water jellyfishes from Japanese waters (Lindsay & Miyake, Reference Lindsay and Miyake2009). Navas (Reference Navas1969) reported a total of 13 individuals (5.5–15.0 mm diameter), which included six mature males and four mature females. Although only two specimens were captured in the present study, one was a mature male (2.4 cm diameter) and the other a mature female (2.5 cm diameter), both much larger than the specimens in the original description, and correspondingly having more tentacles. The number of tentacles in medusae of the genus Earleria are known to increase with growth (Widmer et al., Reference Widmer, Cailliet and Geller2010).
DISCUSSION
Water mass and macro-zooplankton community
Several different distributional patterns were observed – Nanomia bijuga and salps were distributed over a wide depth range inside the caldera, Bargmannia spp. were similar but slightly deeper, while Earleria bruuni were patchy in their occurrence and were restricted to the near-bottom layer. On the other hand, Colobonema sericeum occurred both inside and outside the caldera but in higher numbers and over a wider depth range inside. The diel vertical migrator N. bijuga has been reported from the epipelagic and mesopelagic zones in many places in the Pacific Ocean (Hunt & Lindsay, Reference Hunt and Lindsay1999; Minemizu et al., Reference Minemizu, Kubota, Hirano and Lindsay2015), including down to 300 m (9°C) and 400 m (12°C) in the warm Celebes and Sulu Seas, respectively (Grossmann et al., Reference Grossmann, Nishikawa and Lindsay2015). In the present study, N. bijuga was observed only inside the caldera between 271 m (11.4°C) and 742 m (11.1°C) but it has also been reported from depths down to 800–900 m (4.5°C) in Monterey Bay (Robison et al., Reference Robison, Reisenbichler, Sherlock, Silguero and Chavez1998). The reason it was not observed outside the caldera may just be an artefact due to the high descent speed and low resolution of the ROV's camera compared with that of the human eye, since all occurrences during the present study were during the HOV survey. In contrast, the large, easily recognizable Colobonema sericeum was observed both inside (483–693 m, 11.2–11.1°C) and outside (584–606 m, 5.5–4.9°C) the caldera, with depth seemingly more important than temperature in determining its distribution and reinforcing its status as a cosmopolitan species, except in polar regions, the deep, warm Mediterranean (e.g. Larson et al., Reference Larson, Mills and Harbison1991; Minemizu et al., Reference Minemizu, Kubota, Hirano and Lindsay2015) and possibly the Red Sea. It has been reported to occur between 500–600 m in the Celebes Sea at temperatures of 5.0–5.5°C and in the deep, warm Sulu Sea between 300–400 m at temperatures of 12–13°C (Grossmann et al., Reference Grossmann, Nishikawa and Lindsay2015). Small rhopalonematid medusae that resembled C. sericeum were observed inside the Kurose Hole caldera at 133 and 302 m depth and although specimens were not captured to allow positive identification it may be that this species exhibits an ontogenetic migration to depth as it matures. Bathocyroe spp. were only observed below 550 m depth outside the caldera, even though the volume of water observed inside the caldera by the ‘Shinkai 2000’ was comparatively much greater than that observed by the ROV. Solmissus incisa sensu lato (including several different morphotypes of this species, see Lindsay et al., Reference Lindsay, Umetsu, Grossmann, Miyake, Yamamoto, Ishibashi, Okino and Sunamura2015) was similarly also only observed outside the caldera, even though it is easily recognizable to the naked eye even from a distance. The cold water-adapted deep-sea scyphomedusae Atolla spp. and Periphylla periphylla (Péron & Lesueur, 1810) (Lindsay et al., Reference Lindsay, Furushima, Miyake, Kitamura and Hunt2004) were only observed in the deeper layers outside the caldera. Consequently, the present study revealed that the gelatinous macrozooplankton community inside the closed, warmer environment of the caldera was considerably different to that outside with regards to both the community composition and the vertical distributions of the fauna.
Iwabuchi et al. (Reference Iwabuchi, Ashi and Fujioka1989) suggested that rather than the Kurose Hole being filled by a warm water mass derived from influx and vertical mixing of Kuroshio current waters into the caldera, it was more likely, based on the salinity values inside the caldera being lower than the values of Kuroshio Water, that the internal water mass was formed through geothermal heating and subsequent vertical mixing. The low salinity values presumably derive from the pore waters of the subducted sediments from which the geothermally heated waters originate. The occurrence of macrozooplanktonic taxa in the deeper waters of the caldera is therefore considered not to be due to active transport to deeper depths within a surface- or subsurface-derived current of Kuroshio origin but rather due to movements by the animals themselves. This warm, lower salinity water mass seems to provide an ‘oasis’ for the gelatinous macrozooplankton living inside.
Earleria bruuni
Earleria bruuni occurred at a high population density, being observed between 519 and 777 m depth, where salinity ranged from 34.38 to 34.40 and temperature was stable at 11.1°C. They were limited to the near bottom layer, where isothermic water occurred inside the Kurose Hole caldera. Minimum depth distribution revealed by the ‘Shinkai 2000’ dive was slightly shallower (519 m) than by the ROV ‘Dolphin-3K’ (612 m) but as only one dive was carried out by each platform the difference in observed distributions could just as easily have been due to inherent patchiness as to the greater ease of detecting rare (low density) individuals through the greater volume scanned by the human eye. According to Navas (Reference Navas1969) and Navas & Vannucci (Reference Navas and Vannucci1991), E. bruuni were collected in the 125–1000 m depth strata with the most accurate depth record being for 125–250 m depth. In that strata salinity ranged from 34.7 to 35.0 and temperature from 12.2 to 17.35°C (Navas, Reference Navas1969), being sandwiched between Bay of Bengal Surface Water (BBSW) and Bay of Bengal Sub Surface Water (BBSSW) in the maximum salinity layer and E. bruuni were never caught in the surface layer (Navas & Vannucci, Reference Navas and Vannucci1991). Earleria bruuni from the Kurose Hole and the Bay of Bengal were observed under similar water temperatures but different salinities and it can therefore be characterized as a warm water mass species. Although E. bruuni was not observed outside the caldera, based on the similar environmental factors to those associated with its occurrence in the Bay of Bengal, it should be able to occur. Their dark pigmented gut suggests that they are adapted to catch deep-living bioluminescent prey and we speculate that the E. bruuni report from the Bay of Bengal was due to them being upwelled from the near-bottom layer to near the surface.
Most of the reports on the occurrence of congeners are from semi-closed environments such as canyons or fjords. Earleria antoniae (Foersteria antoniae: Gili et al., Reference Gili, Bouillon, Pagès, Palanques, Puig and Heussner1998; Bouillon et al., Reference Bouillon, Pagès, Gili, Palanques, Puig and Heussner2000) and Earleria araiae (Foersteria araiae: Gili et al., Reference Gili, Bouillon, Pagès, Palanques and Puig1999; Bouillon et al., Reference Bouillon, Pagès, Gili, Palanques, Puig and Heussner2000) were reported from canyons in the Mediterranean sea. Earleria purpurea (Foersteria purpurea: Mackie, Reference Mackie1985; Larson et al., Reference Larson, Matsumoto, Madin and Lewis1992) were reported as one of the benthopelagic species present in fjords of British Columbia in the 50–450 m depth strata, and deeper than 200 m depth in Monterey Bay. Earleria quadrata (Foersteria quadrata, Hosia & Pagès, Reference Hosia and Pagès2007) were reported from a fjord in Norway deeper than 500 m. In this respect, it can be hypothesized that the genus Earleria favours deep, isolated water masses. An advantage of the steep-walled concave geography of the fjords and canyons they inhabit is that the particle concentration should increase with depth. Indeed, the cross-sectional area at the 700 m isobath in the Kurose Hole is 30 times smaller than at the caldera rim, and this would act to concentrate sinking particles and vertically migrating plankton, increasing ambient food concentrations.
In addition, the detailed life cycle of the congeneric species Earleria corachloeae has been revealed and their polyps were found on the head of the midwater shrimp Pasiphaea pacifica Rathbun, 1902 (Widmer et al., Reference Widmer, Cailliet and Geller2010). Correspondingly, Pasiphaea spp. were observed during all dives in the present study, some individuals were passing through the patch of E. bruuni inside the Kurose Hole between 730 and 777 m depth. In terms of reproduction, the topography of the Kurose Hole retains the water it contains and the organisms within it, presumably enhancing blooming of E. bruuni and providing favourable substrates for the polyps.
Differences between ROV vs HOV observations
In order to clarify the distribution of gelatinous macrozooplankton, which are transparent and fragile animals, the advantages and disadvantages of various survey methods must be assessed. Direct observations, such as are possible with ROV or HOV dives, can provide accurate information on the distribution of gelatinous macrozooplankton and associations with other organisms, but the amount of data/volume of water sampled is not as high as with net sampling and not all specimens can be identified to species level. Furthermore, when community structure is surveyed through net sampling vs ROV observation, the sampled or observed species composition is different (Raskoff et al., Reference Raskoff, Hopcroft, Kosobokova, Purcell and Youngbluth2010), because of individual sizes, fragility and/or transparency. Likewise, differences between ROV observations and HOV observations were found in the present study because a human's eyes have better resolution than that of a video camera. During ‘Shinkai 2000’ dive 1227, more individuals were observed and more species-level identifications were possible than with the ‘Dolphin-3K’ dives. However, the technical restrictions imposed by the ROV actually lead to a more quantitative evaluation, since camera angle, imaged volume, etc. are restrained and recorded, unlike with the human eye. Consequently, it seems that HOV investigations by an experienced researcher are effective for biodiversity surveys, while ROV investigations may be more efficient for an environmental impact assessment (EIA), which requires a repeatable, uniform method. With the development of high resolution imaging technology such as 4 and 8 K video camera systems, we can expect more detailed and quantitative investigation methods to be developed for gelatinous macrozooplankton surveys, finally bridging the gap between naked-eye and camera-based investigations.
CONCLUSIONS
Comparative ROV dives revealed that semi-closed systems such as deep-sea calderas can have unique environmental factors and gelatinous macrozooplankton communities. In the Kurose Hole, Izu-Ogasawara Islands, extremely low taxon diversity was found inside the caldera, and large numbers of the leptomedusa Earleria bruuni were observed inside the caldera, being the predominant species. Blooming of Earleria bruuni was also observed during the additional HOV dive, which was performed 3 weeks later. Surprisingly, the current report of E. bruuni is the first in 50 years, even though the medusa was extremely abundant in the Kurose Hole. Two major causes for this phenomenon may be, firstly, the isolated habitat formed inside the caldera with an isothermic warm water mass when compared with outside the caldera may be a highly suitable habitat for the medusa, and, secondly, the steep, concave topography could concentrate sinking particles and vertically migrating plankton, thereby increasing ambient food concentrations and leading to the observed blooming of E. bruuni.
ACKNOWLEDGEMENTS
We are grateful to the reviewers for critical and constructive comments on the manuscript. Thanks are due to the captain, crew and scientific parties of RV ‘Natsushima’ cruises NT00-10 and NT00-11. We also thank Dr Hiroyuki Yamamoto of the Environmental Impact Assessment Research Group, within the Research and Development Center for Submarine Resources, JAMSTEC, for his support. This study is a contribution to the International Network for Scientific Investigations of Deep-Sea Ecosystems (INDEEP), and the Deep Ocean Stewardship Initiative (DOSI). Contributors: Maps of the research areas used to assemble Figure 1 were plotted by Kazuya Kitada using the software package GMT: The Generic Mapping Tools (Wessel & Smith, Reference Wessel, Smith, Scharroo, Luis and Wobbe2013). Vertical profiles of environmental physicochemical parameters (Figure 3) were plotted by Kei Sunahara using the software package R. A draft of Figure 5 was prepared by Ryota Nakajima using the software package ArcGIS.
FINANCIAL SUPPORT
This work was partially funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI: (grant numbers 24248032, 26304030 and 23405031) and JST grant CREST, the fund for Interdisciplinary Collaborative Research by the Atmosphere and Ocean Research Institute, University of Tokyo, and the Cross-ministerial Strategic Innovation Promotion Program (SIP) for the Development of New-generation Research Protocols for Submarine Resources; Core Research for Evolutionary Science and Technology.









