INTRODUCTION
Pâté is a food prepared from a minced mixture of offal (particularly liver), muscle, fat, vegetables, herbs and spices which is cooked at relatively low temperatures [Reference Hutchison1]. A variety of constituents from different animal species are used in pâté production, particularly pigs or poultry (e.g. chickens and ducks), although fish and shellfish can also be used. Following a period of chilling, pâté is usually served as a ready-to-eat spreadable paste, often together with bread or other farinaceous products. The major risks from microbiological hazards for liver pâté consumption are associated with the low cooking temperatures (including the possibility of undercooking), contamination after cooking and the growth of bacteria in the product during storage. In England and Wales in the 1980s, outbreaks of food poisoning caused by the consumption of liver pâté were associated with Listeria monocytogenes [Reference McLauchlin2] and Salmonella [Reference Threlfall, Hall and Rowe3]. More recently there has been an increase in liver pâté outbreaks associated with Campylobacter [Reference Little4–Reference Edwards6] in England and Wales, as well as in other countries [Reference Parry, Fearnley and Denehy7–Reference Lahti10]. Outbreaks of foodborne illness associated with pâté consumption outside the UK have also been reported due to botulism [Reference Lafuente11], hepatitis A virus [Reference Schwarz12] and Aeromonas hydrophila [Reference Krovacek13].
A previous report [Reference Elson14] detected differences in the microbiological quality of in-house made pâté as compared with that produced on a larger commercial scale. However, there are no recent data to support this observation. Therefore, in addition to the increase in outbreaks due to Campylobacter and the lack of recent data on microbiological quality, this study was undertaken to provide an assessment of the microbiological quality of liver pâté from catering and retail settings and allow comparison of in-house and large scale produced product.
METHODS
Sample collection
Samples of ready-to-eat pâté with liver as the major ingredient were collected during April 2012 to March 2013, and all were independent of any outbreak investigations or incidents associated with foodborne illness. Sampling officers from Environmental Health Departments in England collected liver pâté either from retail (national supermarkets, butchers, delicatessens, farm-shops, and other small- and medium-sized retailers), from catering (public houses, cafes, restaurants, hotels, guest houses, mobile food units and takeaways), or from the point of manufacture where this was separate to the point of sale or serving. Data on temperature of storage were collected by the sampling officer as outlined in the Food Standards Agency Food Law Practice Guidance [15]. Initially, temperature of storage data were obtained by an assessment of the food business operator's own refrigeration equipment. The specific temperature of storage was collected either via an external/surface thermometer or via the food business operator's or the sampling officer's own temperature probe. Information on retailer, vendor or caterer, the sample type, country of origin and details of the storage at the point of sale were collected by the sampling officers: data were collected using a standardised study questionnaire.
At least 100 g of pâté was sampled and transported in accordance with the Food Standards Agency Food Law Practice Guidance [15]. All samples were examined by one of the five Health Protection Agency (HPA) Official Control Laboratories in England (Food, Water and Environmental Microbiology Laboratories) located at Birmingham, London, Preston, Porton and York.
Microbiological examination
Pâté samples were examined using internationally recognised standard methods. These comprised: detection of Salmonella spp. (BS EN ISO 6579:2002); detection of Campylobacter spp. (ISO 10272-1:2006); detection and enumeration of Listeria spp., including L. monocytogenes (BS EN ISO 11290-1:1996 and 11290-2:1998); enumeration of Clostridium perfringens (ISO 7937:2004); enumeration of coagulase-positive staphylococci, including Staphylococcus aureus (BS EN ISO 6888-1:1999 +A1:2003); enumeration of Bacillus spp., including Bacillus cereus (BS EN ISO 7932:2004); enumeration of Escherichia coli (based on BS ISO 16649-2:2001 but using a surface spread rather than pour plate technique); enumeration of Enterobacteriaceae (BS EN ISO 21528-2 2004); enumeration of aerobic colony counts (ACCs; BS 4833-2:2013). All presence/absence tests were performed on 25 g samples.
The identification of isolates of Bacillus spp., C. perfringens, L. monocytogenes and S. aureus was performed in each of the individual laboratories as outlined in the standard methods above. Cultures of L. monocytogenes were sent to the HPA GBRU (Gastrointestinal Bacteria Reference Unit), for confirmation and further characterisation [Reference Nogva16–Reference Roussel19]. Comparison of L. monocytogenes types was carried out using the UK national surveillance database of results generated from analysis of isolates from both human cases of listeriosis and food.
Microbiological results were interpreted according to the HPA Guidelines for assessing the microbiological safety of ready-to-eat foods placed on the market as outlined in Table 1 [20] to categorise the results as unsatisfactory, borderline, and satisfactory. Regulation (European Commission, EC) No. 2073/2005 on microbiological criteria for foodstuffs [21] was also used to interpret microbiological results where relevant. Samples for which results were not available for one or more of the microbiological parameters specified in the study protocol were excluded from this analysis.
Statistical analysis
Contingency tables were used to provide both descriptive and inferential analysis of the study data. Initially χ 2 tests were performed to assess associations between variables collected on the sample and microbiological quality. The microbiological outcome of the ordered categorical variable; satisfactory, borderline and unsatisfactory was used in an ordered logistic regression analysis to quantify the strength of association in terms of estimated relative odds. These refer to a specific outcome category compared with the category immediately lower, e.g. the odds of satisfactory compared with the odds of borderline. The assumption of proportional odds was assessed to ensure the validity that the odds ratio across all categories as approximately equal. A P value of 0·05 or lower was defined as indicating statistical significance. All analyses were performed using Stata v 13 (Stata Corp. College Station, Texas).
RESULTS
A total of 870 liver pâté samples were tested: data from a further 56 samples were submitted but these were not included in the analysis since results from one or more of the microbiological parameters specified in the study protocol were missing. Following interpretation according to the HPA Guidelines for assessing the microbiological safety of ready-to-eat foods [20], 73% were of satisfactory microbiological quality, 18% were borderline and 9% were unsatisfactory (Table 2). Neither Salmonella spp. nor Campylobacter spp. was isolated from any sample. The most common causes of unsatisfactory results were elevated ACCs (6% of the samples) and high Enterobacteriaceae counts (5% of samples). The remaining unsatisfactory results were due to elevated counts of either E. coli (three samples), B. cereus (one sample at 2·6 × 105 cfu/g) or L. monocytogenes (one sample at 2·9 × 103 cfu/g). Amongst the 18% of samples that were of borderline quality, the commonest individual causes (in order of numbers of samples) were elevated: ACCs, Enterobacteriaceae, Bacillus spp., E. coli, B. cereus, S. aureus, C. perfringens and L. monocytogenes (Table 2). L. monocytogenes at <20 cfu/g was recovered from a further seven samples, and nine samples contained Listeria spp. (not L. monocytogenes) also at <20 cfu/g. The sample containing L. monocytogenes at >102 cfu/g was the only sample that did not comply with the microbiological criteria as laid down in EC Regulation No. 2073 [21].
Table 2. Microbiological quality of 870 ready-to-eat pâté by different parameters and premises types

NK, not known.
* L. monocytogenes detected in 10 samples including two samples categorised as borderline where the bacterium was present at 40 and 80 cfu/g and one sample categorised as unacceptable where the bacterium was present at 2·9 × 103 cfu/g and is potentially injurious to health.
† Listeria spp. detected in nine samples, six L. welshimeri, two L. seeligeri, 1 L. innocua.
‡ Four samples from mobile vendors and two from takeaways.
§ Sixteen from farm shops, 6 from market stalls and 17 from other shops.
Samples from catering, retail and manufacture
Samples from catering (those where liver pâté was served for direct consumption by consumers) included public houses, cafes, restaurants, hotels, guest houses, mobile food units and takeaways. Retail establishments (those where liver pâté was sold for consumption elsewhere) included national supermarkets, butchers, delicatessens, farm-shops, and other small- and medium-sized retailers. The point of manufacture was included where this was separate to the point of sale or serving. Amongst all 870 samples, 46% were from catering, 53% were from retail and the remaining 1% were from the point of manufacture. The 399 samples from catering were collected from: restaurants and cafés (48%), public houses (30%), hotels and guest houses (21%) and the remainder from mobile vendors or takeaways (1%). Amongst the 462 pâté samples from retail, 61% were from national retail chain supermarkets, 13% from delicatessens, 8% from butchers and 17% from other shops (farm shops, market stalls and other types of retailers). An ordered logistic regression model was employed to assess differences in microbiological quality between samples from catering, retail supermarkets and other retail settings, and the likelihood ratio test showed a significant difference (χ 2 test 13·2 on 2 degrees of freedom, P = 0·001). The microbiological quality of pâté sampled from supermarkets was significantly better than those from catering establishments (odds ratio 0·57, 95% CI 0·40–0·84, P = 0·004): there were no significant differences between the results obtained from pâté collected from catering with those from other retailers.
An ordered logistic regression model was employed to assess if there was evidence for differences in microbiological quality between results from samples collected from within all catering and all retail settings. Within catering, the microbiological quality of samples from mobile vendors and from takeaways was significantly worse than those from restaurant and café establishments (95% CI 1·00–20·62, P = 0·05). There were no significant differences in sample microbiological quality between all other catering settings. For samples from different retail settings, the likelihood ratio test showed a significant difference (χ 2 test 19·04 on 3 degrees of freedom, P = 0·0003). Samples from delicatessens were of significantly worse microbiological quality than those from supermarkets (estimated odds ratio 2·95 95% CI 1·65–5·29, P < 0·001). The microbiological quality of samples from butchers was significantly worse than those from supermarkets (estimated odds ratio 3·14, 95% CI 1·55–6·37, P = 0·001). Comparison of results for samples from all other retail settings showed no significant differences in microbiological quality.
Declared animal species used for liver
Amongst all 870 samples, the most common declared animal species used for liver was chicken in 40%, pork in 31%, duck in 9% and other animals (including mixtures) was declared for 7%: information was not available in the remaining 13% (Table 2). An ordered logistic regression model showed that there was a significant difference in the quality between the declared animal species (χ 2 test statistics 9·43 on 4 degrees of freedom, P = 0·05). The microbiological quality of pork liver pâté was significantly better than that of poultry (chicken and duck; estimated odds ratio was 0·66, 95% CI 0·46–0·94, P = 0·02). For all other comparisons between pâté manufactured from the livers of different animal species there were no significant differences in sample quality and this effect was independent of the setting.
Temperature of storage
Data on specific temperature of storage were available for 318 (37%) of all samples (Table 3) and 5% were stored frozen, 78% were recorded as between 0 and <8 °C, and the remaining 17% were stored at ⩾8–22 °C. In addition, descriptive data were available for 460 (53%) of the samples which described these as collected from refrigerated storage. The main effects region model provided estimated odds ratios for microbial quality and there were no significant differences between the quality of samples collected frozen, as compared with those stored refrigerated at 0–<8 °C and ⩾8–22 °C.
Table 3. Temperature of storage for 318 samples of pâté at the point of collection
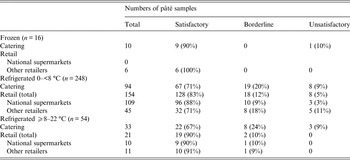
Excluding those stored frozen, the percentage of samples stored at between 0 and <8 °C (as compared with those stored at ⩾8 °C) was 74% for catering, 92% for supermarkets and 80% for other retail outlets. An ordered logistic regression model was employed to assess whether there was evidence to suggest that the microbiological quality differed between the storage temperature groupings in the different retail settings. The likelihood ratio test was used to provide an overall test of association (χ 2 test statistics 0·43 on 2 degrees of freedom, P = 0·81) and there was no significant difference in the microbiological quality between the storage types. An interaction between the storage groups and retail groups was fitted in the model to assess whether there was any evidence to suggest that the association between storage temperature and microbial quality differed between the retail groups. A likelihood ratio test was used to test this interaction (χ 2 test statistics 1·51 on 2 degrees of freedom, P = 0·68) and found no evidence for a difference between retail settings.
Remaining shelf life
For liver pâté made in-house, the shelf-life data collected were the number of days after production before consumption. For commercially produced liver pâté both on retail sale or collected at catering, the remaining shelf life was the time in days between the day of collection and the use-by date on the packaging. Remaining shelf-life data were available for 176 (20%) of all the 854 refrigerated samples, and these comprised 79 collected at catering, 65 from supermarkets and 32 from other retail outlets (Table 4). The shelf-life data on the outside of the packaging for samples collected at retail varied from 0 to 211 days. The percentage of samples with a shelf life of 5 or fewer days was 81% for samples collected at catering, 15% from supermarkets and 19% from other retail establishments. Initially each retail group was modelled individually using mid-point of the number of days of shelf life remaining as a continuous predictor variable. In order to assess if the relationship between days of shelf life remaining and microbiological quality was non-linear, the groups were fitted as a categorical predictor, and an ordered logistic regression model was used in this analysis. For samples from catering outlets there was no significant association between days of shelf life remaining and microbiological quality. The likelihood ratio test was used to provide an overall test of association (χ 2 test statistics 1·95 on 5 degrees of freedom, P = 0·86). When considering days of remaining shelf life as a continuous predictor there was no evidence of an association (χ 2 test statistics 0·03 on 1 degree of freedom, P = 0·87). For samples collected from retail supermarkets, there was no evidence of an association between days of remaining shelf life and microbiological quality. The likelihood ratio test was used to provide an overall test of association (χ 2 test statistics 1·69 on 5 degrees of freedom, P = 0·89). When considering days of remaining shelf life as a continuous predictor there was again no evidence of an association (χ 2 test statistics 0·17 on 1 degree of freedom, P = 0·68). For other retailers, there was no evidence of an association between days of remaining shelf life and microbiological quality. The likelihood ratio test was used to provide an overall test of association (χ 2 test statistics 3·89 on 5 degrees of freedom, P = 0·57). When considering days of remaining shelf life as a continuous predictor there was again no evidence of an association (χ 2 test statistics 1·96 on 1 degree of freedom, P = 0·16).
Table 4. Remaining shelf-life data for 176 samples of refrigerated pâté

S, satisfactory; B, borderline; U, unsatisfactory.
* Range 41–211 days, of all 29 samples, eight had a remaining shelf life of >100 days.
Comparison of results for individual microbiological parameters
A comparison of results for individual microbiological parameters obtained from 234 samples of pâté collected at retail or from catering which were of borderline or unsatisfactory microbiological quality is shown in Table 5. For B. cereus there was weak evidence to suggest a difference in the percentages where B. cereus was present at an unsatisfactory level in slightly more samples from catering than from retail (Fisher's exact test, P = 0·07). There was no evidence for differences in the distributions of Bacillus spp., C. perfringens, L. monocytogenes or S. aureus between samples collected at retail or from catering which were of borderline or unsatisfactory microbiological quality. There was strong evidence to suggest a difference in the percentages with elevated levels of E. coli and Enterobacteriaceae in catering samples (Fisher's exact test, P < 0·001). There was strong evidence to suggest a difference in the percentages where the ACC was considered borderline or unsatisfactory (Fisher's exact test, P = 0·001) in both catering and other retail samples compared with samples from supermarkets. There was a greater proportion of samples contaminated with L. monocytogenes from catering as compared with retail (1·5% vs. 0·9%): although this was not significantly different (Fisher's exact test, P = 0·2), this difference was consistent with the elevated E. coli, Enterobacteriaceae and ACC results from catering establishments outlined above and suggesting poorer hygiene.
Table 5. Comparison of results for individual microbiological parameters obtained from 234 samples of pâté collected at catering or retail and of borderline or unsatisfactory microbiological quality
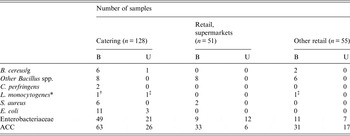
B, borderline; U, unsatisfactory.
* Four samples with L. monocytogenes and five with other Listeria spp. recovered at <20 cfu/g were in-house produced and recovered from catering. Three samples contaminated with L. monocytogenes at <20 cfu/g and collected at retail were all pre-packaged and manufactured in Belgium: The four samples from retail contaminated with other Listeria spp. at <20 cfu/g three were from supermarkets and one was from a butcher.
† Produced in-house.
‡ Pre-packed and produced in another EU Member State.
Samples contaminated by Listeria including L. monocytogenes
Amongst the 10 samples contaminated by L. monocytogenes, in all three where this bacterium was detected at ⩾20 cfu/g, borderline Enterobacteriaceae result were obtained. One of the samples where L. monocytogenes was detected at ⩾20 cfu/g also had an unsatisfactory ACC. Of the remaining seven samples were L. monocytogenes was present at <20 cfu/g, three were satisfactory for all other parameters, and of the remaining, two had a borderline Enterobacteriaceae result and three had either an unsatisfactory or borderline ACCs. Six of the samples contaminated by L. monocytogenes were collected from catering establishments (including one of the two borderline results and the one unsatisfactory result) and four were collected at retail. There was no evidence of a significant difference in the percentages where the L. monocytogenes was present in catering compared with retail outlets (Fisher's exact test, P = 0·92). Of the six samples from catering contaminated by L. monocytogenes, four were chicken and two pork liver pâté and all were collected from public houses and produced in-house, apart from the one sample contaminated at 2·9 × 103 cfu/g which was manufactured in Belgium and was purchased within 1 day before the end of shelf life. Of the four samples from retail, all were pre-packaged pork liver pâté which were externally manufactured, three were from different Belgian manufacturers (all were obtained from supermarkets) and one was collected from a delicatessen and manufactured in France (L. monocytogenes detected at 80 cfu/g): shelf-life data were not available for any of these samples.
Amongst the nine samples contaminated at <20 cfu/g by Listeria spp. other than L. monocytogenes, five of these were collected from catering establishments (all were produced in-house and prepared in a hotel, a public house and three restaurants) and the remaining four from retail: three were from supermarkets and one was from a butcher. Amongst these nine samples contaminated with Listeria spp., three where satisfactory for all other parameters. In the remaining six samples: four had a borderline or unsatisfactory Enterobacteriaceae results, one a borderline E. coli and three unsatisfactory or borderline ACCs.
Nine of the 10 L. monocytogenes isolates were tested by molecular serotyping: four were of serogroup 1/2a, two 1/2c, and the remaining three serogroup 4 (Table 6). Using fluorescent amplified fragment length polymorphism type (fAFLP) analysis, each isolate was characterised as of a distinct type. A comparison was carried out with results from all 333 human listeriosis cases in the UK with onsets during 2011 and 2012 and where L. monocytogenes isolates were submitted for typing. Amongst four of the L. monocytogenes types from pâté, there were no isolates of the same type from any case. Of the remaining five ‘pâté types’, there were 20 cases (between 2 and 10 cases per type) where isolates were indistinguishable: no epidemiological links were identified with pâté consumption in any of these cases. Furthermore, the one type with the greatest number of cases (fAFLP types IV4·6), comprised two temporal and geographical clusters of five cases each which were consistent with two common source outbreaks associated with sandwich consumption which did not include pâté as a filling.
Table 6. Characterisation of L. monocytogenes isolates from pâté and comparison with isolates from human cases in the UK (2011–12)

* Total 333 cases of listeriosis.
DISCUSSION
Outbreaks of food poisoning in England which have been reported in the peer-reviewed literature to have been caused by the consumption of liver pâté were associated with infections due to L. monocytogenes, Salmonella and Campylobacter [Reference McLauchlin2–Reference Edwards6]. Furthermore, a review was carried out of the Public Health England (PHE) electronic Foodborne and non-Foodborne Gastrointestinal Outbreak Surveillance System (e-FOSS) database which includes a total of 2869 foodborne outbreaks and 71 507 cases reported for England between 1992 and 2014. Amongst the reports in the e-FOSS database, 49 outbreaks (814 cases, 2–59 cases per outbreak) were associated with consumption of chicken or duck liver mousse, parfait or pâté. All except 1 of the 49 outbreaks were due to Campylobacter (the remaining outbreak was due to Salmonella Typhimurium) and 48 occurred in catering institutions (universities/colleges, hotels/guest houses, pubs/bars, restaurants and other food service premises). The majority of the Campylobacter outbreaks occurred between 2009 and 2014 (PHE unpublished data).
In this study, a total of 870 liver-based pâté samples were tested as part of a continuing programme of PHE national co-ordinated food studies. This programme involves two to three studies per year and relies on the close working relationship with Environmental Health Departments throughout England to carry out the sampling as part of their routine monitoring. This approach allows sampling officers some flexibility to investigate premises and sample the product types that occur in their local food businesses, rather than being prescriptive about the numbers of each sample type to collect. This approach has been used in previously published studies [e.g. Reference Elson14, Reference Nichols, McLauchlin and de Louvois22–Reference Jørgensen26] and although the sampling strategies are not precisely defined, the study described here presents the results of more than 850 liver pâté samples collected from across all areas of England and throughout each month over an entire year (at least 21 samples were collected each month). Data on the overall consumption of liver pâté were not available in the planning stage of this study (indeed this would be difficult for the in-house prepared products), and we recognise this as a limitation of this study design. However, samples representing the range of retail and catering settings were collected and consequently the data presented here provides a useful overall picture of the microbiological quality of liver pâté products during a 12-month period in 2012–13.
Overall, 870 ready-to-eat pâté samples were tested in this study, and 73% were classified as of satisfactory microbiological quality, 18% were borderline and 9% were unsatisfactory. It is reassuring that the current major hazards associated with liver pâté and identified from surveillance of outbreaks (i.e. Salmonella spp. and Campylobacter spp.), were not isolated from any sample. In addition, for the major hazard previously identified with this food type in the 1980s (i.e. L. monocytogenes), there was only one (0·1%) sample with levels of the bacterium considered to be unsatisfactory and potentially injurious to health.
Commercially prepared pork liver pâté was associated with a large outbreak of listeriosis in the 1980s [Reference McLauchlin2] and longitudinal studies performed over the past 25 years have shown a considerable reduction in the occurrence and levels of L. monocytogenes detected in the UK [Reference McLauchlin2, Reference Elson14, Reference Nichols, McLauchlin and de Louvois22, Reference Gilbert, McLauchlin and Velani27]. Amongst the 10 samples contaminated by L. monocytogenes in this study, six were collected from catering establishments (including one of the two borderline results and the one unsatisfactory result) and four were collected at retail. The single sample collected almost at the end of its shelf life and with 2·9 × 103 cfu/g L. monocytogenes demonstrates the continued risk of contamination of the product with harmful levels of this bacterium, albeit that the overall risk is now lower compared with other types of foods. Furthermore, the four samples contaminated with L. monocytogenes and collected at retail were all manufactured in other European Union (EU) Member States and comprised pre-packaged pork liver pâté where either the bacterium has survived the cooking process or was present as a result of cross-contamination prior to packaging. This is a further concern since this product can support the growth of L. monocytogenes to levels which are unacceptable and potentially hazardous, even under ideal refrigeration and shelf-life conditions [Reference Uyttendaele28] and this includes the range of temperature and remaining shelf lives recorded in this study. The epidemiological and subtyping analysis did not allow the identification of links between consumption of pâté and cases of listeriosis. However, the present surveillance strategies now include whole genome sequencing for the characterisation of L. monocytogenes isolates and this will provide the potential for improved identification of associations between infected patients and contaminated foods as well as better recognition of foodborne outbreaks [Reference Awofisayo-Okuyelu29].
A previous report from the UK indicated that there were differences in the microbiological quality of in-house produced pâté as compared with that produced on a larger commercial scale [Reference Elson14] and this is supported by the results from this study. Mobile vendors and markets were here identified as more likely to sell foods of borderline or unsatisfactory microbiological quality and this observation is similar to that for other food types [Reference Little, Omotoye and Mitchell23, Reference McLauchlin24]. As stated above, 98% of the foodborne outbreaks associated with pâté consumption were from catering outlets. The microbiological quality of pâté sampled from supermarkets was better than those from other retailers. Furthermore, samples from retail were less likely to be contaminated with L. monocytogenes and had lower levels of Enterobacteriaceae and ACCs than those from catering, suggesting poorer hygiene at catering establishments. Neither of these observations could be explained by the types of ingredients and although the temperature control (i.e. proportion of samples stored at >8 °C) was better in supermarkets than in other retail establishments or in catering, this was not reflected in the microbiological quality which was similar in samples stored under different temperatures. It was not possible to identify all those products prepared in-house and those prepared commercially, however, products sold in supermarkets are likely to be commercially prepared in industrial settings with a high degree of control of the process. Products sold at catering, together with those from other retail establishments, are more likely to be prepared in-house and a higher proportion had short shelf lives (<6 days) with poorer microbiological quality suggesting a lower level of control of the hygiene and cooking process. This observation is consistent with the recent data from outbreaks outlined above which identified problems of safety with consumption of liver pâté at catering establishments.
Campylobacter spp., L. monocytogenes and Salmonella spp. occur in raw poultry liver prepared for human consumption [Reference Rodrigo30–Reference Harrison32] although Salmonella is likely to be less common in the UK (as well as other parts of the EU) as a result of control programs [Reference Messens33]. These three genera of bacteria will also occur in raw mammalian liver for human consumption, although less commonly than for poultry [Reference Swanenburg34]. In this study there was some evidence that pork liver-based pâtés were of better microbiological quality than those manufactured from poultry livers. Campylobacter spp., L. monocytogenes and Salmonella spp. will be eliminated by proper cooking such as should be applied to pâté [Reference Hutchison1]. However, as illustrated by data from foodborne outbreaks, undercooking is a major risk factor, particularly for Campylobacter and to a lesser extent Salmonella. Contamination of pâté post-cooking is a risk for all these three groups of pathogens [Reference Sofos35] but is of particular concern for L. monocytogenes since this hazard can multiply in liver pâté even under ideal refrigerated storage conditions [Reference Uyttendaele28]. The presence of S. aureus may also provide evidence for contamination post-cooking from food handlers, especially if there are poor practices later in the food chain [Reference Seo, Bohach, Doyle and Beuchat36]. Anaerobic and aerobic spore bearing bacteria (e.g. C. perfringens and B. cereus) are also potential contaminants from the post-cooking environment. However, since C. perfringens and B. cereus produce endospores and can be present in raw ingredients including those of non-animal origin such as herbs and spices which are commonly added to pâté prior to cooking, these groups of bacteria may survive the cooking process [Reference Sagoo25].
A previous study carried out by us in 2011 on 356 samples of ‘lightly cooked’ foods involved testing samples collected after regeneration and as would be served to a consumer [Reference Jørgensen26]. In the study of lightly cooked foods, 18% were of borderline and 12% were of unsatisfactory microbiological quality, demonstrating an overall similar quality to that reported here for pâté. However, in contrast to the pâté study, 2% (6 samples) of the lightly cooked foods were unsatisfactory and potentially injurious to health due to the presence of: Salmonella spp. (one duck breast); Campylobacter spp. (two pink duck breast and one chicken liver pâté); L. monocytogenes at 4·3 × 103 cfu/g (one duck confit with foie gras ballotine) or C. perfringens at 2·5 × 105 cfu/g (chicken liver pâté; [Reference Jørgensen26]). The results from these two studies, together with epidemiological evidence already presented from reported outbreaks, reconfirms the potential public health risks from liver pâté both from Salmonella and Campylobacter when lightly or under cooked and from L. monocytogenes when conditions allowed multiplication. A study in 2014 reviewed pâté manufacturing recipes, as well as identifying procedures likely to eliminate, or at least reduce, the presence of Campylobacter, and included washing livers with organic acids, freeze thawing and flambé in alcohol [Reference Hutchison1]. Furthermore the Food Standards Agency has provided advice including a liver pâté recipe ‘for caterers that's free from the bacteria Campylobacter’ [37]. A study in 2012–13 of chicken liver on retail sale detected Campylobacter in 87% with a level of contamination up to >10 000 cfu/g [Reference Harrison32]. At the time of writing (2016) efforts by the poultry industry have shown some reduction in the levels of Campylobacter contamination on the surface of chickens at retail [38]. However, it is not known if this reduction has also been reflected in the presence and levels of contamination in chicken livers. Furthermore a study in 2015 showed a tendency for both chefs and the general public to undercook liver [Reference Jones39] and although the highest numbers of campylobacteriosis outbreaks associated with consumption of poultry liver pâté were reported in England in 2010, these have continued to be reported each year up to 2016 (PHE unpublished data) demonstrating a continuing public health risk.
In summary, we report here results of a study on the microbiological quality of 870 liver pâté samples on sale in catering and retail premises in England during 2012–13. Seventy-three percent of samples were of satisfactory microbiological quality, 18% were borderline and 9% were unsatisfactory. Although the presence of pathogens in this study was rare, there are continued risks of contamination of this food product with levels of this bacterium which are unsatisfactory and potentially injurious to health. There is therefore a continued need to control microbiological hazards in this food type including by maintaining adequate cooking regimes and high standards of hygiene to prevent cross-contamination.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. The authors thank the staff in Environmental Health Departments throughout England and staff in the PHE Food, Water and Environmental Microbiology Laboratories for their contributions to this study. They also thank Dr Kathie Grant of the PHE Gastrointestinal Bacteria Reference Unit, for discussion on data on the typing of L. monocytogenes isolates and Lukeki Kaindama and Richard Elson from the PHE Gastrointestinal, Emerging and Zoonoses Infections for provision of e-FOSS outbreak data.
DECLARATION OF INTERESTS
None.










