Short Overview of the DTR
With more than 85,000 twin pairs, the Danish Twin Registry (DTR) is one of the largest registers containing multiple births, and also the oldest nation-wide twin register in the world. The registry has grown from initially including twin cohorts from 1870 to 1910 to now covering all birth cohorts of twins since 1870 (Christensen et al., Reference Christensen, Kyvik, Holm and Skytthe2011; Harvald & Hauge, Reference Harvald and Hauge1999; Harvald et al., Reference Harvald, Hauge, Kyvik, Christensen, Skytthe and Holm2004; Hauge, Reference Hauge, Mednick, Baert and Bachmann1981; Kyvik et al., Reference Kyvik, Christensen, Skytthe, Harvald and Holm1996; Skytthe et al., Reference Skytthe, Kyvik, Holm, Vaupel and Christensen2002, Reference Skytthe, Kyvik, Holm and Christensen2011).
Twins have been ascertained using four different methods depending on the birth cohorts. Despite the different methods employed, the ascertainment always included at least two steps: identification of the twins, that is, date of birth and names of the twins; and tracing of the twins, that is, their vital status and addresses, if alive. A detailed description of the methods employed can be found in (Skytthe et al., Reference Skytthe, Kyvik, Holm, Vaupel and Christensen2002, Reference Skytthe, Kyvik, Holm and Christensen2011).
Zygosity of a twin pair is key in most twin studies. In the majority of birth cohorts, zygosity assessment has been based on the questionnaire method that uses four questions on similarity to determine zygosity among same-sex twin pairs. This method classifies the zygosity correctly in more than 95% of same-sex twin pairs (Christiansen et al., Reference Christiansen, Frederiksen, Schousboe, Skytthe, von Wurmb-Schwark, Christensen and Kyvik2003). During the past decade, zygosity assessment using genetic markers has been increasingly used, especially in smaller studies with detailed phenotyping, and also as a result of the decreasing cost for genetic testing
By January 1, 2012, the DTR comprised 86,398 twin pairs born from 1870 to 2009, with a total of 105,000 twin individuals alive. The DTR has complete coverage of all twin births (including still births) since 1973, comprising a total of 35,205 twin pairs. Information about zygosity is available for 75.3% of all twin pairs; missing zygosity information is due partly to death of twins before zygosity could be assessed (before age 6 years), and partly to missing response to questionnaires. Data on the twins have been collected mainly through a number of large questionnaire surveys, longitudinal interview surveys, and a large number of clinical investigations of different subgroups of the twin cohorts. In addition, data on health have been collected by linkage to national registers of cancer, hospitalization, birth characteristics, and mortality using the unique personal identification number for every Danish resident.
Combining Twin Questionnaire and Survey Data With National Registers
Over the past 60 years, Danish twin research has been based on questionnaires, surveys, and clinical investigations and over more recent decades also on register research. Previously, these approaches were run in parallel, but in the new millennium we have merged data from questionnaires, surveys, and clinical investigations with data from Statistics Denmark, which has provided additional leverage and research opportunities.
Among the first uses have been studies of twin-singleton differences, that is, the ‘long-term prognosis’ of being a twin compared with being a singleton. In a study based on a linkage with the DTR to Statistics Denmark data regarding test scores in 9th grade in the Students Register, we demonstrated that contemporary Danish adolescent twins have school test results similar to singletons (Christensen et al., Reference Christensen, Petersen, Skytthe, Herskind, McGue and Bingley2006). Moreover, a recent study of diabetes prevalence over a decade in 77,000 twins and 215,000 controls, based on data from the DTR and three nation-wide Danish health registers, showed similar diabetes prevalence in twins and singletons and no MZ–DZ differences (Petersen et al., Reference Petersen, Nielsen, Beck-Nielsen and Christensen2011b). Finally, in a sample of 36,000 twins and 92,000 singletons, a study comparing the waiting time to first marriage as well as the waiting time to divorce from first marriage indicated that twins had slightly lower marriage and divorce rates compared with singletons, and that the results were similar for DZ and MZ twins (Petersen et al., Reference Petersen, Martinussen, McGue, Bingley and Christensen2011a).
The merging of questionnaire/survey data with Statistics Denmark has also enabled large-scale co-twin control studies that controlled for the effect of rearing environment and genetic factors in studies of the association between exposures and health. A recent study on the relationship between social class and health yielded results that were mostly compatible with an effect of early family environment in explaining the Danish educational inequalities in mortality (Madsen et al., Reference Madsen, Andersen, Christensen, Andersen and Osler2010). Furthermore, the linkage between survey data and register data has enabled validation studies of data obtained through questionnaires. In a study from 2009, self-reported use of medication in twins was examined. The self-reported medication use was compared with information on prescription medication, which has been registered in the Register of Medicinal Products Statistics since 1994. This comparison revealed an under-reporting of the number of used medications that was independent of age and sex, but positively linearly dependent on the registered number of medications (Oksuzyan et al., Reference Oksuzyan, Petersen, Stovring, Bingley, Vaupel and Christensen2009).
The Infrastructure Initiative 2008–2011
The Infrastructure initiative 2008–2011 is the umbrella project for a number of subprojects that together comprise the assessment of 14,000+ twins born 1931–1969, including sampling of biological material. The most notable are: the Middle Age Danish Twin (MADT) study follow-up and the MIddle age Danish Twin (MIDT) study.
The Middle Age Danish Twin (MADT) Study Follow-Up
Most longitudinal twin studies have focused on either the early or the late stages of life. Midlife, a developmental period that has drawn increasing attention as setting the stage for functioning throughout the entire second half of the lifespan (Lachman, Reference Lachman2001), has been relatively neglected by twin researchers. The MADT study was initiated in order to investigate how health, lifestyle, and functioning at midlife contribute to differences in late-life health and mortality. The original MADT sampling framework targeted 240 twins from 120 intact twin pairs (i.e., both members alive and living in Denmark) randomly selected from all available twin pairs from 22 consecutive birth years (1931 through 1952). The 120 twin pairs from each birth year consisted of 20 pairs each of monozygotic males (MZM), monozygotic females (MZF), like-sex dizygotic males (DZM), and like-sex dizygotic females (DZF), and 40 pairs of opposite-sex dizygotic pairs (OSDZ). Of the 5,280 individual twins in the sampling framework, 90 individuals died prior to the time the survey was undertaken, and 4,314 (83%) of the 5,190 surviving twins completed an in-person intake assessment, usually in the twin's home, in late 1998 or early 1999. The intake sample included 1,884 twin pairs where both members participated including 664 MZ, 603 same-sex DZ (SSDZ), and 617 OSDZ and ranged in age from 46 to 68 years, with an average of 56.9.
Beginning in 2008 and ending in 2011, the Infrastructure initiative at the DTR provided an opportunity to complete a follow-up assessment of the MADT twins. Follow-up assessment involved three tasks: (1) completion of a questionnaire survey consisting of approximately 100 questions, (2) completion of an in-person interview and examination at one of five assessment centers located throughout Denmark, and (3) provision of a blood sample that could be used as a source for DNA analysis and other biological assays. A total of 2,402 MADT twins completed at least one of these three stages, with the vast majority of participating twins (N = 2,298) completing all three stages. A total of 2,387 twins completed the questionnaire, 2,393 completed the in-person examination, and 2,314 provided a biological sample. In total, 816 intact twin pairs, including 308 MZ, 250 SSDZ, and 258 OSDZ participated in the follow-up. The follow-up sample ranged in age from 56 to 80 years, with an average of 66.7.
Table 1 provides a summary of the major assessment domains at the two waves and documents the substantial overlap that exists between these two assessments. The MADT assessment of cognitive functioning essentially duplicates the assessment used in our parallel study of older twins, the Longitudinal Study of Aging Danish Twins (LSADT; McGue & Christensen, Reference McGue and Christensen2007), with the exception that we did not administer the Mini Mental State Examination in MADT because the examination is designed to identify cognitive impairment in samples older than the MADT. The MADT assessments of health, depression, and physical functioning also overlap with LSADT and consequently provide an opportunity to investigate twin resemblance comprehensively throughout the second half of life. One unique feature of the MADT follow-up was inclusion of a 20-item activity self-report, which provides an opportunity to investigate how physical, social, and intellectual engagement is associated with important life outcomes using a twin study design (McGue et al., Reference McGue, Osler and Christensen2010). In addition, for male participants in MADT born after 1939, conscript records that include an assessment of physical, medical, and intellectual functioning when they were approximately 19 years old are currently being identified for inclusion in the MADT database.
TABLE 1 Overview of Major MADT Assessment Domains at Intake and Follow-Up

BMI: body mass index.
Table 2 gives a summary of the follow-up status of the 4,314 twins that completed an intake MADT assessment. Nearly 10% of the sample had died in the interval between the intake and follow-up assessments. Although 55.7% of the original 4,314 MADT twins participated at follow-up, a better estimate of the participation rate at follow-up is given by the number of follow-up participants divided by the number of surviving intake participants, or 61.8%. The rate of follow-up participation is nonetheless substantially lower than the participation rate at intake (83%), no doubt a consequence of a difference in assessment burden. Intake assessments were arranged to take place at a location convenient for the twin, usually her or his place of residence. Follow-up in-person assessments had to take place in a clinic and so required the twin to travel. As expected, those who were deceased by the time of the follow-up assessment were on average older at intake than those who were alive at follow-up. Somewhat less expected is the observation that rate of follow-up participation was greater among men than women.
TABLE 2 Characteristics of the MADT Follow-Up Sample
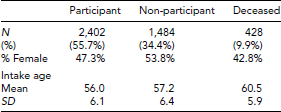
In order to further investigate whether attrition has contributed to bias in the follow-up sample, we compared participants, non-participants, and those who were deceased on the following intake measures: (1) a composite of the cognitive ability measures; (2) body mass index (BMI); (3) grip strength; (4) subjective assessment of health; (5) depression symptomatology; and (6) level of physical activity. To facilitate comparisons among groups, we transformed all six of these variables to have a mean of 50 and a standard deviation (SD) of 10 in the total MADT intake sample. All variables have been adjusted for age and sex effects. Figure 1 gives the comparison of the three groups on these six variables. There was a significant group effect for all variables except BMI. However, for all of these variables the differences were much larger between the participant and deceased groups than between the participants and non-participants. The differences between the participant and deceased groups were generally moderate in magnitude, on the order of 0.4–0.7 SDs, except for grip strength where it was only 0.1 SD. In contrast, the differences between the participants and non-participants were all modest, in no case exceeding 3 SDs. These data on attrition suggest that even though the rate of participation was lower at follow-up than at intake, the low rate of participation did not result in significant bias in the follow-up MADT sample.
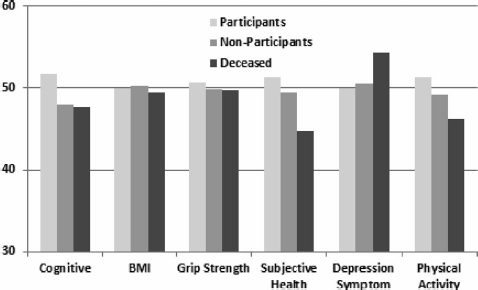
FIGURE 1 Comparison of MADT follow-up participants (N = 2,402), non-participants (N = 1,484), and deceased (N = 428) on several key intake measures. To facilitate comparison, all measures were adjusted for age and sex effects and then scaled to have a mean of 50 and a standard deviation of 10 in the total MADT intake sample. The group effect is significant for all measures except BMI. However, the magnitude of the difference between participants and non-participants is uniformly small, suggesting little bias due to attrition.
The MIddle age Danish Twin (MIDT) Study
The MIDT study comprises twins from the birth cohorts 1931–1969 who are not included in the MADT study or other substudies of the Infrastructure initiative. The MADT and MIDT studies share the assessment instrument including tests and biological samples, thus enlarging the size of the available study population.
The MIDT study was conducted in parallel with the MADT study from mid-2008 to the end of 2011. The total number of eligible twins for the MIDT study at the beginning of 2008 was 40,723 twin individuals, of whom 26,075 were invited during the study period. Priority was given to twins from the oldest cohorts, twins whose co-twin was alive, and twins who had previously shown willingness to participate in research projects.
A total of 10,281 MIDT twins completed at least one of the three stages: completion of a questionnaire, completion of in-person interview, and provision of a blood sample, again with the vast majority of participating twins (N = 10,006) completing all three stages. The MIDT sample comprised 3,018 twin pairs with both twins participating, including 707 MZ, 1,132 SSDZ, and 1,080 OSDZ. A higher proportion of women than men (53.5% vs. 46.5%) participated, and among the 1,839 same-sex twin pairs with both twins participating, the proportion of female twin pairs was 60.8% among MZ and 57.1% among DZ. The age of participants ranged between 40 and 80 years with an average of 56.6 years.
The participation rate in the MIDT study was lower than in the MADT follow-up study: about 40% of invited twins participated. The lower participation rate can be attributed to a number of factors: the participants being required to travel to one of the examination centers; the invited twins being younger, meaning that a higher proportion were still in their active work life, and the majority of the younger cohorts not having been approached as often as the participants in the MADT follow-up study.
The Danish Twin Registry Biobank
The biobank connected to the DTR was established in 1997 with the collection of biological material from twin members of intact pairs (Bathum et al., Reference Bathum, Fagnani, Christiansen and Christensen2004) as a part of the second wave of the LSADT survey (McGue & Christensen, Reference McGue and Christensen2007). The purpose of the DTR biobank was to expand the potential of the epidemiologic research in late-life health and mortality to also include the exploration of contributing genetic and biochemical factors. This extension has allowed for more comprehensive studies of complex diseases and phenotypes and is expected to facilitate the identification of predisposing genes and epigenetic factors and to provide support for a better understanding of disease etiology, including gene–gene and gene–environment interactions.
Presently, the DTR biobank has grown to now comprise more than 51,000 sample specimens from approximately 20,000 twins, as well as specimens from participants from surveyed cohorts of Danes living to exceptional ages, altogether constituting a valuable resource for a variety of national and international research.
Tables 3 and 4 provide a summary of sample types drawn within each of the twin studies conducted over the period 1997–2011. Following the initial sampling of full blood for storage of leukocyte DNA and plasma from the LSADT-1997 participants, collection of biological material from all non-proxy participants was added as a regular component in all follow-up surveys of LSADT, as well as other follow-up studies with recurring assessment, and in most of the subsequent twin studies.
TABLE 3 Overview of Material Collected Within Population-Based Twin Studies
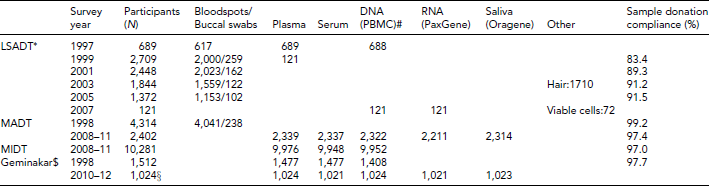
LSADT: Longitudinal Study of Aging Danish Twins; MADT: Middle Age Danish Twin; MIDT: MIddle age Danish Twin; *participants overlap partially between LSADT waves; #PBMC = peripheral blood mononuclear cells. $Plasma collected using various anticoagulants; §ongoing collection.
TABLE 4 Overview of Ancillary Twin Studies With Inclusion of Biological Material

BMI: body mass index; *CLP: cleft lip and palate; #COL: chronic obstructive lung disease.
Particularly in the first half of the DTR biobank life, the majority of the twin studies were larger nation-wide interview surveys typically conducted in the participant's own home (Christensen et al., Reference Christensen, Holm, McGue, Corder and Vaupel1999; Gaist et al., Reference Gaist, Bathum, Skytthe, Jensen, McGue, Vaupel and Christensen2000). Based on financial and logistic considerations, the biological specimens collected within these surveys were thus primarily in the form of dried blood spots, sampled by means of self-administered fingerprick blood stored on filter cards (Guthrie cards). If participants were reluctant to provide a blood sample, buccal swab samples were collected as an alternative DNA source. The actual quantity and quality of the DNA that can be extracted from the dried blood spots is highly variable, but has for the most part been adequate to allow for a minimum of several hundreds of genotypings. Newly employed whole genome amplification techniques have further enabled a successful increase in the amount of obtainable DNA from collected blood spots, thus securing continual use of these valuable samples.
While long-term archiving of material for extraction of DNA was considered of main importance in the initial phases of the DTR biobanking period, there has been an increasing request for material suitable for other biological assays over the years, and the range of material collected for the DTR has broadened accordingly in more recent surveys.
As outlined in Tables 3 and 4, multiple types of samples have been introduced in recent studies, such as serum, plasma, saliva (in the form of Oragene tubes), PaxGene sampling (for later RNA extraction), urine, and viable cells. Additionally, storage of aliquots of high molecular, high-quality DNA has become of increasing importance. In part, this expansion is a result of the change toward conducting large high throughput studies, such as gene expression profiling and genome-wide and epigenome-wide association studies, thus encouraging the accommodation of the future needs of such studies. Furthermore, some substudies conducted within the DTR are dedicated to a more thorough investigation of specific medical conditions, such as twins with rheumatoid arthritis (Svendsen et al., Reference Svendsen, Hjelmborg, Wiik, Houen, Kyvik and Junker2011), or specific research questions, such as the GEMINAKAR study (Schousboe et al., Reference Schousboe, Visscher, Henriksen, Hopper, Sorensen and Kyvik2003) and the study of birth weight discordant twins (Frost et al., Reference Frost, Petersen, Brixen, Beck-Nielsen, Holst, Christiansen and Christensen2012a), both described in more detail below. Such studies may call for specific samples. Thus, a strength of the DTR is the possibility of using the entire register to screen for the most informative twin pairs for a given condition or phenotype. This strategy has been applied in a number of ancillary studies, in which sampling of biological material was determined by the research questions of the particular study.
An issue of possible concern is the participation rate, not only for the surveys that are meant to be representative of the source population, but also for the compliance in providing a biological sample. While phlebotomy was a requirement for participation in the majority of the ancillary studies (see Table 4), this was not the case for the population-based studies like LSADT and MADT. Table 3 gives the blood sampling participation rate in these studies. In general, the willingness to donate blood has been high, ranging from 84% to 99%, with a slight tendency toward a decrease with age. As approximately half of the non-donors were proxy interviewed participants, who were not asked to donate blood due to cognitive impairment to some extent, this implies that including a collection of biological material in surveys is generally accepted by the twins and does not lead to substantially lower participation rates (Christensen et al., Reference Christensen, Bathum, Christiansen, Weinstein, Vaupel and Wachter2008).
Examples of Intensively Studied Twin Cohorts
The Importance of Genes, Familiar and Common Environment for the Development of Insulin Resistance, Abdominal Adiposity, and Cardiovascular Risk Factors (GEMINAKAR)
The GEMINAKAR study is a longitudinal, nation-wide Danish research study investigating the genetic epidemiology of a wide variety of phenotypes among Danish twins, including endophenotypes related to what has been called the metabolic syndrome, that is, diabetes or insulin resistance, obesity, and a number of cardiovascular disease risk factors. The twins were recruited from two cohorts of the twin registry. Cohort I covers the birth cohorts 1931–1952, while cohort II covers the birth cohorts 1953–1982. Requests to participate in a full day, intensive clinical investigation were sent to 2,585 randomly chosen twin pairs who fulfilled the criteria that at least one twin should live within 100 km from one of the two investigation sites (Odense and Copenhagen) and the pair should not participate in other studies at the same time. Cohort II was furthermore chosen based on previous self-report of being healthy. The letter of informed consent contained detailed information about the study and its exclusion criteria (i.e., known diabetes or cardiovascular disease, conditions making a progressive maximal bicycle test impossible, pregnancy, and breast feeding). A reply coupon was enclosed for the twins to give information about their present health status and to either consent or decline telephone contact. If one twin in a pair did not respond or was not willing to participate, the pair as such was excluded. In all, 1,098 complete twin pairs (42.5%) were both willing and able to participate. A stratified sample of 756 twin pairs underwent an extensive full day clinical examination of a variety of phenotypes. The main focus was on phenotypes related to insulin resistance, obesity, and cardiovascular risk factors. The sample included 311 MZ, 314 SSDZ, and 131 OSDZ twin pairs with a mean age of 38 years (range 18–67 years old).
The examinations were conducted from 1997 to 2000. The twins in a pair were examined on the same day. DNA-based microsatellite markers with the PE Applied Biosystems AmpFISTR Profiler Plus Kit were used to determine zygosity of these twins. The participating twins filled in questionnaires on health and health-related behavior and underwent a detailed clinical investigation, including sampling of biological material for biobanking.
GEMINAKAR has so far resulted in more than 10 theses and approximately 70 papers, but these will be too extensive to review here. In short, with regard to the core phenotypes of interest, that is, those related to glucose and insulin metabolism, anthropometry, and cardiovascular risk factors, it was found that especially anthropometric measures were highly heritable, whereas the other phenotypes were moderately heritable. Bivariate and multivariate analyses did not find substantial evidence for common genetic factors underlying these diverse phenotypes, despite the clustering of these in many patients (Benyamin et al., Reference Benyamin, Sorensen, Schousboe, Fenger, Visscher and Kyvik2007; Fenger et al., Reference Fenger, Benyamin, Schousboe, Sorensen and Kyvik2007; Hasselbalch et al., Reference Hasselbalch, Benyamin, Visscher, Heitmann, Kyvik and Sorensen2008; Schousboe et al., Reference Schousboe, Visscher, Henriksen, Hopper, Sorensen and Kyvik2003, Reference Schousboe, Visscher, Erbas, Kyvik, Hopper, Henriksen and Sørensen2004). The DTR participated in the European twin study GenomEUtwin with the data from GEMINAKAR (Kettunen et al., Reference Kettunen, Perola, Martin, Cornes, Wilson, Montgomery and Peltonen2009).
Since 2010, the second wave of GEMINAKAR has been ongoing. This time we have equipped a mobile home as a mobile examination unit, and we offer to visit the twins in their home, at work or any other place they might choose. So far over 80% have chosen to participate again. The core clinical examination is the same as in 1997–2000, but has been expanded with questions on sleep duration and quality as well as working conditions in order to study the relationship between sleep, work, insulin, and cardiovascular risk factors. The investigation of the twins will be completed in autumn 2012.
The Study of Extremely Birth Weight Discordant Monozygotic Twins
The extensive collection of information on twins in the DTR has provided an excellent opportunity to investigate the effects of a broad spectrum of exposures on disease development by use of several different study designs. Recently, the discordant twin design was applied in order to explore the effects of early life factors on adult health by use of a large sample of highly informative twins recruited from the DTR.
Numerous studies have reported inverse association between early life factors; for example, birth weight, a proxy for intrauterine conditions, and adverse health outcomes in adulthood. These associations may be explained by several factors, including fetal programming, genetics, and confounding from shared environment. The discordant twin design was used to study the association between birth weight and adult health, controlling for confounding from genetic effect and shared environment. It was hypothesized that if fetal growth has an independent effect on adult metabolism, then glucose levels and thyroid function would diverge in adult MZ twins who were extremely weight discordant at birth.
Based on information on birth weight retrieved from the Danish Birth Record Registry or midwife records as well as zygosity, the most birth weight discordant MZ twin pairs in the DTR were identified, and a total of 158 twin pairs participated in a clinical study comprising comprehensive testing of glucose metabolism (Frost et al., Reference Frost, Petersen, Brixen, Beck-Nielsen, Holst, Christiansen and Christensen2010a) and assessment of thyroid function (Frost et al., Reference Frost, Petersen, Hegedus, Christiansen, Heiberg and Christensen2012b). Contrary to what could have been expected from a fetal origins hypothesis, neither glucose metabolism nor thyroid function differed between birth weight discordant monozygotic twins. Therefore, early life factors as assessed by birth weight in these MZ twins had no impact on adult metabolism once genetic and environmental factors were controlled for (Frost et al., Reference Frost, Petersen, Brixen, Beck-Nielsen, Holst, Christiansen and Christensen2012a, Reference Frost, Petersen, Hegedus, Christiansen, Heiberg and Christensen2012b).
Conclusion
The new developments in the DTR in the twenty-first century have facilitated ongoing research and laid the groundwork for new research directions. The linking of twin survey data with national demographic, social, and health registers in Statistics Denmark provides opportunities for large-scale studies with minimal selection bias. The in-person assessments of 14,000+ twins born 1931–1969 and sampling of biological material have been the basis for a number of subprojects and have expanded and consolidated the DTR biobank. As the research field of genetic epidemiology continues to develop, the growing number of various bio-specimens kept in the DTR biobank will likely be a central feature of future studies within the settings of the DTR.
Acknowledgment
The DTR is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology and Innovation, the Velux Foundation, and the US National Institutes of Health (P01 AG08761).







