The development of megavoltage-based breast conservation techniques
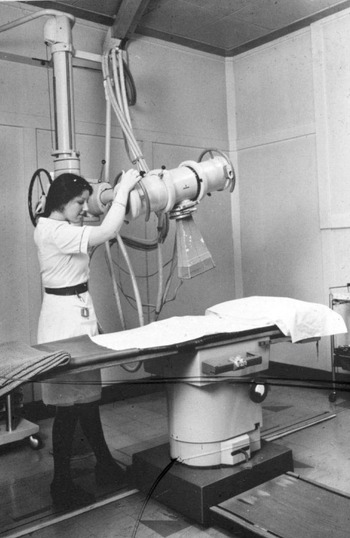
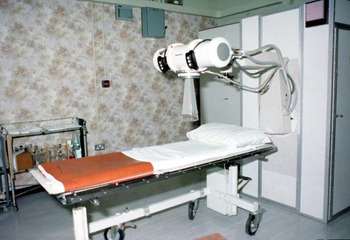
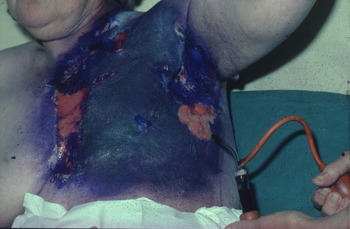
In the 1970s, at the Western Infirmary Glasgow in Scotland (now demolished), early breast cancer was treated by simple mastectomy followed by 250 (Figure 1) or 300 KV (Figure 2) irradiation using a six-field technique. By the end of treatment, all patients had at least very red and sore skin. A substantial number had moist desquamation of the skin. A full-time nursing sister was employed to treat these ‘reactions’ with dressing and lashings of Gentian violet (Figure 3) plus great care and sympathy as the sister had been treated for breast cancer herself.

Figure 1. 250KV ‘DXT’ machine at the Western Infirmary Glasgow in the 1970s.

Figure 2. 300KV X-ray therapy machine in the Western Infirmary Glasgow about 1980.

Figure 3. Areas of moist desquamation treated by Gentian violet and dressing after kilovoltage X-ray treatment to the chest wall after a mastectomy for breast cancer.
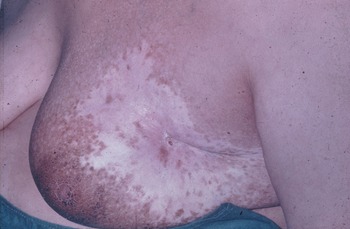
The late and permanent effects were marked especially in the parasternal field with red telangiectatic blood vessels which delineated the irradiated area. Irradiation of the intact breast could result in severe late effects such as telangiectasia, depigmentation and atrophy (Figure 4).

Figure 4. Late effects of atrophy, depigmentation and telangiectasia after irradiation of the intact breast by kilovoltage X-rays.
However, change was coming. A timeline listing the progress in radiation technology and the potential of medical genetics to reduce the late side is shown in Table 1. Between 1973 and 1980, patients in Milan were randomised to either radical mastectomy (including removal of the pectoral muscles as well as the breast) or quadrantectomy (removal of the cancer contained in a quarter of the breast) plus post-operative skin-sparing megavoltage radiotherapy. The results of this study were first published in 1981 and the eventual 20-year survival results in 2002. Reference Veronesi, Cascinelli and Mariani1 Death from breast cancer was 26·1% for those receiving breast conservation treatment and 24·3% in the radical mastectomy group (p = 0·8).
Table 1. Timeline in the evolution of breast conservation and the reduction of side-effects of breast cancer radiotherapy

A similar study was initiated by the European Organisation for Research and Treatment of Cancer (EORTC-trial number10801) in 1980. By 10 years after treatment, survival was very similar in both arms of the study of predominantly stage II breast cancer treated by either radical mastectomy (66%) or lumpectomy (removal of the breast cancer plus 1 cm of normal tissue) followed by radiotherapy (65%). Reference van Dongen, Voogd and Fentiman2
Radiotherapy is necessary in most cases after conservative surgery
The importance of the addition of radiotherapy following local excision to reduce loco-regional recurrence was shown in numerous clinical trials including the Scottish trial. Reference Forrest, Stewart and Everington3 Two hundred and ninety-one patients were treated by lumpectomy and tamoxifen or CMF chemotherapy. A further 294 had additional breast radiotherapy. The loco-regional recurrence rate at 6 years was 6 .1% in the irradiated group and 28·6% in the excision-only group.
The overall value of radiotherapy was shown by the Early Breast Cancer Trialists’ Group in 2011 in a meta-analysis of 10,801 individual patient data from 17 randomised trials. 4 The reduction in loco-regional disease and absolute reduction in mortality varied according to the patient’s age, tumour grade, oestrogen receptor (ER) status and extent of surgery but in broad terms radiotherapy to the conserved breast halved the loco-regional recurrence rate and breast cancer deaths by one-sixth.
Further technological change
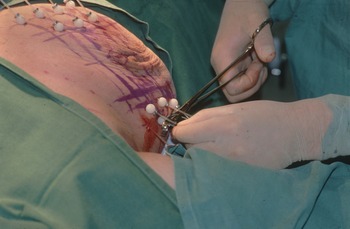
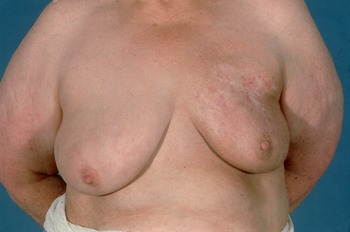
In EORTC trial 10801, the radiotherapy regimen was megavoltage irradiation (50 Gy in 25 fractions) to the whole breast followed by additional brachytherapy dosage from iridium 192 implants of 25 Gy to the lumpectomy site. This was the practice in Glasgow in the 1980s (Figure 5). As electrons became more widely available, iridium implantation was abandoned as electrons offered an easier, cheaper, outpatient-only treatment. An unpublished audit of the cosmetic results of electrons compared to iridium brachytherapy in Glasgow showed that there was much more induration in the tumour bed following implantation with more breast retraction and atrophy but more skin telangiectasia from electron boosts (Figure 6).

Figure 5. Two-plane iridium after-loading implant to the lumpectomy site.

Figure 6. Late changes including breast retraction and atrophy after an iridium implant.
The adverse cosmetic results from boost treatments can be reduced by more selective indications for boosts taking into account an EORTC phase III randomised trial where patients were randomised to whole breast radiotherapy plus or minus a boost. The 20-year follow-up results showed boost treatments had no effect on long-term survival but did improve local control although there was an increase in moderate to severe fibrosis in the boost group. Reference Bartelink, Maingon and Poortmans5 The maximal value of the increased dose to the tumour bed was in younger patients. Improved loco-regional control has to be balanced against the risk of more induration. Current UK indications for boosting are patients aged up to 59 years, high histological grade, node positive disease and stages T3 or T4. Positive or close surgical margins are indications for a higher dose to be delivered to the tumour bed. Reference Kunckler, Symonds, Mills and Duxbury6
It is noteworthy that in the REQUITE cohort Reference Webb, Harper and Rattay14 that the prevalence of boosts was 75–100% in continental European centres. Leicester followed the British practice and only administered field-in-field intensity-modulated radiotherapy (IMRT) boosts in 10% of patients. Radiation toxicity is important as the all stage 10-year survival in the UK has improved from 40% in the 1970s to 78% in those treated in 2011. 7 Stage T1 N0 patients have a 10-year survival of over 90%. The reasons for the improvement in survival are complex and include earlier diagnosis (including screening) and improvements in surgery, radiotherapy, chemotherapy and hormone treatment. At least 35,000 women in the UK undergo post-operative radiotherapy each year, and the majority receive this treatment as part of a breast conservation programme.
Advances in radiation technology particularly due to the skin sparing properties of megavoltage radiation have largely reduced radiotherapy toxicity. The use of field-in-field IMRT boosts rather than electrons have further reduced the prevalence of telangiectasia. Further improvement in cosmesis by using IMRT was demonstrated in the Cambridge randomised controlled trial of either treatment planning using 2D or IMRT methodology. Reference Mukesh, Barnett and Wilkinson8 Overall, cosmesis was improved (p = 0 038) and telangiectasia was reduced (p = 0·031) by IMRT, but significantly there was no reduction in breast shrinkage, oedema, tumour bed induration or pigmentation.
Genetic variation and response to radiotherapy
As only a minority of irradiated patients develop severe or moderate late effects, this suggests some individuals are intrinsically more sensitive to radiation. Mutations of the ATM gene (ataxia-telangiectasia, A-T) cause a multi-system disorder of childhood onset with cerebellar ataxia, conjunctive telangiectasia, immune deficiency and an increase in malignancies, particularly leukaemia and lymphomas. Treatment of lymphomas in these individuals by relatively modest doses of radiation induced severe and sometimes fatal toxicity. Reference Gotoff, Amirimorkri and Leibner9 Owing to the unusual radiation sensitivity of A-T sufferers, variations in the A-T gene have long been considered as strong candidates for contributing to late radiation toxicity. Tanteles and colleagues in the East Midlands Reference Tanteles, Murray and Mills10 in a retrospective study of irradiated breast cancer patients found an A-T variant was associated with increased telangiectasia, fibrosis and overall toxicity. A meta-analysis by Andreassen et al Reference Andreassen, Rosenstein and Kerns11 which included 2,759 breast cancers and 2679 prostate cancer patients from 17 different cohorts found a significant increase in acute toxicity (OR 1·5) and late toxicity (OR1·2) for patients carrying ATM variant r1801516.
As well as genes which predispose to increased acute and late toxicity, individuals may carry genes protective against radiation damage. A German group Reference Seibold, Behrens and Schmezer12 investigated a large cohort of 2600 breast cancer patients in eight studies and found a variant of the base excision repair gene XRRC1 that was associated with significantly both decreased skin (p = 0·02) and overall toxicity (p = 0·016).
Body clock genes and the scheduling of radiotherapy
Genetic testing taking into account the time of day of irradiation may lead to reduction in post-radiotherapy atrophy which, as the Cambridge randomised trial shows, is not reduced by IMRT. This is shown in data from the REQUITE pan-European group whose aim is to develop models and biomarkers that predict the risk of late radiation toxicity. Reference Seibold, Webb and Aguado-Barrera13 In this prospective study, Reference Webb, Harper and Rattay14 late atrophy was defined as worsening of breast shrinkage by at least one grade as defined in the Common Toxicity Criteria Adverse Events (CTCAEv4) scale. There were 1690 evaluable patients from the UK (Leicester), Barcelona and Santiago de Compostela (Spain), Leuven and Gent (Belgium), Mannheim (Germany), Montpellier (France) and Milan (Italy). Overall, there was a 35% incidence of atrophy which varied between 21 and 51% as reported by the various centres in spite of the widespread use of IMRT. The incidence of atrophy was affected by the body mass index (BMI), radiation dose and the extent of pre-radiotherapy surgery (lumpectomy or quadrantectomy). When the time of day of treatment was considered along with variants of three circadian (body clock) genes (PER3, CLOCK and RASD1), there was a striking relationship between the incidence of atrophy and the time of day adjusted to allow for variations in daylight hours and local time zones. The timing associated with the highest and lowest risks was dependent on variation of key circadian genes. The greatest effect was seen where patients had two copies of the T variant in PER3, two copies of the A variant of RASD1 and two copies of the A variant of CLOCK had the highest probability of atrophy when treated close to 3·30 pm. Such patients ideally should be treated in the morning. Potentially, atrophy could be reduced by selecting the optimum treatment time for individual patients according to their circadian (body clock) genotype. For one combination of three gene variants representing 14% of the population, atrophy could be reduced from 70 to 30% simply by treating in the morning rather than the afternoon.
Circadian rhythms or body clocks are 24 h cycles which govern the sleep/wake cycles and numerous cell functions that can affect the response to cancer treatments. The daily rhythms are controlled by the master clock in the brain and respond to environmental factors such as light, but there are also peripheral clocks in every organ under control of circadian genes. Normal variation of circadian genes influence whether the individual is a morning or evening person but crucially affect the time of day when cells are most susceptible to radiation damage. Cells synthesising DNA (S phase of the cell cycle) are the most radioresistant with dividing cells (in G2/M) the most radiosensitive.
The REQUITE consortium findings need at least to be tested by further studies. Ideally, this should be a randomised trial with patients randomly allocated to a treatment time and the outcome compared to those treated at an optimum time to reduce the risk of late effects as predicted by genetic testing. By carefully allocating treatment slots following a cheap DNA blood test, this simple low cost change in practice could improve the quality of life for thousands of patients every year.
In just over 40 years, we have seen a doubling of 10-year survival of breast cancer patients and instead of the virtually inevitable marked acute and late effects of kilovoltage irradiation, acute and late effects have been reduced by megavoltage and IMRT treatment with an improvement in quality of life. There is now the potential to reduce the late effects of breast radiotherapy by tailoring treatment slots to the patient’s body clock gene profile. Personalised radiotherapy for breast cancer is now possible.
Acknowledgements
The authors acknowledge the support of the Leicester ECMC.
Authorship
All authors have contributed to the paper and have seen and agreed with the final draft.
Financial support
The genetics research which was part of the REQUITE programme was funded from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 601826.
Conflict of interest
The authors declare none.