Many probiotic lactic acid bacteria and Bifidobacterium with health benefits to humans, such as improvement of the intestinal environment and allergy symptoms, preventive effects on cancer and lowering of serum cholesterol levels, have been reviewed(Reference Gill and Guarner1–Reference Liong3). Recent studies have revealed that intranasal administration of Lactobacillus species might be effective in protecting against virus infection and decreasing the relevant inflammation(Reference Gabryszewski, Bachar and Dyer4, Reference Youn, Lee and Lee5). Activation of host innate immunity and/or adaptive immunity has been suggested to be important for the protective effect against virus infection(Reference Gabryszewski, Bachar and Dyer4, Reference Youn, Lee and Lee5). An orally administered Lactobacillus acidophilus strain L-92 (L-92) has an anti-allergy effect in a mouse model: it has been shown to modulate a T helper (Th) 2-skewed Th1/Th2 immunobalance by increasing the production of IL-12, known as a Th1-type cytokine, and reducing the production of IL-4, known as a Th2-type cytokine, in a mouse model(Reference Torii, Torii and Fujiwara6). The anti-allergy effects of the oral administration of L-92 have been proven in several previous human trials, including pollen(Reference Ishida, Nakamura and Kanzato7) and perennial allergies(Reference Ishida, Nakamura and Kanzato8), and atopic dermatitis(Reference Torii, Torii and Itoh9). However, immunostimulating effects, such as the antiviral and anti-pathogenic bacterial effects of L-92, have not yet been elucidated.
The recent outbreak of pandemic H1N1 influenza has been a big social problem. Influenza virus infection to the host respiratory tract mucosa leads to high mortality, and there have been many influenza pandemics, such as the 1918 influenza pandemic(Reference Tamura and Kurata10–Reference Korteweg and Gu15). Excessive production of inflammatory cytokines in the lung (cytokine storm) has often been observed in influenza infection, and this causes a severe clinical condition due to an inflammation in the lung(Reference Walsh, Teijaro and Wilker16, Reference Salomon, Hoffmann and Webster17). At present, the use of drugs is the best approach for treatment against virus infection, but there is a risk of the emergence of drug-resistant bacteria due to repeated drug use(Reference Regoes and Bonhoeffer18) or that a drug may not be sufficiently effective if they are not taken within about 48 h of infection(Reference Aokil, Macleod and Paggiaro19). Humans have encountered many pandemics caused by new types of influenza virus and tens of thousands of people have died, even though there are many antiviral drugs(Reference Taubenberger and Morens13, Reference Taubenberger and Morens14, Reference Fraser, Donnelly and Cauchemez20). We are investigating alternative treatments against virus infection. Many challenging studies have been reported in mice using various probiotic strains to investigate the protective effects against influenza virus infections(Reference Kawase, He and Kubota21–Reference Namba, Hatano and Yaeshima23). However, these probiotic effects are thought to be species and strain dependent(Reference Youn, Lee and Lee5). The anti-allergy effect of L-92 is thought to be linked to certain characteristic features in the cell-wall components(Reference Kuwana and Yamamoto24, Reference Ashida, Yanagihara and Shinoda25). In the present study, we report for the first time the potency of the probiotic L-92 against influenza virus infections. Changes in various cytokines and chemokines were analysed to understand specific L-92 defence mechanisms against virus infection using a mouse model. In addition, the antiviral effects of both live and non-live L-92 cells were compared.
Materials and methods
Mice
Female BALB/c mice (4 weeks old) were purchased from Japan SLC and allowed to acclimatise for 1 week. Mice were housed in an air-conditioned animal room at 21–27°C and 40–80 % humidity under a 12 h light–12 h dark cycle. The experiments were performed in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006). All experimental procedures were approved by the Calpis Animal Ethical Committee.
Preparation of a live and non-live Lactobacillus acidophilus strain L-92
L-92 isolated from a healthy Japanese volunteer in our stock culture collection was cultured in a medium consisting of yeast extract (Organotechnie) and casein hydrolysate for 21 h at 37°C. The cell culture was centrifuged at 7000 rpm for 15 min and the pellet cells were washed with sterile PBS. Then, the cells washed with PBS were resuspended in PBS at a concentration of 100 mg wet cells/ml (approximately 4 × 1010 cells/ml: colony-forming units/ml). The number of viable cells was monitored by counting the colonies on the de Man, Rogosa and Sharpe agar plate after the fermentation of the diluted suspension cells for 72 h at 37°C. For the preparation of non-live L-92 cells, the washed cells, prepared by the aforementioned method, were heat-killed until the temperature reached 85°C, then completed freeze-dry treatment and resuspended in PBS at a concentration of 33 mg dried cells/ml.
Experimental design
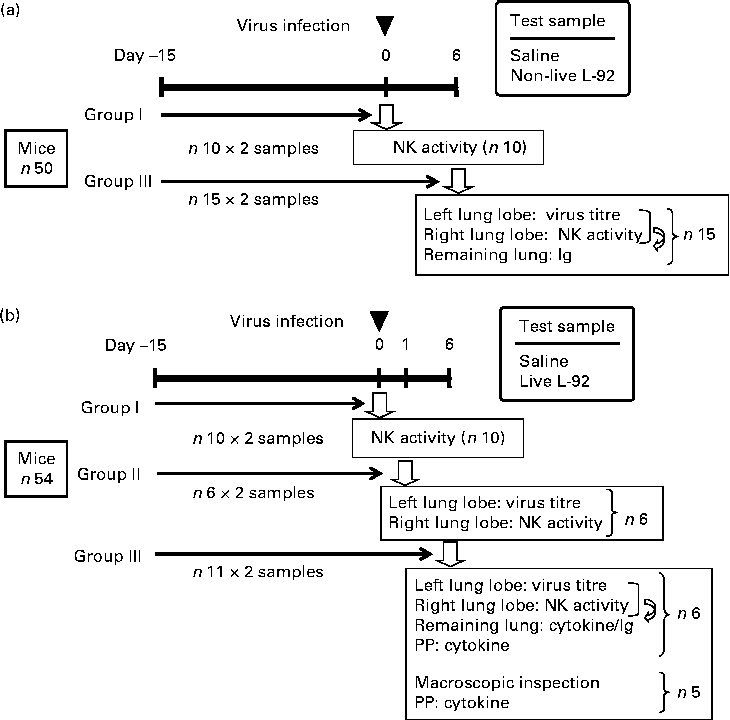
Mice were fed FR-2 (Funabashi Farms) and water was available at all times during the experimental period. As shown in Fig. 1, two sets of experiments were carried out: non-live L-92 was used in Expt I and live L-92 was used in Expt II. In both experiments, L-92 suspension, as prepared above, was administered (Expt I: doses of 300 μl of non-live L-92 suspension/d, corresponding to 10 mg dry cells/mouse per d for both experiments; Expt II: doses of 300 μl of live L-92 suspension/d) to mice by an oral zonde daily for 21 d. Mice in the control groups received doses of 300 μl of saline instead of L-92 suspension. The 1st day of sample administration (L-92 or saline) was defined as day − 15, and mice were infected with the influenza virus on day 0. In Expt I, fifty mice were divided into two groups: group I in which NK assays were performed on day 0 before the virus infection; group III in which virus titre, NK assays and Ig assays were performed on day 6, as illustrated in Fig. 1. In Expt II, fifty-four mice were divided into three groups: group I in which NK assays were performed on day 0 before the virus infection; group II in which virus titre and NK assays were performed on day 1; group III in which virus titre, NK assays, cytokine/chemokine assays, Ig assays and macroscopic inspections were performed on day 6. On day 0, mice in groups II and III were inoculated with the influenza virus. Virus titre analysis of part of the left lung lobe and NK activity measurements from part of the right lung lobe was performed on day 6 in Expt I, and on days 1 and 6 in Expt II (Fig. 1). The remaining left and right lobes were mixed and used for Ig assays on day 6 in Expt I and Expt II, and also cytokine/chemokine analysis on day 6 in Expt II.

Fig. 1 Experimental design and schedules conducted separately for non-live ((a) Expt I) and live ((b) Expt II) Lactobacillus acidophilus strain L-92 (L-92) cells. L-92 or saline was orally administered daily for the whole study period (days − 15 to 6). Mice were intranasally infected by the influenza virus on day 0 (▾) and some of the mice in the group were used for each analysis (![]() ). Details of the analytical points are listed in the text. NK, natural killer; PP, Peyer's patch.
). Details of the analytical points are listed in the text. NK, natural killer; PP, Peyer's patch.
Influenza virus and infection
Influenza A/PR/8/34 (H1N1) was prepared by Japan Biological Science, Inc., as described previously(Reference Kawase, He and Kubota21). Mice were inoculated intranasally in the nasal cavity with a 50 μl drop of influenza virus at 5 × 105 plaque-forming units (PFU)/mouse using a micropipette (Eppendorf Company, Limited).
General symptom score
General symptom scores were calculated by averaging four different health condition scores: eyelid, fur appearance, behaviour and others such as breath and body temperature, as listed in Table 1. Mice were visually inspected for these health conditions, every morning for 21 d, before sample administration, according to the standard operating procedures of Japan Biological Science, Inc. Points were given for each health condition, ranging from 1 (good) to 5 (bad).
Table 1 General symptom scores for the evaluation of health condition of mice

Virus titre
Virus titres in the lung were counted according to the method described previously(Reference Kawase, He and Kubota21). In short, on day 1 or 6, a sliced left lung was homogenised with protease inhibitor cocktail (Sigma-Aldrich), and inoculated into MDCK (Madin–Darby canine kidney) cells (106 cells/ml). Agar medium (1·5 ml) was then added and MDCK cells were incubated for 2 d at 37°C. The number of virus plaques was then counted as PFU.
Lung weight and macroscopic evaluation
Mice were killed and the thorax opened. Lungs were weighed and scored macroscopically, ranging from 1 (no consolidation) to 5 (consolidation through the left or right pulmonary lobe). Scores were averaged if two scores were applied. The consolidation score was calculated by adding the left and right lobe scores, ranging from 2 to 10.
Number of neutrophils in lung slides
The left lung was stained using the Giemsa method to count the number of neutrophils. Thereafter, five sites were counted for each lung and the number of cells at each site was averaged.
Natural killer activity in the lung
NK activity in the lung was evaluated according to a method described previously(Reference Kawase, He and Kubota21). In short, NK activity was determined using a 51Cr release assay that employed 51Cr-labelled YAC-1 cells as target cells. On day 0 before the virus infection, days 1 and 6, right lung cells were incubated with radiolabelled (51Cr) YAC-1 cells. A total of 2 × 105 lung cells were incubated with 1 × 104 target cells at an effector:target ratio of 20:1. NK activity was calculated by measuring the radioactivity released from YAC-1 cells according to the following formula:
Cytokines and chemokines in the lung and Peyer's patch
The following thirty-four different cytokines and chemokines in the lung and Peyer's patch (PP) extracts prepared from mice on day 6 in Expt I were measured using Multiplex based on an immunological method, according to the manufacturer's instructions (Millipore or Affymetrix): eotaxin; granulocyte colony-stimulating factor; granulocyte macrophage colony-stimulating factor; interferon (IFN)-α; IFN-β; IFN-γ; IL-10; IL-12 (p40); IL-12 (p70); IL-13; IL-15; IL-17; IL-1α; IL-1β; IL-2; IL-3; IL-4; IL-5; IL-6; IL-7; IL-9; inducible protein-10; keratinocyte-derived chemokine (KC); leukaemia inhibitory factor; lipopolysaccharide-induced CXC chemokine (LIX); macrophage colony-stimulating factor (M-CSF); monocyte chemoattractant protein-1; monokine induced by IFN-γ; macrophage inflammatory protein (MIP)-1α; MIP-1β; MIP-2; RANTES (regulated on activation, normal T cell expressed and secreted); TNF-α; vascular endothelial growth factor.
Virus-specific IgA and IgG
MDCK cells seeded on a ninety-six-well plate were infected by PR8 influenza and cultured for 2 d at 37°C, according to the method described by Liu et al. (Reference Liu, Hossain and Mori26) with some modification. Then, the influenza-infected cells were immobilised with methanol. The plate prepared above was washed twice with PBS before 100 μl of lung extracts from mice were added to each well and incubated for 30 min at room temperature. Then, a secondary antibody (goat anti-mouse IgA or goat anti-mouse IgG (both peroxidase labelled); Bethyl Laboratories) was added to each well of the plate after being washed with PBS. After the incubation of the plate for 3 h at room temperature, each 100 μl of PBS containing 1 % (w/v) o-phenylenediamine dihydrochloride, 4·3 % (w/v) and 3 % (v/v) H2O2 was added to each well of the washed plate, and incubated for 30 min at room temperature. After the addition of 100 μl of 10 % (v/v) H2SO4 to each well to stop the reaction, optical density at 492 nm was measured to evaluate optical density. The total amount of Ig (IgA and IgG) was quantified, according to the method described previously(Reference Liu, Hossain and Mori26).
Statistical analyses
Data are presented as means with their standard errors. Statistical analyses were calculated using Student's t test, two-way ANOVA or the Mann–Whitney test. Differences were considered significant at P <0·05 or less.
Results
The antiviral effects of live and non-live L-92 were investigated in two sets of mouse model studies (Expt I and II), as illustrated in Fig. 1. There was no significant difference in virus titres on day 6 after the infection between the control groups in Expt I (38 688 (se 3877) PFU) and in Expt II (19 330 (se 10 250) PFU). Throughout the test period, there was no significant difference in body weight or severe fatal damage (data not shown). General symptom scores were high in the control groups of both experiments throughout the test period, while the non-live group tended to have lower symptom scores compared with the control group (P <0·1), but not the live group (P =0·14) (Fig. 2). In Expt I using non-live L-92 cells, virus titres in the lung were significantly (P <0·05) lowered in the L-92 group (1·85 × 104 (se 0·44 × 104) PFU, n 15) compared with the control group (3·87 × 104 (se 0·39 × 104) PFU, n 15) on day 6 (Fig. 3(a)). NK activity in the lung was significantly (P <0·001) higher in the non-live L-92 group (4·70 (se 0·55) %, n 15) compared with the control group (0·90 (se 0·16) %, n 15) on day 6 (Fig. 3(b)). Then, in Expt II using live L-92 cells (viability over 70 %), virus titre and NK activity in the lung were monitored on day 1 to understand the quick host response after the virus infection. As a result, virus titre of the control group on day 1 (300 (se 300) PFU, n 6) increased dramatically on day 6 in the control group (1·9 × 104 (se 1·0 × 104) PFU, n 6), but not in the L-92 group (Fig. 4(a)). NK activity in the lung on day 1 was significantly (P <0·05) higher in the live L-92 group (3·30 (se 0·16) %, n 6) compared with the control group (1·70 (se 0·55) %, n 6) (Fig. 4(b)). However, interestingly, no differences in NK activities were observed between the two groups on day 6 in Expt II (Fig. 4(b)). These results suggested that mice pre-administered either live or non-live L-92 cells showed protective effects against influenza virus infection and/or proliferation.

Fig. 2 General symptom scores of mice administered (a) live (![]() ) or (b) non-live (
) or (b) non-live (![]() ) Lactobacillus acidophilus strain L-92 (L-92) cells and the saline-administered control (
) Lactobacillus acidophilus strain L-92 (L-92) cells and the saline-administered control (![]() ) group from days 0 to 6. L-92 or saline was administered daily for the whole study period (days − 15 to 6). Values are means ((a) n 11 and (b) n 15), with their standard errors represented by vertical bars. * Mean values between groups were marginally significantly different (P< 0·1; two-way ANOVA).
) group from days 0 to 6. L-92 or saline was administered daily for the whole study period (days − 15 to 6). Values are means ((a) n 11 and (b) n 15), with their standard errors represented by vertical bars. * Mean values between groups were marginally significantly different (P< 0·1; two-way ANOVA).

Fig. 3 (a) Virus titres and (b) natural killer (NK) activity in the lung of mice administered non-live Lactobacillus acidophilus strain L-92 (L-92) and the saline-administered control groups on day 6. L-92 or saline was administered daily for the whole study period (day − 15 to 6). Values are means ((a) n 15 and (b) n 15), with their standard errors represented by vertical bars. Mean value was significantly different compared with the control group: * P< 0·05, *** P< 0·001 (Student's t test). PFU, plaque-forming units.

Fig. 4 (a) Virus titres and (b) natural killer (NK) activity in the lung of mice administered live Lactobacillus acidophilus strain L-92 (L-92) and the saline-administered control groups on days 1 and 6. L-92 or saline was administered daily for the whole study period (days − 15 to 6). Values are means ((a) n 6 and (b) n 6), with their standard errors represented by vertical bars. * Mean value was significantly different compared with the control group (P <0·05; Student's t test).
There were similar host responses between the live and non-live L-92 groups in virus titres on day 6; however, host responses in NK activities in the lung of both groups were quite different (Figs. 3(b) and 4(b)). Notably, the repression of the virus titre in the live L-92 group compared with the control group was significantly greater on day 6 but not on day 1; however, NK activity was significantly activated on day 1 and not on day 6 (Fig. 4). NK activity in the non-live group was also significantly activated on day 0 before the infection (after 15 d of L-92 administration) compared with the control group (4·18 (se 0·48), n 10), but was not significantly changed in the live L-92 group (2·68 (se 0·54), n 10) compared with the control group (2·28 (se 0·08), n 10) (Fig. 5). These results suggested that live L-92 cells might have a greater (or quicker) effect in the prevention of the influenza virus infection.

Fig. 5 Natural killer (NK) activity in the lung of mice administered live or non-live Lactobacillus acidophilus strain L-92 cells or saline on day 0 before the virus infection. Values are means (n 10), with their standard errors represented by vertical bars. * Mean value was significantly different compared with the control group (P <0·05, Student's t test).
Thus, live L-92 cells were selected and used in the subsequent study for a more detailed understanding of the host response after the infection of the influenza virus. After the infection of influenza virus, inflammatory host damage in the lung is usually observed as the main host event, so the consolidation score of the lung for the live L-92-treated group was compared with that for the saline-treated control group. The live L-92 group showed a significant decrease in consolidation scores (3·00 (se 0·35), n 5, P <0·001) compared with the control group (6·00 (se 0·55), n 5) (Fig. 6(a)). In addition, the number of neutrophils in the lung was significantly (P <0·05) lowered in the L-92 group (46·7 (se 8·0), n 5) compared with the control group (80·3 (se 11·1), n 5) (Fig. 6(b)). Lung weight is known to be increased by inflammation; here it was significantly (P <0·05) lowered in the live L-92 group (171·3 (se 13·7) mg, n 5) compared with the control group (232·7 (se 26·4) mg, n 5).

Fig. 6 (a) Consolidation score and (b) the number of neutrophils in the lung of mice administered live Lactobacillus acidophilus strain L-92 (L-92) cells and saline on day 6. L-92 or saline was administered daily for the whole study period (days − 15 to 6). Values are means ((a) n 5 and (b) n 5), with their standard errors represented by vertical bars. Mean value was significantly different compared with the control group: * P <0·05, ** P <0·01 (Student's t test).
To understand the host response, the antiviral effect mechanism and anti-inflammatory events most probably linked to the virus infection: the release of various cytokines and chemokines in the lung and PP was measured in the live L-92 group on day 6. The thirty-four different cytokines and chemokines were measured, which showed that eotaxin, M-CSF, IL-1β, RANTES and IFN-α were significantly increased in the lung of the live L-92 group compared with the control group (Fig. 7(a)). Furthermore, there were significant increases in the production of IL-17, while IFN-α tended to be increased and IL-4 and IL-6 tended to be decreased in PP, 6 d after the virus infection (Fig. 7(b)). As expected from the protective anti-influenza virus effect shown in Figs. 2–4, notable increases in IFN-α, which is known to be activated in response to virus infection, were observed in the lung and PP (Fig. 7(a) and (b)). In addition, IFN-β was increased in the lung of mice from the L-92 group (310 (se 280) pg/ml, P= 0·33), although this was not significant when compared with the control group (17·4 (se 3·5) pg/ml). IL-6 was decreased in the lung in the L-92 group (4·4 (se 2·3) ng/ml, P= 0·31), although this was not significant when compared with the control group (8·9 (se 3·5) ng/ml).

Fig. 7 Cytokine and chemokine production in (a) the lung and (b) Peyer's patch (PP) on day 6 of mice administered live Lactobacillus acidophilus strain L-92 cells or saline daily for the whole study period (day − 15 to 6). The production of the thirty-four different cytokines and chemokines was evaluated. Values are means ((a) n 6 and (b) n 11), with their standard errors represented by vertical bars. Mean value was significantly different compared with the control group: * P <0·05, ** P <0·01 (Student's t test). † Mean value was marginally significantly different compared with the control group (P <0·1; Student's t test). M-CSF, macrophage colony-stimulating factor; RANTES, regulated on activation, normal T cell expressed and secreted; IFN, interferon. □, Control; ![]() , live.
, live.
IgA and IgG were measured in the lung on day 6 to understand the effect of live and non-live L-92 on adaptive immunity. A small, but significant (P <0·05) decrease in the amount of IgG was observed in the live L-92 group (0·12 (se 0·004), n 6) when compared with the control group (0·14 (se 0·005), n 6), but not in the non-live L-92 group (0·15 (se 0·005), n 15) when compared with the control group (0·14 (se 0·005), n 15). There was no significant change in IgA in the live and non-live L-92 groups compared with the control group (data not shown).
Discussion
There have been many reports on the protective effects of the probiotic Lactobacillus and Bifidobacterium strains against influenza virus infection in mice(Reference Youn, Lee and Lee5, Reference Kawase, He and Kubota21–Reference Namba, Hatano and Yaeshima23, Reference Oliveres, Díaz-Ropero and Sierra27). These effects seem to be species and strain dependent. Youn et al. (Reference Youn, Lee and Lee5) reported strain-specific clinical efficacy and differences in the effects among strains used in their clinical study. So far, there has been only one report of an antiviral effect of L. acidophilus in a human trial(Reference Leyer, Li and Mubasher28), and no detailed mechanism studies or discussions about the mode of action have been published. This is the first report of the protective effects of a L. acidophilus strain against influenza virus infection in a mouse model and the first mechanism study considering the effect. This is also the first comparative study using live and non-live L. acidophilus cells analysing the antiviral effects. L-92 is known for the immunomodulatory effect in some allergy symptoms, such as pollen(Reference Ishida, Nakamura and Kanzato7) and perennial allergies(Reference Ishida, Nakamura and Kanzato8), and atopic dermatitis(Reference Torii, Torii and Itoh9) in humans. The present study also reveals a potential immunomodulatory effect of L-92, not only for anti-allergy effects, but also for an immunostimulation effect, such as protective effects against pathogenic bacteria and virus infection in vivo.
In the present study, both live and non-live L-92 cells showed antiviral effects in separate mouse model experiments. However, host responses after the oral administration of live and non-live cells differed. NK activity before the virus infection was higher in the non-live group compared with the live group; however, virus titres in the lung 6 d after the infection were more repressed in the live group (more than 99 %) than in the non-live group (55 %) when compared with the control groups. Furthermore, NK activity in the non-live group was stimulated more than 5-fold 6 d after the virus infection when compared with the control group, but not in the live group. These results suggest that elimination of the virus from the lung, based on NK activity-dependent innate immunity, was higher in the non-live group than in the live group. However, elimination of the virus from the lung in the live group was higher than that in the non-live group. One of the possible explanations would be quick and temporary activation of NK activity by the virus infection on day 1 (Fig. 4(b)) that reaches to following thorough elimination of the virus in the live L-92 group observed on day 6 (Fig. 4(a)), and disappeared NK activation on day 6 (Fig. 4(b)). In contrast, the increase of NK activity in the non-live L-92 group appearing on day 6 may be due to a delayed host response compared with the live group, which may affect on the following elimination of the virus in the lung after day 6 (not tested). Higher preventive effects against influenza virus infection in a live bacteria-administered group compared with a heat-killed bacterial group have been reported in a previous study(Reference Youn, Lee and Lee5). With respect to the general symptom score, there seemed to be a greater improvement in the non-live group (P <0·1 v. control group, n 15) than in the live group (P =0·14 v. control group, n 11) throughout the test period (Fig. 2). The possible reason for this difference must be because of the smaller number of mice used in the experiments for the live group. The statistical calculation suggested that the general symptom score in the live group would be significantly lower than that of the control group (P <0·05) if the number of mice was increased to 15. However, a detailed comparative study on the mechanism of host responses against virus infection and immune response including NK activity and some cytokines between live and non-live, and also different administration routes, should be addressed in the future.
In the present study, among the measured cytokines and chemokines, eotaxin, M-CSF, IL-1β, RANTES and IFN-α were significantly increased in the lung of the live L-92 group compared with those in the control group. In a previous study, influenza infection of C57BL/6J mice induced chemokine gene expression for monocyte chemoattractants, monocyte chemotactic protein-1, MIP-1α, MIP-1β, RANTES and IFN-inducible protein 10(Reference Wareing, Lyon and Lu29). RANTES was considered to be important for virus infection because knockout ( − / − ) mice did not demonstrate increased susceptibility to influenza infection(Reference Wareing, Lyon and Lu29). M-CSF enhancement has been suggested to be important for early host resistance to influenza virus(Reference Sever-Chroneos, Murthy and Davis30). A potent eosinophil chemoattractant, eotaxin, well known to be induced after influenza virus infection in nasal epithelial cells(Reference Kawaguchi, Kokubu and Kuga31), was significantly induced in the present study. At 6 d after the virus infection, there was a significant increase in the production of IL-17, IFN-α tended to be increased and IL-4 and IL-6 were decreased in PP. Influenza virus infection was accompanied by IL-1β and IFN-α production in the bronchoalveolar lavage of mice(Reference Hennet, Zilterner and Frei32). The production of antiviral cytokines, such as IFN-α and -β which have been reported to induce NK activity(Reference Biron33–Reference Durbin, Fernandez-Sesma and Lee35), was measured 6 d after the virus infection in the present experiment. The antiviral cytokine IFN-α was significantly increased in both lung and PP compared with the control group. IFN-β in the L-92 group was present at a higher value than that in the control group (P =0·33). In addition, other cytokines (IL-2 and IL-15), which are also known to induce NK activity, were not significantly different, but their average values were both higher in the L-92 group compared with the control group. The enhancement of NK activity in the lung may be caused by stimulating various antiviral cytokines after the oral administration of L-92 cells. An evaluation of various cytokines and chemokines before the virus infection would be of interest in considering the host response against probiotic challenge with or without virus infection.
Mohamadzadeh et al. (Reference Mohamadzadeh, Duong and Hoover36) reported that surface components of a Lactobacillus strain were the most important factor in the intake of active cells via M cells in the gastrointestinal tract and the activation of dendritic cells to induce a host immunomodulation effect. Perdigón et al. (Reference Perdigón, Fuller and Raya37) also reported that several kinds of surface components in lactic acid bacteria, such as surface layer protein (Slp), lipoteichoic acid and peptideglycan, are involved in the interaction with host cells. In addition, the importance of SlpA in L. acidophilus NCFM in association of the cell with immature dendritic cells, which is essential for immunomodulatory action, has been suggested(Reference Konstantinov, Smidt and de Vos38). A recent study has reported on the potential of L. acidophilus ATCC 4365 to inhibit Junin virus entry using Slp on L. acidophilus to bind to dendritic cell-specific intracellular adhesion molecule (ICAM)-3-grabbing nonintegrin that inhibited the entry of the virus(Reference Martínez, Acosta and Candurra39). An inhibitory effect of Slp on the adhesion and entry of several virus families has also been suggested(Reference Martínez, Acosta and Candurra39). In our previous study, probiotic L-92-enriched GroEL and GroES have been considered to have the potential to affect immunomodulation in mouse splenocytes(Reference Kuwana and Yamamoto24). Moreover, a comparison of L. acidophilus strains showed that L-92 expressed higher amounts of cell-wall Slp and produced greater amounts of the Th1-type cytokine IL-12 after incubation with splenocytes(Reference Ashida, Yanagihara and Shinoda25). These reports have suggested that L-92 has the potential to protect against virus infection via Slp binding to DC-specific ICAM-3-grabbing nonintegrin on epithelial cells. There may also be structural changes in the cell wall and membrane components of live and non-live cells after heat treatment, leading to changes in host responses in the gastrointestinal immune system. To further understand the antiviral effect of L-92 cells, clarification of the key active components and the differences between live and non-live cells should be addressed.
In the present study, adaptive immunity was not thought to be involved because there were no significant increases in IgA and IgG in the live L-92 group. IgA in the lung 6 d after the infection in the L-92 group was not significantly changed compared with the control group. IgG in the live L-92 group was slightly, but significantly, lower compared with the control group. The lower adaptive immune responses are thought to be due to the delayed production of IgG and IgA after the virus infection (about 1 week after the infection). Generally, lactobacilli have been reported to induce adaptive immunity by increasing Ig(Reference Wells40); however, in the present study, they were not increased in the live L-92 group. We suggest that pretreatment of L-92 cells activates innate immunity so effectively that it causes immediate elimination of virus infection, or the mode of L-92 action differs from other lactobacilli.
A number of studies in animals have demonstrated the potential of using probiotics as immune adjuvants. Lactobacillus species and other probiotics stimulate both the cellular and innate immune systems(Reference MacDonald and Bell41). Clinical trials have also supported the potential of Lactobacillus in the prevention of influenza infection(Reference Davidson, Fiorino and Snydman22, Reference Oliveres, Díaz-Ropero and Sierra27). In a clinical trial on thirty-nine subjects, Lactobacillus GG showed a promising immunoadjuvant effect against the vaccine H1N1 and B strain(Reference Harata, He and Hiruta42). In contrast, a large clinical study has shown no significant protective effects against respiratory symptoms when volunteers received daily doses of Lactobacillus casei Shirota for 176 d(Reference Puyenbroeck, Hens and Coenen43). It may be necessary to conduct a clinical study using the L-92 strain to investigate the potential protective effects against virus infection.
In conclusion, a protective effect against the nasal infection of influenza virus in both live and non-live L-92 cells showed potential in reducing the risk of the virus infection, and may be extended to other types of virus. The antiviral effect was considered to be caused by the early activation of NK activity in the lung caused by stimulating various antiviral cytokines and chemokines during the infection period.
Acknowledgements
The authors thank A. Morikawa and H. Tsunoda (Nihon Bioresearch, Inc.) for technical assistance throughout the two animal studies. H. G. and N. Y. analysed the data, wrote and revised the paper; A. S., N. A., T. H. and T. S. conceived the design, performed the experiments and acquired the data; S. K. performed the experiments. There was no funding for the present study. The authors have no conflicts of interest to declare.