Observational evidence indicates that an elevated circulating total homocysteine (tHcy) concentration is a risk factor for coronary, cerebral and peripheral vascular disease(Reference McNulty, Pentieva and Hoey1). Homocysteine may be lowered by taking B-vitamin supplements containing folate with or without vitamins B12 or B6(2). However, taking homocysteine-lowering vitamins has generally not reduced vascular events in secondary prevention trials(Reference Bonaa, Njolstad and Ueland3, Reference Toole, Malinow and Chambless4). Recently, it has been proposed that plasma S-adenosylhomocysteine (AdoHcy) might be a more sensitive indicator of cardiovascular risk than plasma tHcy(Reference Kerins, Koury and Capdevila5, Reference Wagner and Koury6). Kerins et al. (Reference Kerins, Koury and Capdevila5) reported that plasma AdoHcy, but not tHcy, was markedly higher among patients with vascular disease (40·0 nmol/l and 12·8 μmol/l, respectively, n 30) than among healthy control subjects (27·0 nmol/l and 11·0 μmol/l, respectively, n 29).
AdoHcy is the immediate precursor of homocysteine and is formed when S-adenosylmethionine (AdoMet) donates a methyl group to a variety of acceptors including DNA, a reaction catalysed by DNA methyltransferases. AdoHcy is an inhibitor of DNA methyltransferase(Reference De Cabo, Santos and Fernandez-Piqueras7). Of importance, elevated concentrations of AdoHcy have been associated with global leucocyte DNA hypomethylation in male patients with atherosclerosis(Reference Castro, Rivera and Struys8), and AdoHcy is also more strongly associated with lymphocyte global DNA methylation levels than plasma tHcy(Reference Yi, Melnyk and Pogribna9). DNA methylation influences gene expression and altered methylation has been implicated in the aetiology of several diseases including atherosclerosis. Whereas high plasma AdoHcy has been associated with unfavourable outcomes, plasma AdoMet has been positively associated with vascular function in elderly people with several CVD risk factors(Reference Spijkerman, Smulders and Kostense10). Using both metabolites, the ratio of plasma AdoMet:AdoHcy was found to be lower in patients with peripheral arterial occlusive disease compared with a control group(Reference Loehrer, Tschopl and Angst11), which prompts the importance of studies to address whether altered AdoMet:AdoHcy ratio can be normalised in individuals at risk with B-vitamin supplementation.
Elevations in plasma tHcy are usually paralleled by an increase in plasma AdoHcy(Reference Castro, Rivera and Struys8, Reference Yi, Melnyk and Pogribna9, Reference Finkelstein, Kyle and Harris12). However, in one study, plasma folate was inversely correlated with tHcy, but not with AdoHcy(Reference Becker, Smulders and Teerlink13) suggesting that plasma AdoHcy may, at least in some conditions, be independent of folate status. If elevated AdoHcy is an important risk factor contributing to atherosclerosis, but is not influenced by supplementation with B-vitamins, then B-vitamin supplementation would not be expected to lower CVD risk(Reference Wagner and Koury6, Reference Becker, Smulders and Teerlink13).
In the present study, our aim was to determine whether lowering tHcy with folate, vitamin B12 and B6 over 2 years in healthy older individuals known to have moderate hyperhomocysteinaemia alters not only plasma tHcy, but also plasma AdoHcy and AdoMet.
Subjects and methods
Participants
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by The University of Otago Human Ethics Committee. The work presented here is part of a larger study that was designed to examine the effects of B-vitamin supplementation on cognition in older people(Reference McMahon, Green and Skeaff14, Reference Green, McMahon and Skeaff15). Briefly, men and women aged 65 years or older were recruited from Dunedin and surrounds. People were ineligible to participate if they had suspected dementia, were taking vitamin supplements containing folic acid, vitamin B12 or B6, were being treated for depression, had a history of stroke or transient ischaemic attacks or had diabetes. At screening, a blood sample was taken after an overnight fast and analysed for plasma tHcy and creatinine. Participation required a tHcy concentration greater than 13 μmol/l. People with a plasma creatinine concentration exceeding 133 μmol/l (men) and 115 μmol/l (women) were excluded.
Study design
Participants meeting the inclusion criteria were stratified using the median values of age and tHcy concentration from the screening population and then randomised to treatment. The treatments were capsules containing either B-vitamins (1000 μg l-5-methyltetrahydrofolate, 500 μg cyanocobalamin and 10 mg pyridoxine) or a placebo. Capsules were supplied by Merck Eprova AG (Schaffhausen, Switzerland). Participants were instructed to take one capsule per day for 2 years. Compliance was assessed by counting returned capsules.
Blood collection and laboratory methods
A fasting blood sample was collected at screening, baseline and at 2 years. Blood was collected into evacuated tubes containing EDTA and kept on ice. Within 2 h of collection, plasma was separated from whole blood by centrifugation at 1650 g, for 15 min at 4°C. Plasma was aliquoted into cryovials and stored at − 80°C until analysed. Plasma samples were analysed for tHcy and creatinine at screening. Plasma samples were analysed for metabolites and vitamins at baseline and at 2 years. Plasma tHcy was measured using an IMx instrument and kits (Abbott Laboratories, Abbott Park, IL, USA). Folate was analysed using the microbiological method on 96-well microplates(Reference O'Broin and Kelleher16). Vitamin B12 was measured using the ADVIA® Centaur™ vitamin B12 assay (Siemens Healthcare Diagnostics Deerfield, IL, USA), and creatinine was analysed colorimetrically using Roche diagnostic kits (Roche Diagnostics, Basel, Switzerland). Genotyping for the methylenetetrahydrofolate reductase 677 C → T polymorphism was performed using methods described previously(Reference Venn, Mann and Williams17).
Plasma samples for AdoHcy and AdoMet were analysed in a subset of the 276 participants who had taken part in the cognition study. One hundred samples were analysed, which consisted of plasma from the first fifty participants randomised to each treatment group who had completed the study and for whom there was an aliquot of plasma which had undergone no freeze–thaw cycles. Samples for AdoHcy and AdoMet were shipped frozen on dry ice from New Zealand to the Metabolomics Laboratories of the Child and Family Research Institute of the University of British Columbia. Samples were analysed for AdoHcy and AdoMet using isotope dilution high-performance liquid chromatograph–tandem MS using a Quattro Micro tandem mass spectrometer configured with an electrospray source coupled to an Aquity high-performance liquid chromatography equipped with a thermostated autosampler. Internal standards were AdoHcy 13C5 prepared as described(Reference Jiang, Liang and Luo18, Reference Struys, Jansen and de Meer19) and AdoMet 2H3 (CDN Isotopes, Inc., Pointe Claire, QC, Canada). The mass spectrometer was operated in positive electrospray in selected reaction mode using the transitions of m/z 390–250 for AdoMet and m/z 390–250, 402–250 for AdoMet 2H3, and 385–136 m/z for AdoHcy and m/z 390–136 for AdoHcy 13C5. The inter- and intra-assay variations for AdoHcy were 5·0 and 4·2 %, respectively, and for AdoMet were 3·0 and 3·1 %, respectively.
In the present study, care was taken to ensure that sample aliquots for analysis of AdoMet and AdoHcy had never undergone any freeze–thaw cycle since this can lead to loss of AdoMet and an increase in AdoHcy; the error introduced is much greater for AdoHcy because of its lower concentration in plasma. Stability studies using the same plasma samples in repeat freeze–thaw cycles, keeping the thaw cycle to a minimum to enable sample aliquoting, showed that under our conditions the loss of AdoMet was 3·5, 9·8 and 18·8 % and gain in AdoHcy in the same samples was 16·6, 31·8 and 39·4 % over 1, 2 and 3 freeze–thaw cycles, respectively. While the loss in nmol/l AdoMet was similar to the nmol/l increase in AdoHcy, the fivefold lower concentration of AdoHcy introduces much greater error to AdoHcy than for AdoMet.
Statistical analysis
Plasma AdoHcy and AdoMet were not normally distributed and were transformed using the natural logarithm. Pearson correlation coefficients were used to determine associations between biochemical measures at baseline. Multiple regression analysis, adjusting for the baseline values age, sex and TT genotype for the methylenetetrahydrofolate reductase 677 C → T polymorphism, was used to estimate the differences at 2 years between the placebo and treated groups. To determine whether renal function influenced response to treatment, we divided baseline plasma creatinine concentration into quartiles. We then ran regression models for each of AdoHcy and AdoMet, which included an interaction term for quartile of plasma creatinine and treatment. Results were considered significant at P < 0·05. All analyses were undertaken using STATA 9.0 software for Macintosh (Stata Corp., College Station, TX, USA)(Reference Vickers and Altman20).
Results
Baseline characteristics of the study participants are presented in Table 1. Overall, 85 % of participants took at least 95 % of their study capsules. Associations between plasma AdoHcy and metabolite and vitamin concentrations at baseline are shown in Fig. 1. One participant with a very high AdoMet (380 nmol/l; >3 sd from the mean) was excluded from the correlation analysis. There were significant positive correlations at baseline between plasma creatinine and each of tHcy (r 0·34; P < 0·001), AdoMet (r 0·48; P < 0·001) and AdoHcy (r 0·65; P < 0·001). Plasma AdoHcy was associated with AdoMet (r 0·61; P < 0·001) and tHcy (r 0·42; P < 0·001). Neither plasma folate (r − 0·10; P = 0·34) nor plasma vitamin B12 (r 0·02; P = 0·80) was associated with AdoHcy. Plasma tHcy was not correlated with plasma folate (r − 0·19; P = 0·10) or vitamin B12 (r − 0·20; P = 0·10).
Table 1 Baseline characteristics of the participants
(Mean values and standard deviations)
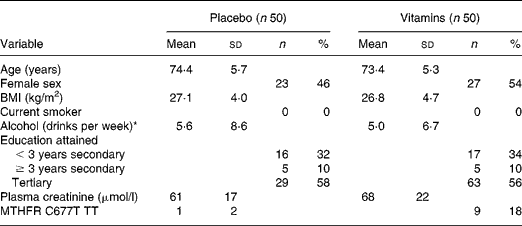
MTHFR C677T TT, methylenetetrahydrofolate reductase C677T, homozygous TT genotype.
* One drink defined as 15 ml of pure alcohol; 360 ml beer, 150 ml wine or 45 ml distilled spirits.

Fig. 1 Correlation between plasma metabolites, creatinine and folate at baseline (n 99). (a) r 0·34; P < 0·001. (b) r 0·48; P < 0·001. (c) r 0·65; P < 0·001. (d) r 0·61; P < 0·001. (e) r 0·42; P < 0·001. (f) r − 0·10; P = 0·34.
The main biochemical results of the intervention are presented in Table 2. Plasma tHcy concentration at 2 years, after adjustment for baseline values, age, sex and MTHFR C677T genotype, was 4·4 μmol/l (95 % CI 3·2, 5·6) lower in the vitamins group compared with the placebo group (P < 0·001). Plasma folate and vitamin B12 concentrations were 54·6 nmol/l (95 % CI 47·7, 61·4; P < 0·001) and 346 pmol/l (95 % CI 280, 413; P < 0·001) higher, respectively, in the vitamins group compared with the placebo group. At 2 years, there was no difference in plasma AdoHcy ( − 1 % (95 % CI − 10, 8 %); P = 0·82), AdoMet (4 % (95 % CI − 2, 11 %); P = 0·19) or the AdoMet:AdoHcy ratio between the groups (0·22 (95 % CI − 0·04, 0·49); P = 0·10). There was no significant interaction (P>0·05) between quartile of baseline plasma creatinine and treatment for either AdoMet or AdoHcy indicating that baseline plasma creatinine did not influence treatment response.
Table 2 Plasma metabolites and vitamins in older adults administered B-vitamins or placebo for 2 years

tHCY, total homocysteine; AdoMet, S-adenosylmethionine;Adohcy, S-adenosylhomocysteine.
* There were no significant differences between the placebo and vitamins groups at baseline (Student's t test).
† Difference between intervention and control groups at 2 years (multiple regression analysis with adjustment for baseline values, age, sex and MTHFR genotype).
‡ Geometric mean.
§ Ratio of geometric means of the intervention groups relative to the control groups at 2 years (multiple regression analysis with adjustment for baseline values, age, sex and MTHFR genotype).
Discussion
Although many investigators have shown that B-vitamins, particularly folic acid, lower plasma tHcy, this is the first study to examine the effects of supplements containing folate, and vitamins B12 and B6 on AdoHcy concentrations in people with moderately elevated hyperhomocysteine concentrations(2). The present study clearly demonstrates that despite a nearly 4·4 μmol/l drop in plasma tHcy in the treated group relative to placebo group, there was no effect of B-vitamin supplementation on plasma concentrations of AdoHcy or AdoMet. Altered DNA methylation has been implicated in the aetiology of atherosclerosis, and a decreased AdoMet:AdoHcy ratio has also been suggested as an indicator of diminished cellular methylating capacity(Reference Hershfield, Kredich and Koller21). B-vitamins did not significantly alter the plasma AdoMet:AdoHcy ratio in the present study, although it approached significance (+0·22; P = 0·10). However, AdoHcy may be a more relevant indicator of cellular methylation capacity than the AdoMet:AdoHcy ratio, as plasma AdoHcy levels have been shown to correlate with lymphocyte global DNA methylation levels(Reference Yi, Melnyk and Pogribna9) and AdoHcy is an inhibitor of methyltransferases.
In apparent contrast to the present findings, Stabler et al. (Reference Stabler, Allen and Dolce22) reported that high-dose oral vitamin B12 (1000 μg/d) over 3 months lowered plasma AdoHcy by about 10 % relative to baseline in older people (n 27). The effect appeared to be confined to people (n 27) with suboptimal vitamin B12 status as indicated by high-serum methylmalonic acid concentrations (>271 nmol/l). Different B12 status of participants in the two studies seems unlikely to account for the contrasting findings of B12 supplementation on AdoHcy, because mean plasma B12 concentrations in the high methylmalonic acid group in the study by Stabler et al. were similar to the mean concentration at baseline in the present study participants (312 pmol/l cf 295 pmol/l, respectively). However, the present findings are in agreement with those of Stabler et al. (Reference Stabler, Allen and Dolce22) who found that vitamin B12 supplementation, irrespective of B12 status, lowered plasma tHcy but had no effect on plasma AdoMet or on the ratio of AdoMet:AdoHcy in people without elevated methylmalonic acid.
Although B-vitamin supplementation did not alter plasma AdoHcy concentrations, plasma tHcy and plasma AdoHcy were moderately correlated in the present study (r 0·4). Correlations between plasma tHcy and plasma AdoHcy in other studies have ranged from r 0·37–0·81(Reference Kerins, Koury and Capdevila5, Reference Becker, Smulders and Teerlink13, Reference Stabler, Allen and Dolce22, Reference Jabs, Koury and Dupont23). The inability of B-vitamins to lower plasma AdoHcy despite large decreases in plasma tHcy suggests that high-dose B-vitamin supplementation does not override the factors that determine plasma AdoHcy across the usual range of plasma tHcy concentrations. Consistent with the findings of Becker et al. (Reference Becker, Smulders and Teerlink13), plasma AdoHcy was not associated with plasma folate or vitamin B12. The lack of an association in the present study between plasma tHcy and plasma folate or B12 differs from other study results(Reference Becker, Smulders and Teerlink13, Reference Stabler, Allen and Dolce22, Reference Selhub, Jacques and Wilson24) and probably reflects the fact that participants in the present study did not cover the full range of plasma tHcy concentrations, rather only the upper range ( ≥ 13 μmol/l). It is possible that this also explains the lack of cross-sectional association at baseline between plasma AdoHcy and plasma folate and plasma vitamin B12.
Despite excluding individuals with high creatinine concentrations from participating in the present study, plasma tHcy and plasma AdoHcy were strongly correlated with plasma creatinine, an indicator of renal function. This relationship is well described(Reference Stabler and Allen25). However, it is interesting to note that in the present study and in those by others, plasma creatinine is more strongly associated with plasma AdoHcy than with plasma tHcy(Reference Stabler, Allen and Dolce22, Reference Stabler and Allen25). If renal function is the primary determinant of plasma AdoHcy, this may explain why B-vitamins failed to lower plasma AdoHcy concentrations in the present study group. Plasma tHcy and plasma AdoHcy are markedly elevated in renal disease. Folic acid will lower plasma tHcy in renal patients but not to normal levels(Reference van Guldener and Robinson26). The effect of folic acid on plasma AdoHcy in renal patients is unknown.
Strengths of the present study include the long duration (2 years), high compliance and high baseline plasma tHcy concentrations that dropped substantially with B-vitamin supplementation. New Zealand does not have mandatory folic acid fortification so that any effect of folate supplementation on plasma AdoHcy should be more apparent than in a country with mandatory fortification such as the US, where blood folate concentrations are higher and plasma tHcy is lower(Reference Ganji and Kafai27). For example, in the study by Stabler et al. (Reference Stabler, Allen and Dolce22), baseline plasma tHcy was 10·7 μmol/l and serum folate was 46 nmol/l. In comparison with that study, our participants had higher tHcy (17·1 μmol/l) and lower serum folate (22 nmol/l). We acknowledge that the present study has a number of limitations, the first being that plasma AdoHcy may not reflect tissue intracellular AdoHcy concentrations. Mouse models of hyperhomocysteinaemia have increased AdoHcy and reduced AdoMet:AdoHcy ratios in liver(Reference Dayal, Bottiglieri and Arning28, Reference Devlin, Bottiglieri and Domann29); however, it is not known whether this is also accompanied by changes in plasma AdoMet/AdoHcy and AdoHcy. Furthermore, the tissue source of plasma AdoHcy is not known. Second, we screened at baseline for plasma tHcy and not for plasma AdoHcy. It is possible that individuals with higher baseline plasma AdoHcy may respond to B-vitamin supplementation. However, plasma AdoHcy is correlated with plasma tHcy and although not directly comparable because of methodological differences, it would appear that plasma AdoHcy concentrations in the present study were higher or similar to those reported in other studies of older adults(Reference Kerins, Koury and Capdevila5, Reference Becker, Smulders and Teerlink13, Reference Stabler, Allen and Dolce22). Third, based on plasma folate and vitamin B12 concentrations, there was no evidence of frank folate or vitamin B12 deficiency in our participants. It is possible that an effect of B-vitamin supplementation on plasma AdoHcy might occur in people with poorer B-vitamin status, as suggested by the study by Stabler et al. (Reference Stabler, Allen and Dolce22). Finally, we did not assess the effect of B-vitamin supplementation on either global DNA methylation or gene-specific changes in DNA methylation. Hyperhomocysteinaemia and reduced AdoMet:AdoHcy ratio and increased AdoHcy are associated with gene-specific changes in DNA methylation in the liver of mice(Reference Devlin, Bottiglieri and Domann29). Studies in human subjects have been equivocal. Although low folate status or elevated plasma tHcy has been associated with lymphocyte DNA global hypomethylation(Reference Jacob, Gretz and Taylor30) especially in those TT for the common C677T MTHFR polymorphism(Reference Friso, Choi and Girelli31), giving folic acid has not consistently altered global DNA methylation in blood cells(Reference Jacob, Gretz and Taylor30, Reference Auxume, Smith and Poribgny32–Reference Fenech, Aitken and Rinaldi35).
In conclusion, although plasma AdoHcy was correlated with plasma tHcy, B-vitamin supplementation taken over a 2-year period did not lower plasma AdoHcy concentrations in older people despite a substantial drop in plasma tHcy concentrations. If elevated plasma AdoHcy is detrimental, either through a direct effect or through disturbances in cellular methylation capacity, this may explain why B-vitamins have generally failed to reduce vascular events in secondary prevention clinical trials. Another possibility is that AdoHcy is a sensitive indicator of renal insufficiency, which itself may be a much more important determinant of vascular disease than increased tHcy concentrations due to vitamin inadequacy.
Acknowledgements
An Otago Research Grant and a Child and Family Research Institute Nutrition Research Unit Award from Michael Smith Foundation for Health Research and Bristol-Myers Squibb Foundation Unrestricted Discovery Grant funded the present project. Eprova AG (Switzerland) provided the supplements. T. J. G., S. M. I. and C. M. S. conceived of the idea of the metabolite study; T. J. G., C. M. S. and J. A. M. conceived of the design of the larger randomised controlled trial; J. A. M. recruited the participants and carried out the intervention study; S. M. I. developed the assays for AdoMet and AdoHcy and supervised their analysis for the present study; S. M. W. performed the statistical analyses; B. J. V. and A. M. D. helped to interpret the data. T. J. G. wrote the first draft of the manuscript and all authors contributed to writing the final manuscript. None of the authors had a personal or financial conflict of interest.





