Flavonoids are a broad group of polyphenolic compounds with anticarcinogenic(Reference Niedzwiecki, Roomi and Kalinovsky1,Reference Vue, Zhang and Chen2) and antioxidant properties(Reference Costea, Nagy and Ganea3) and are widely distributed in edible plants(Reference López and Ma4,Reference Vauzour, Vafeiadou and Spencer5) . They are classified into six subclasses: flavonols, flavones, flavanones, flavanols, anthocyanidins and isoflavones(Reference Guo, Liang and Liu6,Reference Ross and Kasum7) . Major dietary sources of flavonoids include fruits, vegetables, cereals, tea, wine, fruit juices and some aromatic herbs, such as parsley and celery(Reference Manach, Scalbert and Morand8). Nevertheless, the intake of flavonoids and their subclasses shows marked differences around the world(Reference Rodríguez-García, Sánchez-Quesada and Gaforio9), and their beneficial properties vary according to their chemical structure(Reference Vue, Zhang and Chen2).
The role of flavonoids in several types of cancer, for example, breast(Reference Niedzwiecki, Roomi and Kalinovsky1,Reference Ross and Kasum7) , colon(Reference Niedzwiecki, Roomi and Kalinovsky1,Reference Ross and Kasum7) and prostate cancer (PC)(Reference Niedzwiecki, Roomi and Kalinovsky1,Reference Castro and Cambeiro10) , has been evaluated in experimental studies. Regarding PC, there is experimental evidence in relation to the role of flavonoids as androgen receptor inhibitors, as well as their capacity to suppress cell cycle progression, induce apoptosis and inhibit metastasis, invasion and angiogenesis(Reference Costea, Nagy and Ganea3,Reference Kallifatidis, Hoy and Lokeshwar11–Reference Connors, Chornokur and Kumar14) . Additionally, recent studies have suggested a potential synergistic interaction between some flavonoids(Reference Gray, Stephens and Bigelow15).
Epidemiological evidence is limited and suggestive of differences depending on the type of evaluated flavonoids. Prospective cohort studies in the Netherlands(Reference Geybels, Verhage and Arts16) and in the USA(Reference Wang, Stevens and Shah17) suggest that individual compounds (catechin, epicatechin, kaempferol and myricetin), subclass flavonoids (flavan-3-ols and proanthocyanidins) and consumption of specific foods (black tea) could be associated with a decreased risk of advanced(Reference Geybels, Verhage and Arts16) or high-grade PC(Reference Wang, Stevens and Shah17). Meanwhile, retrospective studies, mainly conducted in Italy, show contradictory results. Bossetti et al.(Reference Bosetti, Bravi and Talamini18) analysed a larger sample size and did not observe any association between total intake or any intake of specific flavonoid components and PC risk; in contrast, in a recent study from Sicily(Reference Reale, Russo and Di Mauro19), using an updated flavonoid database, a reduced risk of PC was found to be associated with a higher intake of flavonols and catechin, and a probably PC risk reduction was associated with flavanol and flavone consumption.
In Mexico, PC is the leading cause of cancer morbidity and mortality in males(20), with a high proportion classified as high-grade PC at the time of diagnosis(Reference Gomez-Guerra, Martinez-Fierro and Alcantara-Aragon21). The Mexican diet is characterised by a high intake of fruits and vegetables, whole grains, legumes and aromatic herbs(Reference Santiago-Torres, Tinker and Allison22); most of which are important dietary sources of flavonoids. According to a study of women(Reference Zamora-Ros, Biessy and Rothwell23), flavonoids (28·8–40·9 %) are the second most common type of polyphenols consumed in the country, mainly at the expense of flavones and flavonols. To the best of our knowledge, in Latin America, only the association between individual dietary sources of flavonoids(Reference Torres-Sánchez, López-Carrillo and López-Cervantes24) and isolated flavonoid intake(Reference Torres-Sanchez, Galvan-Portillo and Wolff25) with breast cancer has been studied. The potential anticarcinogenic role of mixtures of flavonoids in the context of PC has not been evaluated. Our objective was to evaluate the association between dietary patterns of flavonoid intake and PC, as well as histological PC differentiation, in a population-based case–control study carried out in Mexico City.
Materials and methods
Study population
Detailed information about the study methodology has been previously published(Reference Vázquez-Salas, Torres-Sánchez and López-Carrillo26). Briefly, from November 2011 to August 2014, we carried out a case–control study in Mexico City. Cases and controls were residents of Mexico City for at least 1 year and had no previous history of cancer. Of 468 incident and histologically confirmed PC cases at any clinical stage identified in four tertiary-level and two second-level public hospitals, 402 (85·90 %) agreed to participate. Based on the Gleason score at the time of diagnosis, PC cases were classified as well differentiated (Gleason ≤ 6), moderately differentiated (Gleason = 7) and poorly differentiated (Gleason ≥ 8).
After a case was identified, two population controls matched by age ((sd 5) years) were selected. Males with symptoms possibly related to PC (e.g. dysuria and haematuria) or those with a previous history of prostate-specific antigen ≥ 4 ng/ml were not considered appropriate controls. Using the master sample framework of the National Health Surveys, we identified thirty-three basic geostatistical areas, and ten blocks were selected in each area. All households were visited to verify how many males met the inclusion criteria. If more than one male was eligible, we randomly selected one of them to participate in the study. If a potential control was not present at the home, we made up to three attempts to locate him before selecting another control. Of 920 eligible controls, 805 agreed to participate in the study (87·50 %).
The final sample included 395 cases and 797 controls (Fig. 1); six cases and eight controls were excluded from the analysis because they reported extremes in energetic intake (<3347·2 or >18828 kJ/d). The 3347·2–18828 kJ/d (800–4500 kcal/d) range is considered plausible for life and disqualifying individuals outside of it prevented a possible measurement error(Reference Willet, Stampfer and Willet27). Moreover, one case declined to provide dietary information.
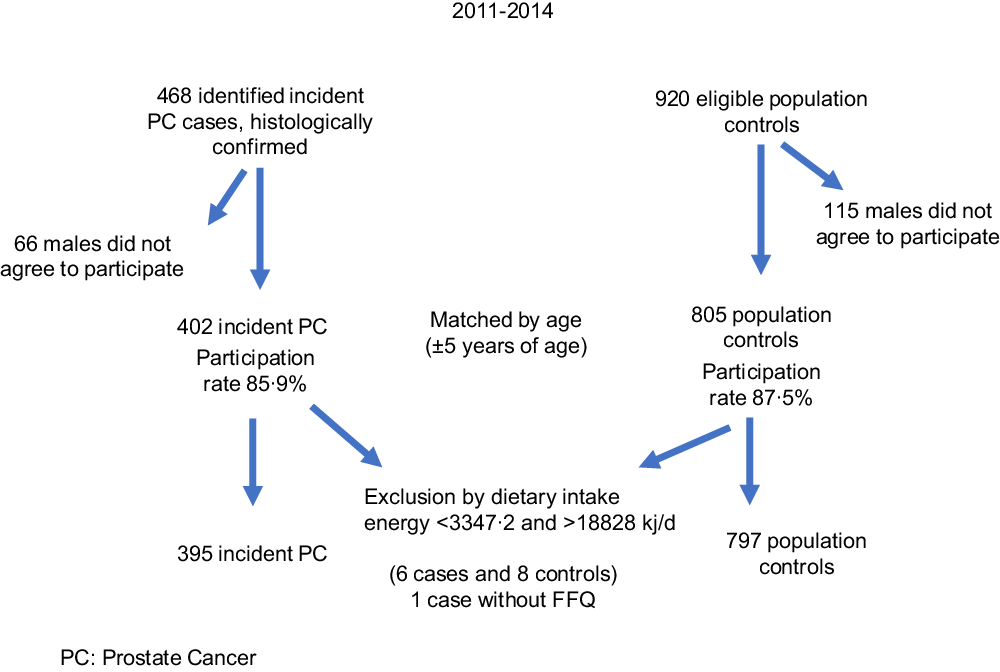
Fig 1. Recruitment of cases and controls in Mexico City.
The study was conducted in accordance with the principles established by the Declaration of Helsinki and was approved by the Research Ethics Committee of the National Institute of Public Health of Mexico (CI: 980), as well as by each of the participating hospitals. All participants received information about their participation in the study, and after receiving answers to their questions, they signed an informed consent form.
Interview
Through a direct interview using a structured questionnaire, information was obtained on the participants’ socio-demographic characteristics as follows: age, maximum level of education attained, usual occupation, marital status and place of birth. Each participant was also asked about a history of PC in first-degree relatives and their personal history of pathologies, focusing on chronic diseases (hypertension, diabetes and dyslipidaemia) and sexually transmitted diseases (gonorrhoea, syphilis, genital warts, herpes or chancre). With respect to lifestyle characteristics, the participants were asked about history of smoking and the practice of leisure-time physical activity during three life stages, as well as dietary habits. Anthropometric measurements of weight, height, and waist and hip circumferences were taken at the time of the interview. Through the questionnaire, information on weight 2 years prior to diagnosis or interview was also obtained. With this information, the BMI was estimated.
Cases were interviewed in the hospital, while the controls were interviewed at their home.
Dietary information
We used the semi-quantitative FFQ as previously described(Reference Hernández-Ramírez, Galván-Portillo and Ward28). The reference frame was 3 years prior to the diagnosis or interview for cases and controls, respectively. Briefly, this questionnaire contains information on the consumption frequency of 127 food items distributed in ten general groups (dairy products and derivatives; legumes and seeds; fruits; chilies and stews with chili; eggs, meats and cold cuts; vegetables; sweets, cereals and snacks; beverages; fats and oils, and traditional Mexican dishes).
Each food item has a predetermined portion and ten consumption frequency options, ranging from ‘never’ to ‘six times a day’. Consumption of fruits and vegetables was adjusted according to their availability in the market throughout the year; for example, plums are available 6 months a year; therefore, the frequency of consumption was divided by two.
The frequency of consumption of each of the food items reported by the participants was converted to g or ml of daily consumption using the standardised measures of the 1999 National Nutrition Survey(Reference Ramírez-Silva, Jiménez-Aguilar and Valenzuela-Bravo29).
Flavonoid content in selected Mexican foods
We updated the number of food sources and the content of three major flavonoid subgroups and their main components using a previously reported database(Reference Galvan-Portillo, Wolff and Torres-Sánchez30). The subgroups and main components were flavones (apigenin, luteolin); flavonols (quercetin, myricetin, kaempferol) and flavanols ((+)-catechin, (+)-gallocatechin, (–)-epicatechin, (–)-epigallocatechin, (–)-epicatechin-3-O-gallate, (–)-epigallocatechin-3-O-gallate). This updated database has information on the flavonoid contents present in fifty-seven food items identified taxonomically(31).
For each food item, we assigned the average value of the selected flavonoids reported in the following databases: (1) database of flavonoid content in selected USDA foods, Release 3.1(Reference Bhagwat, Haytowitz and Holden32), and (2) database of polyphenol content: Phenol-explorer 3.6(33). The average values of each selected flavonoid were reported in mg/100 g of the fresh weight of the edible portion of each food.
Daily consumption of dietary flavonoids
Daily consumption of each selected flavonoid was estimated by matching daily consumption of food flavonoid sources (g/d) with their corresponding flavonoid content.
Total daily consumption of each major flavonoid subgroup was calculated as the sum of each component and expressed in mg/d and adjusted by the total energy intake using the residual method proposed by Willet(Reference Willet, Stampfer and Willet27). Intake of each flavonoid was regressed based on total energy intake, and residuals were estimated. The residual from the regression is uncorrelated with total energy intake and allows for a direct evaluation of variation due to flavonoid composition(Reference Willet, Stampfer and Willet27).
Dietary flavonoid patterns
Energy-adjusted intake residuals of each flavonoid component were used to determine dietary patterns. To identify dietary patterns, a principal component analysis was performed using orthogonal transformation (varimax rotation) to reduce the variance. Scree plot tests, eigenvalues ≥ 1·5 and component interpretability were used to determine the factors to be retained. The factor scores for each dietary pattern were estimated by adding the standardised consumption of a component within each group, weighted by their load factor. Each participant received a factorial score for each of the three identified patterns, which were labelled according to the component with the highest value in the pattern.
Some vegetables are sources of other nutrients, such as lycopene, fibre and β-carotene, which have been associated with PC. For that reason, we also considered the intake of raw tomato, green-yellow leafy vegetables (lettuce, purslane, spinach, pumpkin flowers) and green-yellow nonleafy vegetables (summer squash, smooth-skinned chayote, carrot, maize on the cob).
Physical activity and smoking history
Regarding physical activity, we obtained information on moderate (≥ 3 metabolic equivalents) and vigorous intensity (≥ 6 metabolic equivalents) leisure-time activities during three different life stages, 15–18, 19–29 and > 30 years old. Using KmL packages (k-means + method) for longitudinal data in R software, we identified three individual leisure-time life course physical activity patterns: pattern A was characterised by men who reported consistently low physical activity; pattern B referred to moderate physical activity throughout life and pattern C referred to consistently high physical activity levels. Similarly, the smoking history was determined using the estimated smoking index (packs/year) at three different life stages, and two life course smoking patterns were identified: pattern A, including males who reported low and constant smoking intensity (87·80 %), and pattern B (12·20 %) for males with an initial period of low intensity, followed by an increase during the second period(Reference Jiménez-Mendoza, Vázquez-Salas and Barrientos-Gutierrez34). Participants who reported no leisure-time physical activity practice or smoking throughout life were considered reference categories.
Statistical analysis
The socio-demographic, lifestyle and pathological background characteristics of cases and controls were compared using Student’s t test for continuous variables or the χ 2 test for categorical variables. Based on the observed distribution among the controls, each dietary pattern of flavonoid daily intake was categorised into tertiles, and the lowest tertile represented the reference category.
The association between the major flavonoid subgroups and their components, as well as each dietary flavonoid pattern, with total PC and Gleason scores was estimated using independent unconditional logistic regression models. All models were adjusted by age at interview.
As potential confounders, variables that were known a priori to be risk factors for PC were evaluated. The final model included only variables changing the crude estimator by > 10 %, which were educational level, history of chronic disease, history of sexually transmitted disease, history of PC in first-degree relatives, leisure-time physical activity and smoking patterns, and consumption of raw tomato, green-yellow leafy vegetables and green-yellow nonleafy vegetables. In addition, a sensitivity analysis was performed, adjusting each flavonoid intake pattern model with each of the other patterns. To estimate a trend, in each model, the categorical pattern of intake was included as a continuous variable, where the lowest tertile had a value of 1 and the highest tertile had a value of 3. A significant trend was considered when the P value corresponding to the estimator was < 0·05.
Because of the available sample size, the power calculation (power = 0·90) for this analysis was based on a two-sided α-level test: 0·05 and an estimated OR = 0·4 between the highest v. lowest tertile of flavonoid pattern intake. All statistical analyses were carried out using the STATA/IC 14.2 statistical programme.
Results
The average age at the time of the interview was similar between the cases and controls. At the time of diagnosis, a high proportion of cases was classified as having moderately (36·0 %) or poorly differentiated PC (37·0 %) (data not shown in tables).
Compared with the controls, a greater proportion of cases had a university-level education or more (20·7 % v. 11·7 %), a higher prevalence of chronic diseases (58·2 % v. 41·4 %) and a history of sexually transmitted disease (26·6 % v. 10·9 %). Additionally, a history of PC in first-degree relatives (10·4 % v. 2·5 %) and no practice of physical activity throughout life (14·7 % v. 9·3 %) were reported more frequently by cases than by controls. Energy consumption was significantly higher among cases than in controls (2211·7 (sd 730·9) v. 1998·3(sd 701·6) kcal/d) (Table 1).
Table 1. Selected characteristics of the study population according to cases and controls (Numbers and percentages; mean values and standard deviations)
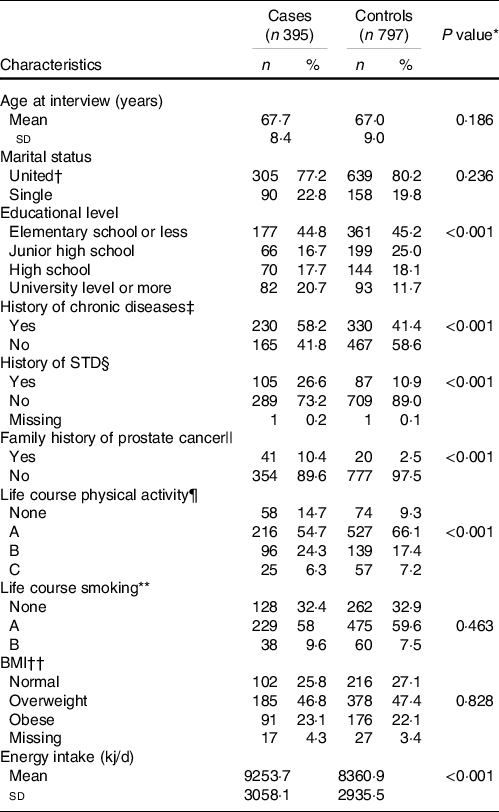
* P value for the Chi or Fisher exact test.
† Married and common law marriage.
‡ Hypertension, diabetes or dyslipidaemia.
§ History of at least one sexually transmitted disease (STD) throughout life.
|| Family history of prostate cancer in first-degree relatives.
¶ Life course physical activity patterns: None: males who do not practice any physical activity during their lifetime; pattern A showed a consistently low physical activity; pattern B presented moderate physical activity throughout life and pattern C showed consistently high physical activity level.
** Life course smoking patterns: None: males who had never smoked during their lifetime; pattern A: males who reported low and constant smoking intensity; pattern B: males with an initial period of low smoking intensity, followed by an increase during the second period.
†† 2 years before the interview.
The gallate pattern was characterised by a higher contribution of (–)-epicatechin 3-O-gallate, (–)-epigallocatechin-3-O-gallate and (+)-gallocatechin. The luteolin pattern was characterised by a higher contribution of luteolin and (–)-epigallocatechin-3-O-gallate. Finally, the mixed pattern (MP) was characterised by a higher consumption of quercetin, (+)-catechin and (–)-epicatechin. The three patterns accounted for 80·66 % (32·82, 25·26 and 22·58 %, respectively) of the total variance in flavonoid intake in the study population (Table 2).
Table 2. Flavonoid dietary patterns identified in the study population (loading factors)
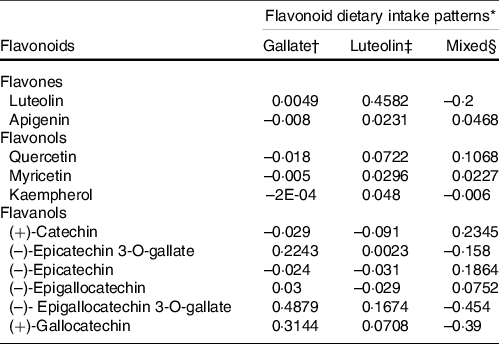
* % total variance explained for all patterns = 80·66.
† Eigenvalue = 4·59: % of variance explained = 32·82.
‡ Eigenvalue = 3·53: % of variance explained = 25·26.
§ Eigenvalue = 3·16: % of variance explained = 22·58.
The main food sources of dietary flavonoid intake patterns were fruits and vegetables, such as cooked tomato with garlic and onion for the gallate pattern and watermelon, cantaloupe, spinach, broccoli, carrot and purslane for the luteolin pattern. In the MP, the main food source was apples (online Supplementary Table S1).
Table 3 shows the distribution between cases and controls in each dietary flavonoid intake pattern and other selected dietary characteristics. A higher daily intake of dietary gallate (ORT3 v. T1 = 0·45; 95 % CI 0·33, 0·62) and luteolin patterns (ORT3 v. T1 = 0·47; 95 % CI 0·35, 0·64) was associated with a decrease in PC risk. Meanwhile, a higher intake of the MP (ORT3 v. T1 = 2·46; 95 % CI 1·82, 3·32) was associated with an increase in PC likelihood. The consumption of green-yellow leafy and nonleafy vegetables showed a no significant reduction in PC risk. After adjusting by age, educational level, history of chronic disease and sexually transmitted disease, history of PC in first-degree relatives, leisure physical activity and smoking patterns, as well as consumption of raw tomato, green-yellow leafy vegetables and green-yellow nonleafy vegetables, all observed associations between dietary flavonoid intake patterns and PC remained and showed significant trends; the lowest risk for PC was observed in the participants classified in the highest tertile of the dietary luteolin pattern. These associations were similar when we stratified by Gleason scale at the time of diagnosis (Table 4). The mutual adjustment between patterns did not change these outcomes (data not shown).
Table 3. Selected dietary characteristics according to cases and controls (Odd ratios and 95% confidence intervals; numbers and percentages)
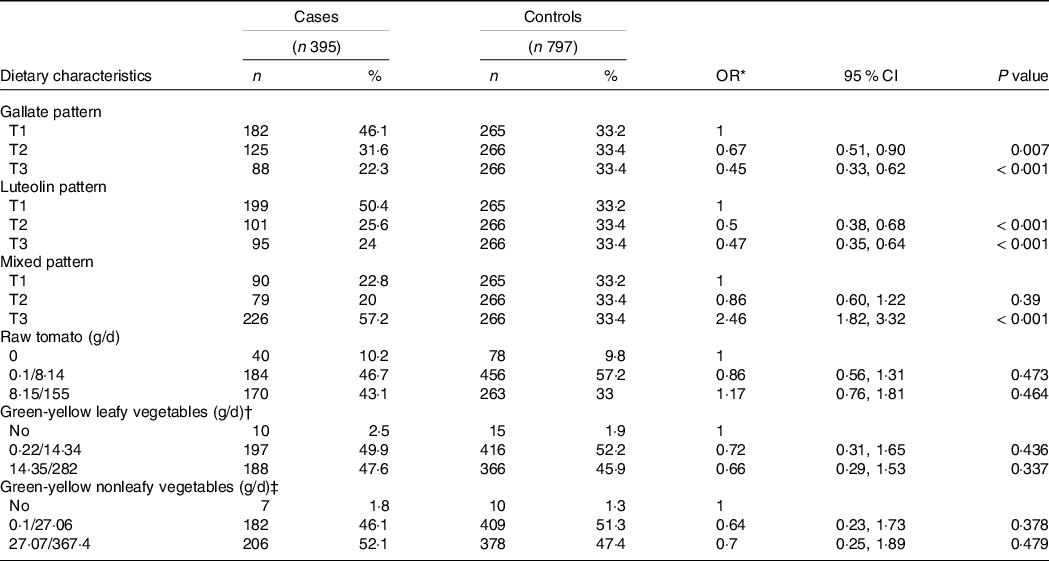
* Adjusted by total energy intake and age.
† Green-yellow leafy vegetables include lettuce, purslane, spinach, pumpkin flowers.
‡ Green-yellow nonleafy vegetables include broccoli, summer squash, smooth-skinned chayote, carrot and maize on the cob.
Table 4. Adjusted association between daily consumption of flavonoids (mg/d) and prostate cancer and PC histological differentiation (Odd ratios and 95% confidence intervals)
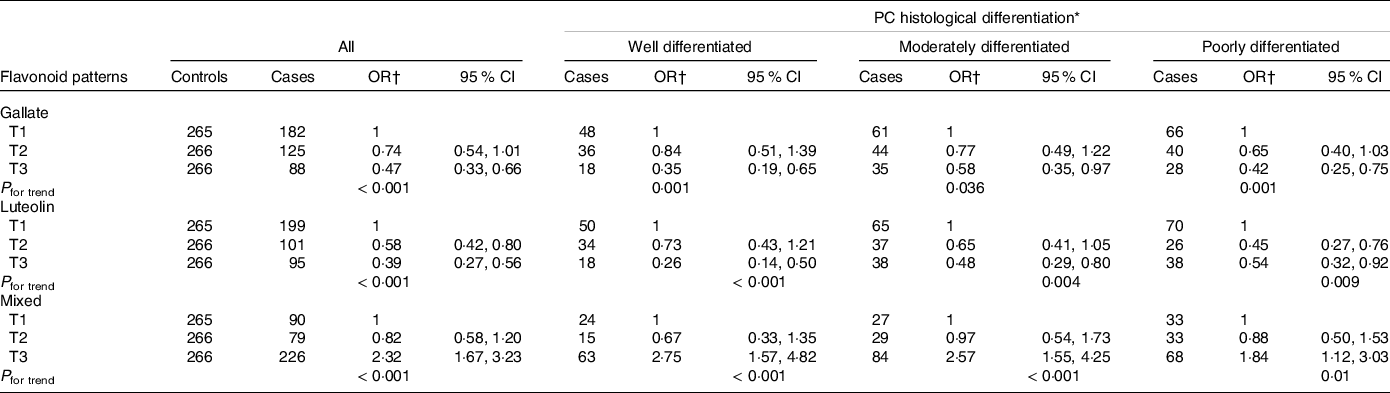
* Gleason score at the time of diagnosis, classified as differentiated (Gleason ≤ 6); moderately differentiated (Gleason 7 (3 + 4 or 4 + 3)) and poorly differentiated (Gleason ≥ 8).
† Adjusted by age, educational level, history of chronic disease, history of sexually transmitted disease, history of prostate cancer in first-degree relatives, leisure physical activity and smoking patterns throughout life, raw tomato, green-yellow leafy vegetables and green-yellow nonleafy vegetables.
Some differences were observed in relation to the association between the intake of the main flavonoid subgroups and their respective compounds and PC risk (online Supplementary Table S2). A higher total flavone intake was associated with a significant reduction in PC risk (OR T3 v. T1 = 0·45; 95 % CI 0·32, 0·65; P for trend = 0·000); nevertheless, the greatest PC risk reduction was observed with luteolin intake (OR T3 v. T1 = 0·40; 95 % CI 0·28, 0·57; P for trend = 0·000). In contrast, a higher intake of total flavonols was associated with a 72 % higher likelihood of PC (OR T3 v. T1 = 1·72; 95 % CI 1·20, 2·47; P for trend = 0·003); however, this result varied by component. While a higher intake of (+)-gallocatechin (OR T3 v. T1 = 0·49; 95 % CI 0·34, 0·71; P for trend < 0·001), or other similar compounds, such as ((–)-epicatechin 3-O-gallate, (–)-epigallocatechin 3-O-gallate and (–)-epigallocatechin), reduced PC likelihood, a higher intake of (+)-catechin and (–)-epicatechin (OR T3 v. T1 = 1·47; 95 % CI 1·05, 2·04; P for trend = 0·019; OR T3 v. T1 = 1·61; 95 % CI 1·16, 2·23; P for trend = 0·001, respectively) was associated with a higher probability of PC (online Supplementary Table S2).
Discussion
To the best of our knowledge, this is the first study in a Latino population to evaluate the association between flavonoid intake and PC. According to our results, higher intakes of flavonoid dietary patterns, characterised mainly by consumption of (–)-epicatechin 3-O-gallate, (–)-epigallocatechin3-O-gallate and (+)-gallocatechin, as well as (–)-epicatechin 3-O-gallate and luteolin, respectively, were associated with a lower PC risk. In contrast, an increase in PC risk was associated with a higher consumption of the MP, whose main components were quercetin, (+)-catechin and (–)-epicatechin.
It is difficult to compare our results with those of other studies because we are the first to have used this pattern approach. However, the reduction in PC risk observed with the gallate pattern is consistent with that observed in studies that have evaluated (–)-epigallocatechin 3-O-gallate intake(Reference Lee, Ng and Liu35), consumption of flavonoid subgroups such as flavan-3-ols, and flavones(Reference Reale, Russo and Di Mauro19) or consumption of epigallocatechin-rich foods such as green tea(Reference Lee, Ng and Liu35,Reference Jian, Xie and Lee36) . Evidence regarding the anticarcinogenic potential role of (+)-gallocatechin(Reference Du, Zhang and Wen37) and luteolin(Reference Chiu and Lin38) has been presented based on experimental studies. Luteolin’s effect seems to occur at relatively low doses and its effect could be higher in androgen-sensitive PC cells(Reference Chiu and Lin38).
The main anticarcinogenic role of flavonoid compounds is based on their capacity to promote cell cycle arrest, autophagy and apoptosis and to reverse adverse epigenetic regulation(Reference Niedzwiecki, Roomi and Kalinovsky1,Reference Costea, Nagy and Ganea3) . Additionally, some evidence suggests a differential anticarcinogenic effect for compounds of the same group of flavonoids, as well as a potential interaction between flavonoid compounds(Reference Phan, Paterson and Bucknall39) and some antiandrogenic PC treatments. The flavones luteolin and apigenin both limit the metastatic capacity of PC cells by suppressing fatty acid synthase; however, the luteolin effect is greater.(Reference Chiu and Lin38) Studies in vitro have revealed a synergistic mechanism between (–)-epigallocatechin3-O-gallate and luteolin in PC cells, as well as in the surrounding tumour microenvironment, reversing myofibroblast activation(Reference Gray, Stephens and Bigelow15). Myofibroblasts can stimulate tumour epithelial cell proliferation by secreting high levels of growth factors that can promote migration and invasion(Reference Polanska and Orimo40,Reference Orimo, Gupta and Sgroi41) . PC cells positive and negative for androgen treated with (–)-epigallocatechin-3-O-gallate and bicalutamide (alone and in combination) showed a dose-dependent decrease in cell number with each separate treatment; however, this effect was significantly greater in the cells treated with a combination of bicalutamide and (–)-epigallocatechin-3-O-gallate(Reference Morrissey, Brown and O’Sullivan42).
We do not have a clear biological explanation for the unanticipated association between MP intake and PC risk. Nevertheless, some potential mechanisms for this finding could be related to oestrogenic and differential anticarcinogenic effects of the main compounds in this pattern (quercetin, epicatechin and catechin). Quercetin exhibits a potential prostate cell proliferation effect as a consequence of its high affinity for the β-oestrogen receptor(Reference van der Woude, Ter Veld and Jacobs43), and it also inhibits catechol-O-methyltransferase activity, allowing a longer cell exposure time to the carcinogenic metabolites of E2 and to oxidative stress(Reference Singh, Mense and Bhat44). In rats with breast cancer induced by oestradiol implants, the consumption of quercetin (2·5 g/kg food) did not inhibit E2-induced oxidative stress and significantly reduced tumour latency. Additionally, an epidemiological study of African American men also revealed an elevated PC risk among men with high quercetin intake and normal vitamin D levels(Reference Paller, Kanaan and Beyene45); however, there is no clear biological explanation for this potential modifying effect. Likewise, in vitro and in vivo studies suggest that epicatechin has no or a weak anticarcinogenic effect(Reference Vue, Zhang and Chen2).
The main strength of this study is the characterisation of the three dietary flavonoid intake patterns, which explain 80·66 % of the total flavonoid intake variability. This approach is highly recommended in observational studies because the effect of a single dietary component on the risk of disease is difficult to assess; dietary flavonoid subgroups or individual components are not found separately in food, and their complex effects are likely to be interactive or synergistic(Reference Phan, Paterson and Bucknall39,Reference van Breda and de Kok46) . Therefore, this strategy allows us to consider the cumulative and complex effects of multiple flavonoids contained in food or in the daily diet.
Nonetheless, to interpret our results, some methodological aspects should be considered. It is unlikely that our results are a consequence of selection bias. Participation rates between cases (85·90 %) and controls (87·50 %) were similar, and we did not observe differences between participants and nonparticipants in relation to age, birthplace, marital status and educational level(Reference Vázquez-Salas, Torres-Sánchez and López-Carrillo26). It is difficult to evaluate whether our controls are representative of the population from which the cases arose because, in Mexico, information about flavonoid intake is only available among females; however, the median intake of selected macronutrients (i.e. energy 1930·1 v. 1782 kcal/d, fat 55·0 v. 53·1 g/d and carbohydrates 272·2 v. 274·7 g/d) among our controls was similar to those reported in the 2006 Mexican National Health and Nutrition Survey for an adult population residing in Mexico City(Reference Barquera, Hernández-Barrera and Campos-Nonato47). In addition, the prevalence of ever smokers (67·2 % v. 65·6 %) among our controls was similar to that reported nationally in males over 60 years of age who resided in Mexico City(48). Compared with flavonoid consumption among Mexican women(Reference Zamora-Ros, Biessy and Rothwell23), the average consumption varied according to the flavonoid subgroup and could be a consequence of differences in reported and preferred consumption according to sex. Usually, women are more aware of their health, so they consume more healthy foods such as fruits and vegetables (main sources of flavonoids), while men tend to engage in risky behaviours that lead them to consume less healthy food(Reference Fagerli and Wandel49).
Flavonoid intake was evaluated retrospectively using a semi-quantitative FFQ, and we cannot rule out the existence of a measurement error. The FFQ tends to underestimate usual consumption(Reference Denova-Gutiérrez, Ramírez-Silva and Rodríguez-Ramírez50); nonetheless, our participants and interviewers were unaware of the specific study hypothesis. If there exists a measurement error, it could have underestimated the observed association. The main known risk factors for PC were included in the analysis, such as physical activity and smoking, which were measured in a manner that considered temporality and intensity throughout life. However, we cannot reject the potential for the presence of residual confounding because we did not have information about alcohol consumption throughout life or Se, supplement and antioxidant intake. Additionally, instead of adjusting by specific compounds with similar activities on cell cycle arrest (β-carotene, lycopene and others), we used food sources of these compounds. Finally, our relatively small sample size and the lack of available information about vitamin D and/or oestrogen status prevented us from evaluating potential interactions with flavonoid patterns.
Conclusion
Our findings support the existing evidence of the anticarcinogenic capacity of some flavonoids, particularly (–)-epigallocatechin-3-O-gallate and luteolin. Moreover, the safety of these compounds supports their potential use as chemopreventive and adjuvant therapeutics in PC management; however, well-designed clinical trials are needed to further demonstrate their beneficial effects.
Acknowledgements
The authors thank the following hospitals for the use of their facilities: General Hospital of Mexico (SSA); National Cancer Institute (SSA); Salvador Zubirán National Institute of Medical and Nutrition Sciences (SSA), the XXI Century National Medical Center Oncology Hospital (Centro Médico Nacional Siglo XXI) (IMSS), Dr Carlos McGregor Sánchez-Navarro Regional General Hospital (IMSS) and the Aldolfo López Mateos Regional Hospital (ISSSTE).
This project was supported by the National Council for Science and Technology (CONACYT) (L. T. S., grant numbers 140482, 272810).
M. V. G. P. participated in the study design, analysis and interpretation of data and in drafting the manuscript. R. A. V. S. participated in the study design, the acquisition, analysis and interpretation of data and in the critical review. J. G. H. P. participated in the analysis and interpretation of data, drafting the reviewed manuscript, and in the response to the editor and to reviewers’ observations. J. B. M. participated in the study design and critically revised it for important intellectual content. L. L. C. participated in the study design and the critical review of important intellectual content. L. T. S. conceptualised and designed the study; supervised the acquisition, analysis and interpretation of data; and participated in critically revising it for important intellectual content. Finally, all the authors agreed to be accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final version to be published.
The authors declare no conflicts of interest, and the funder had no participation in the design collection, analyses, interpretation of data, final version of the manuscript or decision to publish the results.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521002646







