The history of the American West is deeply intertwined with the domestic horse. The genus Equus first evolved in the Pliocene (Blancan) steppes of the Americas, radiating outward into Eurasia and Africa sometime during the last approximately three million years (Orlando et al. Reference Orlando, Ginolhac, Zhang, Froese, Albrechtsen, Mathias Stiller, Cappellini, Petersen, Moltke, Johnson, Fumagalli, Vilstrup, Raghavan, Korneliussen, Malaspinas, Vogt, Szklarczyk, Kelstrup, Vinther, Dolocan, Stenderup, Velazquez, Cahill, Rasmussen, Wang, Min, Zazula, Seguin-Orlando, Mortensen, Magnussen, Thompson, Weinstock, Gregersen, Røed, Eisenmann, Rubin, Miller, Antczak, Bertelsen, Brunak, Al-Rasheid, Ryder, Andersson, Mundy, Krogh, Thomas P, Gilbert, Sicheritz-Ponten, Jensen, Olsen, Hofreiter, Nielsen, Shapiro, Wang and Willerslev2013). The ecological landscape of North America evolved with these wild equids, and the first people to move into the continent had a relationship with horses—both as prey species (Bourgeon et al. Reference Bourgeon, Burke and Higham2017; Kooyman Reference Kooyman, Hills, McNeil and Tolman2006) and as a source of raw material for bone tools (Webb and Hemmings Reference Webb, Hemmings, Olsen, Grant, Choyke and Bartosiewicz2006)—along with other megafauna up to the end of the Pleistocene. Current radiocarbon dates of fossil equids in North American place their disappearance about approximately 10,000 years ago (Haile et al. Reference Haile, Froese, MacPhee, Roberts, Arnold, Reyes, Rasmussen, Nielsen, Brook, Robinson, Demuro, Thomas P, Gilbert, Austin, Cooper, Barnes, Möller and Willerslev2009), and the Americas were without horses until the Spanish introduced the domestic horse (Equus caballus) and donkey (Equus asinus) after AD 1492.
Horses were first reintroduced to the Americas by Spanish colonists in the sixteenth century, and they were swiftly integrated into the culture, economy, and lifeways of many Indigenous peoples, especially in the North American West (Mitchell Reference Mitchell2015). Some Indigenous groups established territorially dominant politics and became “horse nations” rivaling European powers on the continent (Hämäläinen Reference Hämäläinen2008; Mitchell Reference Mitchell2015). Although historic records shed some light on the development of riding and breeding horses among Indigenous North Americans, these processes took place largely outside the attention of European observers—limiting the explanatory power of history to address when, why, and how horses were first integrated into Indigenous societies in the Americas (Jones and Gabe Reference Jones and Gabe2015).
The limitations of the Euro-American historical record in sixteenth- and seventeenth-century North America parallel challenges in understanding early human-horse relations in prehistoric Eurasia, where zooarchaeology has proven to be a valuable source of data about the early stages of horse domestication, management, and use. Osteological study of horse remains in natural history collections and comparison with the archaeological record have revealed characteristic damage patterns to the equine skeleton caused by metal bit use (Anthony et al. Reference Anthony, Telegin and Brown1991; Bendrey Reference Bendrey2007), riding with or without a frame saddle (Bartosiewicz and Gal Reference Bartosiewicz and Gal2013; Levine et al. Reference Levine, Whitwell and Jeffcott2005), heavy exertion (Taylor et al. Reference Taylor, Treal and Tuvshinjargal2015), and bridle nosebands (Taylor and Tuvshinjargal Reference Taylor, Tuvshinjargal, Bartosiewicz and Gal2018). Biomolecular study of equid remains can provide sex and species identifications of bone fragments with a high success rate, even in the absence of sufficient DNA for more extensive sequencing (Schubert et al. Reference Schubert, Mashkour, Gaunitz, Fages, Seguin-Orlando, Sheikhi, Alfarhan, Alquraishi, Al-Rasheid, Chuang, Erminia, Gamba, Weinstock, Vedat and Orlando2017), whereas stable isotope analysis can shed light on seasonal movements, trade, and supplementary feeding practices (Bendrey et al. Reference Bendrey, Hayes and Palmer2009; Makarewicz et al. Reference Makarewicz, Winter-Schuh, Byerly and Houle2018). Such a combination of zooarchaeological and biomolecular analyses of horse remains promises to reveal detailed insights into how horses were managed, ridden, and integrated into ancient lifeways—even in the absence of historical records.
One of the obstacles to the application of a multiproxy zooarchaeological approach to the early historic era in the North American West is the limited sample of horse remains identified in relevant museum collections. For example, in the entire state of Wyoming just two early historic horse specimens plausibly associated with Indigenous cultures have been identified by Thornhill (Reference Thornhill2016). It is possible, however, that North America's rich paleontological record of Pleistocene horses, combined with the well-known significance of horses in historic Euro-American societies, has resulted in the regular misclassification of Indigenous archaeological horses as either Euro-American or Ice Age in origin. Indeed, one of the two early historic horses identified by Thornhill from the site of Black's Fork, Wyoming, was originally suspected to be the remains of a modern or ancient wild horse that died in that location from a natural death—until it became clear that the horse had been buried as part of a ritual feature, along with three coyote skulls (Eckles et al. Reference Eckles, Lockwood, Kumar, Wedel and Walker1994). Similarly, Scott and colleagues (Reference Scott, Stafford, Graham and Martin2010) identified an early historic horse skull that had remained misidentified for a century, even earning its own Pleistocene taxon—Equus laurentius—before radiocarbon dating and a thorough morphological comparison revealed it to be historic. Furthermore, undated “intrusive” horse burials are sometimes reported from Indigenous archaeological sites after being ascribed to non-native ranchers or settlers (Supplemental Figure 1), despite clear archaeological evidence documenting that horses were sometimes integrated into Indigenous funerary and ritual inhumation (e.g., O'Shea and Ludwickson Reference O'Shea and Ludwickson1992). These examples suggest that, when confronted with ancient horse assemblages, researchers may often misidentify archaeological horse remains associated with Indigenous cultures.
The Lehi Horse
In the spring of 2018, residents of Lehi, Utah—a city located at the foot of the northern Wasatch Front in the Provo metropolitan area at an elevation of approximately 1,390 m asl (Figure 1)—discovered equine skeletal remains during new construction. The discovery was reported to researchers at the nearby Museum of Ancient Life, who visited the site and determined that the horse was located in sandy lacustrine deposits of Pleistocene Age. Researchers noted several skeletal pathologies, including arthritis, and determined that the animal was small, about “the size of a Shetland pony” (Holson Reference Holson2018). Although no formal analysis was conducted, the animal was determined to be an elderly Pleistocene horse—perhaps Haringtonhippus francisci (Heintzman et al. Reference Heintzman, Zazula, MacPhee, Scott, Cahill, McHorse, Kapp, Stiller, Wooller, Orlando, Southon, Froese and Shapiro2017)—which had died a natural death on the Provo shoreline of what was then Lake Bonneville (Holson Reference Holson2018) and thought to date to approximately 16,000 yr BP (Benson et al. Reference Benson, Lund, Smoot, Rhode, Spencer, Verosub, Louderback, Johnson, Rye and Negrini2011; Oviatt Reference Oviatt2015).

Figure 1. Lehi horse site within Utah Valley and locations mentioned in the text, along with topography and a simplified geologic map derived from Hintze and colleagues (Reference Hintze, Willis, Laes, Sprinkel and Brown2000).
Although the evidence for an Ice Age origin was intriguing, given that Pleistocene horse remains have been previously reported from shoreline deposits along the Wasatch Front associated with Lake Bonneville (Milligan and McDonald Reference Milligan and McDonald2017; Nelson and Madsen Reference Nelson, Madsen, Kopp and Cohenour1987), we identified several key aspects of the Lehi horse (reposited at the BYU Museum of Paleontology, specimen #BYU 43000, locality #2428) that raised questions about its age, paleontological associations, and context. The available excavation documentation (Figure 2) suggests a pit-like feature in the likely Pleistocene sands, partially infilled with a dark organic layer and horizontally bedded sands and silts (Supplemental Text 1). The presence of rip-up clasts overlying the horse remains supports at least some fluvial incision and deposition during or following burial. The feature's irregular morphology led the original investigators to conclude that this pit was not produced by industrial machinery and was thus Pleistocene in origin (i.e., that the horse remains rest at the base of a small channel fill rather than in an anthropogenic pit). During excavations, however, investigators also recovered a human-modified chert flake (specimen BYU 43001) from the “overburden” (Figure 3)—either the fluvial sand and gravel deposits or organic overburden units shown in Figure 2. A large amount of cortex and large flake scars indicate that this flake was probably produced during early-stage reduction. The precise provenience of this object was not recorded. To us, however, this indicated an Indigenous presence at the location of the Lehi horse after burial. Moreover, our preliminary examination of the horse's vertebral column revealed osteophytes, cracking, and impinging spinous processes—pathological features that are not commonly observed in wild equids and that are typically associated with mounted horseback riding (Figure 4; Levine Reference Levine, Levine, Rassamakin, Kislenko and Tatarintseva1999; Li et al. Reference Li, Zhang, Taylor, Chen, Flad, Boivin, Wang, Liu, You, Ren, Xi, Han, Wen and Ma2020). These findings raised the possibility that the Lehi horse was historic or protohistoric rather than Pleistocene in age.
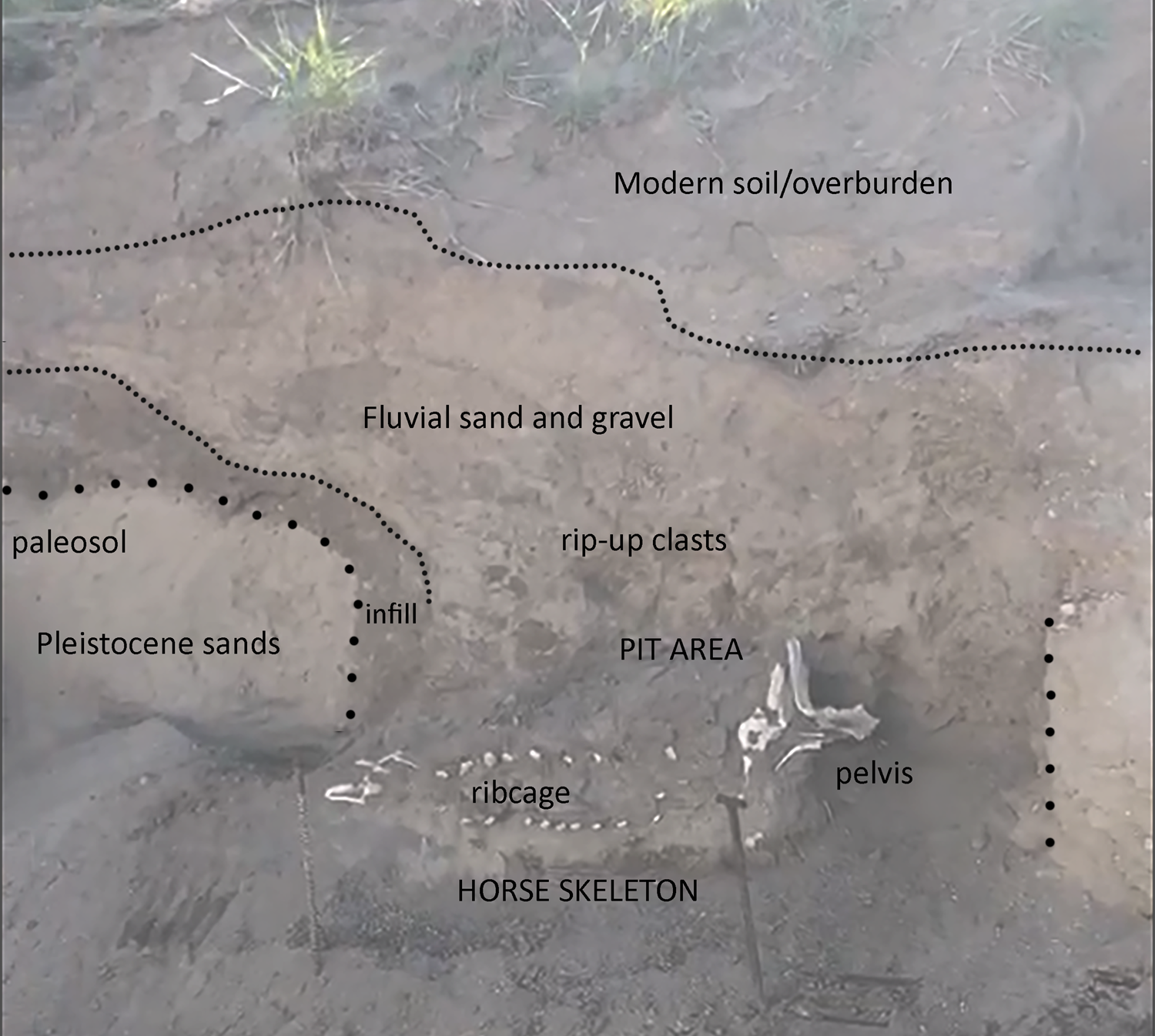
Figure 2. Stratigraphic profile of the Lehi horse excavation, showing a probable pit incised into Pleistocene sands and filled with dark organic soil below horizontally bedded sands and muds, and overlain by recent fluvial deposits. (Color online)

Figure 3. Modified broken chert flake recovered from the “overburden” during initial excavations of the Lehi horse (unprovenienced). (Color online)
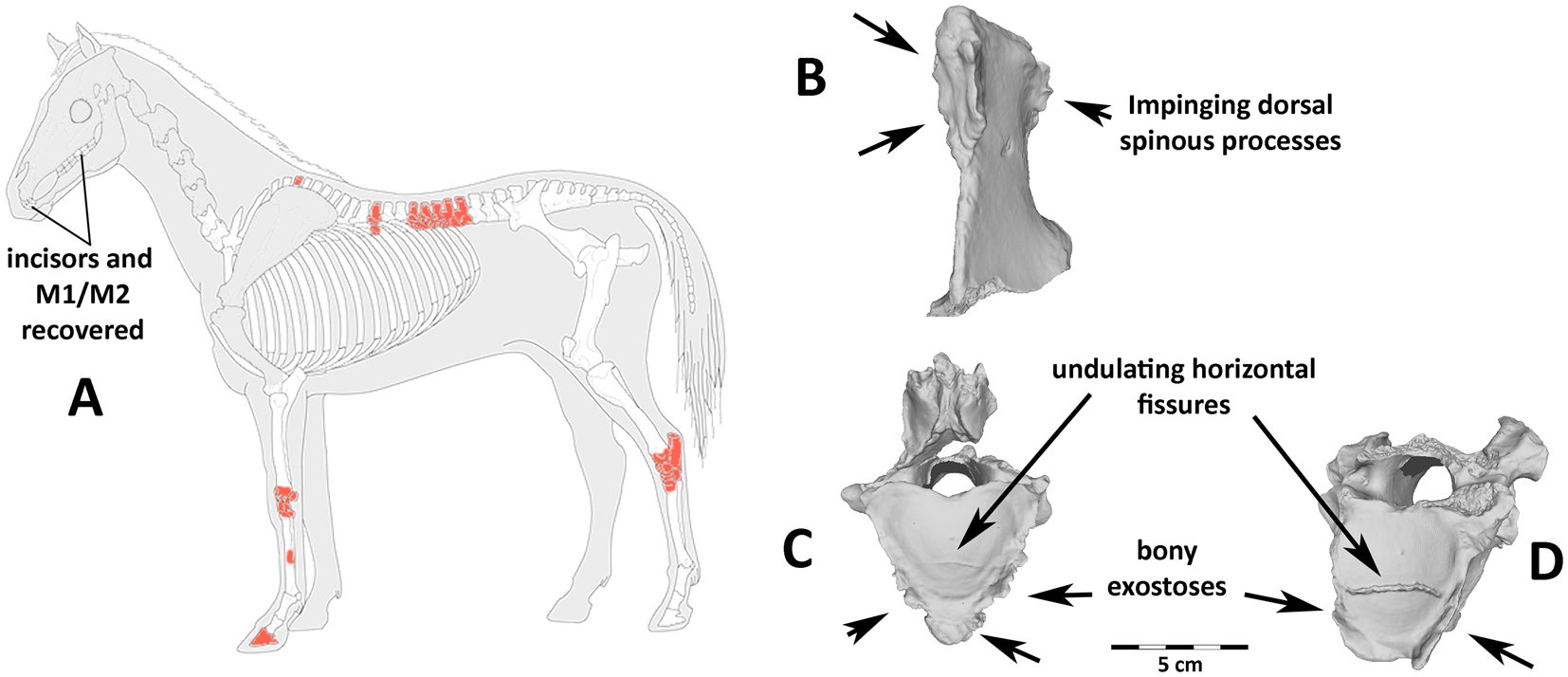
Figure 4. Recovered bones and osteo pathologies: (a) stylized horse skeleton showing missing bones (gray), recovered bones (white), and recovered pathological bones (red); many teeth were recovered, but much of the head/neck was destroyed before excavation. Examples of diagnostic vertebral pathologies (b–d) linked with mounted horseback riding. These include (b) impinging dorsal spinous processes on thoracic vertebra 13, (c, d) undulating, irregular horizontal fissures in the articular surface on the caudal end of the centra, and a proliferation of bony exostoses on thoracic vertebrae (c) 16 and (d) 17.
If the Lehi horse was indeed a misidentified, early historic horse managed by Indigenous peoples, it represents a unique opportunity to explore how horses were used, managed, and cared for in Native societies beyond the limited documentation in Euro-American historical records. Here, we apply osteological and biomolecular analyses to evaluate the age and domestic status of the Lehi horse, and we explore the implications of our findings for the sixteenth-century reintroduction of horses to the Americas.
Materials and Methods
Radiocarbon Dating
To assess the age of the Lehi horse, we selected an intact upper molar (the upper right M1, or 109 in the modified Triadan system) for radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (ORAU) in Oxford, UK. The ORAU performed routine pretreatment and measurement procedures (Brock et al. Reference Brock, Higham, Ditchfield and Ramsey2010), extracting dentine and combusting it in an elemental analyzer before graphitizing and measuring results on an HVEE accelerator, alongside blanks and standards, which were used to perform contamination calculations and data corrections. We calibrated our resulting radiocarbon measurement in OxCal using the IntCal13 calibration dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Warren Beck, Blackwell, Ramsey, Buck, Cheng, Lawrence Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Felix Kaiser, Kromer, Manning, Niu, Reimer, Richards, Marian Scott, Southon, Staff, Turney and van der Plicht2013) to produce a calendrical estimate for the horse's age.
Osteological Study
We assessed the age of the Lehi horse using dentition (Evans et al. Reference Evans, Jack, King and Jones2006) and evaluated the skeleton for evidence of pathologies (Bartosiewicz and Gal Reference Bartosiewicz and Gal2013). We focused particularly on identifying health conditions or osteological changes indicative of—or related to—human activity, including vertebral pathologies associated with mounted horseback riding (Levine et al. Reference Levine, Whitwell and Jeffcott2005) and anthropogenic modifications to the dentition associated with human veterinary care (Taylor et al. Reference Taylor, Treal, Bayarsaikhan, Tuvshinjargal, Bender, Tromp, Clark, Lowry, Houle, Staszewski, Whitworth, Fitzhugh and Boivin2018). Because the cranium of the horse had been shattered, we were unable to assess premolar changes caused by metal bit use (Bendrey Reference Bendrey2007), nasal or premaxillary remodeling associated with bridle use (Taylor and Tuvshinjargal Reference Taylor, Tuvshinjargal, Bartosiewicz and Gal2018) and heavy exertion (Taylor et al. Reference Taylor, Treal and Tuvshinjargal2015), or bone formation at the nuchal crest often associated with horseback riding or confinement (Bendrey Reference Bendrey2008; Taylor et al. Reference Taylor, Treal and Tuvshinjargal2015).
Isotope Analysis
Analyses of the stable isotope composition of ancient horse remains have the potential to provide important environmental context and reveal aspects of forage, pastoral care, and movement. To shed light on the life history of the Lehi horse, we analyzed the carbon, oxygen, and strontium isotope chemistry of Lehi horse dentition, comparing these data to modern climate and environmental reference data from the region to assess how the horse moved during its lifetime and identify sources of dietary food and water.
δ13C Background
In the terrestrial ecosystems of the Intermountain West, the primary source of variation in stable carbon isotopes (δ13C) is the photosynthetic pathway (C3, C4, CAM) employed by native plant taxa. The distribution of C3 versus C4 taxa in the region is the result of complex interactions between a combination of environmental factors, including temperature, precipitation regime, soil moisture, snow cover, vegetation, and evapotranspiration—all of which vary significantly through space and across time (Notaro et al. Reference Notaro, Liu, Gallimore, Williams, Gutzler and Collins2010). In general, C4 grasses and forbs are more abundant in the American Southwest, with high summer temperatures and a precipitation regime dominated by the North American Monsoon (NAM), whereas C3 taxa dominate the Great Basin, with its cooler environments, significant winter precipitation, and associated spring snowmelt events (Cotton et al. Reference Cotton, Cerling, Hoppe, Mosier and Still2016; Wertin et al. Reference Wertin, Reed and Belnap2015). Elevation has a less significant impact on C3/C4 abundance in this region than do temperature and seasonality of precipitation (Paruelo and Lauenroth Reference Paruelo and Lauenroth1996). Within the C3 pathway, plants respond to reductions in water availability by stomatal closure, leading to a slight increase in leaf δ13C values (Farquhar et al. Reference Farquhar, Ehleringer and Hubick1989). Environmentally driven shifts in intra-taxonomic plant δ13C caused by moisture stress are typically relatively minor—around 2‰ or less (Ehleringer Reference Ehleringer, Rundel, Ehleringer and Nagy1989).
Current native grasses in the Lehi region consist primarily of C3 taxa. Cotton and colleagues (Reference Cotton, Cerling, Hoppe, Mosier and Still2016) characterize the Lehi region as between 0% and 10% C4 vegetation. Northern Utah is devoid of C4 grasses, but C4 forbs such as Atriplex—also consumed by horses—are present and begin greening in mid-spring, providing a seasonal source of higher δ13C values during this time. Although plants expressing the CAM photosynthetic pathway (Lee Reference Lee2010) can have values overlapping those of C4 plants, causing interpretive challenges in some settings (e.g., Smith et al. Reference Smith, Mauldin, Munoz, Hard, Paul, Skrzypek, Villanueva and Kemp2014), CAM plants in the study area consist largely of desert succulents unlikely to be consumed by grazers such as horses. Variation in forage δ13C is passed up the food web, with isotopic enrichment between diet and tooth enamel carbonates averaging +14.1‰ (±0.5) in a wide range of ungulate mammals (Cerling and Harris Reference Cerling and Harris1999:352) and +13.8‰ (±1.9) in horses specifically (Cerling and Harris Reference Cerling and Harris1999:349; see also Passey et al. Reference Passey, Robinson, Ayliffe, Cerling, Sponheimer, Dearling, Roeder and Ehleringer2005). Prior to fossil fuel depletion of atmospheric CO2, fractionation during photosynthesis led to C3 and C4 grasses expressing an average δ13C value of approximately −25‰ and −10‰ respectively—about 2‰ enriched over modern plant taxa (Dombrosky Reference Dombrosky2020).
δ18O Background
Accurate interpretation of archaeological δ18O relies on contextualization with modern and ancient climate data (e.g., Hamilton et al. Reference Hamilton, Lee Drake, Wills, Jones, Conrad and Crown2018). δ18O of environmental water is influenced by a suite of factors, including temperature, altitude, rainfall amount, aridity, seasonal precipitation patterns, and distance from the coast (Cerling et al. Reference Cerling, Harris, Leakey, Passey, Levin, Werdelin and Sanders2010). For example, as water-vapor masses move upslope, H218O preferentially rains out, accelerated by declines in temperature. H218O also rains out as water-vapor masses move inland, progressively depleting δ18O values relative to coastal moisture, although this effect is less marked in cold (versus warm) precipitation events. Thus, the δ18O value of water is influenced by temperature both within and across altitudinal and latitudinal gradients (Daux et al. Reference Daux, LéCuyer, Adam, Martineau and Vimeux2005; Fricke and O'Neil Reference Fricke and O'Neil1999). Generally, colder winter temperatures and precipitation lead to lower water δ18O values, whereas summer aridity and increased temperatures enrich water δ18O (Dansgaard Reference Dansgaard1964). In the Lehi region, most precipitation is concentrated in winter and early spring (Figure 5). Here, deep snowpack with lower δ18O values accumulates at elevations in the Wasatch range, reaching 3,650 m asl (~12,000 feet). Accordingly, as snowpack melts during late spring and early summer, modern δ18O data from the Jordan River near Lehi (Coplen and Kendall Reference Coplen and Kendall2000) exhibit consistently lower δ18O values due to discharge of snowmelt into numerous alpine streams flowing downslope into Utah Lake and the Jordan River drainage.

Figure 5. (a) monthly temperature at Lehi, Utah (1,391 m asl), and at the nearby peak of Mount Timpanogos, roughly 15 km east of the Lehi site (3,582 m asl), showing warmest temperatures in the summer, June–August; (b) monthly total precipitation at Lehi and Mt. Timpanogos, showing very low precipitation year-round at Lehi itself and higher precipitation with a pronounced decrease in the summer months (June–August) at higher elevations; (c) stable oxygen isotope values for the Jordan River between 1984 and 1987 (Coplen and Kendall Reference Coplen and Kendall2000), showing δ18O depletion during spring snowmelt.
Although other processes, such as leaf evapotranspiration, may have minor impacts on the δ18O value of forage (Pederzani and Britton Reference Pederzani and Britton2019), horses are obligate drinkers, deriving most of their moisture intake from imbibed water. During summer months, whether drinking from standing or flowing sources, imbibed water will likely express evaporatively enriched δ18O values relative to snowmelt—the primary source of spring and early-summer drinking water. Thus, in temperate regions, the δ18O values of obligate drinkers—such as horses—primarily reflect seasonally driven variation in δ18O values of water bodies (Levin et al. Reference Levin, Cerling, Passey, Harris and Ehleringer2006; Pederzani and Britton Reference Pederzani and Britton2019; Roberts et al. Reference Roberts, Stewart, Alagaili, Breeze, Candy, Drake, Groucutt, Scerri, Lee-Thorp, Louys, Zalmout, Al-Mufarreh, Zech, Alsharekh, al Omari, Boivin and Petraglia2018). Significantly, the process of enamel mineralization and maturation allows chronologically ordered isotopic changes to be studied (Balasse Reference Balasse2002; Sponheimer et al. Reference Sponheimer, Passey, de Ruiter, Guatelli-Steinberg, Cerling and Lee-Thorp2006), documenting seasonal patterns of movement or environmental conditions (Balasse Reference Balasse2002; Makarewicz et al. Reference Makarewicz, Winter-Schuh, Byerly and Houle2018). Although water cisterns may also bias archaeological δ18O values, there is no evidence of the use of such systems for horse management by Indigenous peoples (Ewers Reference Ewers1955). As a result, δ18O values from the Lehi horse should assess the input of local water sources and/or associated movements during the animal's life.
87Sr/86Sr Background
Strontium isotope ratios (87Sr/86Sr) of tooth enamel have been used to track mobility based on the principle that bedrock of different ages and compositions have distinctive strontium isotope values that do not fractionate from the bedrock to the biosphere (Graustein and Armstrong Reference Graustein and Armstrong1983; Montgomery Reference Montgomery2010). Older lithic formations have relatively higher 87Sr/86Sr in comparison to younger bedrock, while distinctive mineral content leads to characteristic 87Sr/86Sr based on rock “type” (Capo et al. Reference Capo, Stewart and Chadwick1998). The surface of Utah Valley is draped by Late Pleistocene Lake Bonneville–related sediments, and the bases of the surrounding mountains are covered with the lake's shoreline deposits, wave-cut terraces, and related deltaic deposits and alluvial fans. The city of Lehi lies at the north end of Utah Valley, and the burial is incised into a Lake Bonneville shoreline deposit. The mountains encompassing Utah Valley are composed primarily of Paleozoic marine carbonates, which on the north end of the valley were injected by intrusive (granite)—and overlain by extrusive (tuff)—igneous rock units. Tens of kilometers to the northeast and at higher elevations, the headwaters of the Provo River, which drains into Utah Lake, flow along Late Precambrian strata that have been metamorphosed to varying degrees (Biek Reference Biek2005). These mixed source terrains result in a landscape expected to vary in bedrock strontium-isotope content (Figure 1).
Differential weathering of rocks with varied 87Sr/86Sr ratios, as well as the geographic variability of hydrology and aeolian transport, suggests that the 87Sr/86Sr of soils and plants—and individuals consuming them—may not be identical to bedrock ratios (Montgomery Reference Montgomery2010). In the absence of detailed baseline data, the sampling of different points along the vertical axis of a tooth can still provide insight into whether an individual moved during enamel formation. This is because the strontium isotope ratio of animal forage, taken up primarily from soils, is incorporated into tooth enamel during the process of mineralization.
Horse Tooth Mineralization and Development
The first molar of the horse begins to mineralize at around two weeks of age, and it continues to mineralize over the first 24 months of life (Hoppe et al. Reference Hoppe, Stover, Pascoe and Amundson2004). Between the ages of 8 and 12 months, this tooth erupts through the gums and begins to wear (Evans et al. Reference Evans, Jack, King and Jones2006; Hoppe et al. Reference Hoppe, Stover, Pascoe and Amundson2004), and the tooth continues to mineralize away from the crown vertically at a rate of roughly 3.5–4.0 cm/year (Hoppe et al. Reference Hoppe, Stover, Pascoe and Amundson2004). The tooth reaches a total “crown height” (the distance between the lowest portion of the root juncture and the occlusal surface) of 80–90 mm or more (Levine Reference Levine, Wilson, Grigson and Payne1982). Because our sample is derived from the lower portion of this tooth, we infer that our signal derives from the horse's second year of life. The oblique orientation of enamel formation suggests that each sample taken perpendicular to the vertical axis of a tooth averages a short but thus far undocumented period of mineralization of up to several months (Hoppe et al. Reference Hoppe, Stover, Pascoe and Amundson2004).
Horses typically complete the process of weaning by the age of roughly 8–9 months, although this process can be as short as 4 months in domestic animals and may last as long as a year (Waran et al. Reference Waran, Clarke and Farnworth2008). Given that mammalian milk is elevated in δ18O and lower in δ13C relative to imbibed water and herbivore forage, respectively, the interpretation of equid stable isotope data can be confounded by enamel values laid down prior to weaning (Zazzo et al. Reference Zazzo, Mariotti, Lécuyer and Heintz2002). This bias is particularly problematic with equid M1 data because the tooth mineralizes during the first two years of the animal's life. The advanced age of the Lehi horse, however, means that the upper 40+ mm or so of tooth has been worn away through occlusal wear. We sampled the remaining 18 mm of enamel on a very worn M1 crown; this vertical section almost certainly mineralized in the second phase of tooth growth after weaning (Hoppe et al. Reference Hoppe, Stover, Pascoe and Amundson2004). As a result, although suckling clearly impacts both enamel δ13C and δ18O, and may bias strontium ratios, these processes are unlikely to have impacted our data.
Protocols
The lower right first molar (M1/409 in the modified Triadan system) was sequentially sampled for enamel stable carbon and oxygen isotope values (δ13C and δ18O) at the Stable Isotope Laboratory Facility in the Department of Archaeology, Max Planck Institute for the Science of Human History in Jena, Germany. Sampling was conducted at the base of the tooth root on the lingual margin, moving up at 2 mm intervals to a distance of 18 mm above the root, immediately below the occlusal surface of this heavily worn M1.
Enamel powder was pretreated by a wash in 1.5% sodium hypochlorite for 60 minutes, followed by three rinses in purified H2O, before 0.1 M acetic acid was added for 10 minutes, followed by another three purified H2O rinses (per Lee-Thorp et al. Reference Lee-Thorp, Likius, Mackaye, Vignaud, Sponheimer and Brunet2012; Roberts et al. Reference Roberts, Perera, Wedage, Deraniyagala, Perera, Eregama, Petraglia and Lee-Thorp2017; Sponheimer et al. Reference Sponheimer, de Ruiter, Lee-Thorp and Späth2005). The resulting residues were freeze-dried overnight before reaction with 100% phosphoric acid. Gases evolved from the samples were analyzed for their stable carbon and oxygen isotopic composition using a Thermo GasBench II connected to a Thermo Delta V Advantage Mass Spectrometer at MPI-SHH. δ13C and δ18O values were compared against International Standards (IAEA-603 [δ13C = 2.5; δ18O = −2.4]; IAEA-CO-8 [δ13C = −5.8; δ18O = −22.7]; USGS44 [δ13C = −42.2]) and in-house standard (MERCK [δ13C = −41.3; δ18O = −14.4]). Replicate analysis of MERCK standards indicates that machine measurement error is c. ± 0.1‰ for δ13C and ± 0.2‰ for δ18O. Overall measurement precision was assessed by measurement of repeat extracts from a bovid tooth enamel standard (n = 20, ± 0.2‰ for δ13C and ± 0.2‰ for δ18O).
We also sampled the same tooth in three locations for strontium isotope analysis—the base of the tooth root (0 mm), the middle of the sampled section (10 mm), and the crown (18 mm). Chemical sample preparation for strontium isotope analysis was performed in the clean chemistry laboratory of the MC-ICP-MS facility, Department of Geological Sciences, University of Cape Town. Strontium isotope ratio analysis was performed using the Nu Instruments Nu Plasma HR MC-ICP-MS in this facility (after Copeland et al. Reference Copeland, Sponheimer, le Roux, Grimes, Lee-Thorp, de Ruiter and Richards2008). Twenty milligrams of the sampled tooth enamel powder were dissolved in 2 mL concentrated HNO3 in a closed Teflon beaker. The beaker was placed on a hotplate at 140°C for an hour, after which the sample was dried down and redissolved in 1.5 mL 2M HNO3 for strontium separation chemistry, following Pin and colleagues (Reference Pin, Briot, Bassin and Poitrasson1994). The separated strontium fraction was dried down, dissolved in 2 mL 0.2% HNO3 and diluted to 200 ppb strontium concentration prior to MC-ICP-MS isotope ratio analysis. Analyses of NIST SRM987 were used as a bracketing reference standard using a 87Sr/86Sr value of 0.710255. The strontium isotope data was corrected for isobaric rubidium interference at 87 amu using the measurement of 85Rb and the natural 85Rb/87Rb ratio. Instrumental mass fractionation was corrected using the measured 86Sr/88Sr ratio, the exponential law, and a true 86Sr/88Sr ratio of 0.1194. Analysis of an in-house carbonate reference material processed and measured with samples from this study (87Sr/86Sr = 0.708908; 2σ = 0.000018) agreed well with long-term results (87Sr/86Sr = 0.708911; 2σ = 0.000040, n = 414).
DNA Analysis
Two independent DNA extractions were carried out at the ancient DNA research facilities of the Laboratoire AMIS CNRS UMR5288, Université de Toulouse III Paul Sabatier. DNA was extracted from 200 and 350 mg of bone powder following method Y from Gamba and colleagues (Reference Gamba, Hanghøj, Gaunitz, Alfarhan, Alquraishi, Al-Rasheid, Bradley and Orlando2016), with the slight modifications from Gaunitz and colleagues (Reference Gaunitz, Fages, Hanghøj, Albrechtsen, Khan, Schubert, Seguin-Orlando, Owens, Felkel, Bignon-Lau, de Barros Damgaard, Mittnik, Mohaseb, Davoudi, Alquraishi, Alfarhan, Al-Rasheid, Crubézy, Benecke, Olsen, Brown, Anthony, Pitulko, Kasparov, Brem, Hofreiter, Mukhtarova, Baimukhanov, Lõugas, Onar, Stockhammer, Krause, Boldgiv, Undrakhbold, Erdenebaatar, Lepetz, Mashkour, Ludwig, Wallner, Merz, Merz, Zaibert, Willerslev, Librado, Outram and Orlando2018). Aliquots of DNA extracts were treated with the USER enzyme mix (NEB), following Librado and colleagues (Reference Librado, Gamba, Gaunitz, Sarkissian, Pruvost, Albrechtsen, Fages, Khan, Schubert, Jagannathan, Serres-Armero, Kuderna, Povolotskaya, Seguin-Orlando, Lepetz, Neuditschko, Thèves, Alquraishi, Alfarhan, Al-Rasheid, Rieder, Samashev, Francfort, Benecke, Hofreiter, Ludwig, Keyser, Marques-Bonet, Ludes, Crubéz, Leeb, Willerslev and Orlando2017), in order to reduce the impact of postmortem DNA damage in downstream analyses. Triple-indexed DNA libraries were constructed following the methodology from Fages and colleagues (Reference Fages, Hanghoej, Khan, Gaunitz, Seguin-Orlando, Leonardi, Contantz, Gamba, Al-Rasheid, Albizuri, Alfarhan, Allentoft, Alquraishi, Anthony, Benecke, Sánchez, Berrocal-Rangel, Biglari, Boessenkoo, Boldgiv, Brem, Brown, Burger, Crubéz, Daugnora, Davoudi, de Barros Damgaard, Maríade los Ángeles de Chorro y de Villa-Ceballos, Deschler-Erb, Detry, Dill, Oom, Dohr, Ellingvåg, Erdenebaatar, Fathi, Felkel, Fernández-Rodríguez, García-Viñas, Germonpré, Granado, Hallsson, Hemmer, Hofreiter, Kasparov, Khasanov, Khazaeli, Kosintsev, Kristiansen, Kubatbek, Kuderna, Kuznetsov, Laleh, Leonard, Lhuillier, von Lettow-Vorbeck, Logvin, Lõugas, Ludwig, Luis, Arruda, Marques-Bonet, Silva, Merz, Mijiddorj, Miller, Monchalov, Mohaseb, Morales, Nieto-Espinet, Nistelberger, Onar, Pálsdóttir, Pitulko, Pitskhelauri, Pruvost, Sikanjic, Rapan Papeša, Roslyakova, Sardari, Sauer, Schafberg, Scheu, Schibler, Schlumbaum, Serrand, Serres-Armero, Shapiro, Seno, Shevnina, Shidrang, Southon, Star, Sykes, Taheri, Taylor, Teegen, Trbojević Vukičević, Trixl, Tumen, Undrakhbold, Usmanova, Vahdati, Valenzuela-Lamas, Viegas, Wallner, Weinstock, Zaibert, Clavel, Lepetz, Mashkour, Helgason, Stefánsson, Barrey, Willerslev, Outram, Librado and Orlando2019), corresponding to the procedure described in Gaunitz and colleagues (Reference Gaunitz, Fages, Hanghøj, Albrechtsen, Khan, Schubert, Seguin-Orlando, Owens, Felkel, Bignon-Lau, de Barros Damgaard, Mittnik, Mohaseb, Davoudi, Alquraishi, Alfarhan, Al-Rasheid, Crubézy, Benecke, Olsen, Brown, Anthony, Pitulko, Kasparov, Brem, Hofreiter, Mukhtarova, Baimukhanov, Lõugas, Onar, Stockhammer, Krause, Boldgiv, Undrakhbold, Erdenebaatar, Lepetz, Mashkour, Ludwig, Wallner, Merz, Merz, Zaibert, Willerslev, Librado, Outram and Orlando2018) applied with the DNA adapters from Rohland and colleagues (Reference Rohland, Harney, Mallick, Nordenfelt and Reich2015; which include 7 bp long internal indexes). We amplified DNA libraries in 25 uL reactions using the AccuPrime Pfx DNA polymerase following Gaunitz and colleagues (Reference Gaunitz, Fages, Hanghøj, Albrechtsen, Khan, Schubert, Seguin-Orlando, Owens, Felkel, Bignon-Lau, de Barros Damgaard, Mittnik, Mohaseb, Davoudi, Alquraishi, Alfarhan, Al-Rasheid, Crubézy, Benecke, Olsen, Brown, Anthony, Pitulko, Kasparov, Brem, Hofreiter, Mukhtarova, Baimukhanov, Lõugas, Onar, Stockhammer, Krause, Boldgiv, Undrakhbold, Erdenebaatar, Lepetz, Mashkour, Ludwig, Wallner, Merz, Merz, Zaibert, Willerslev, Librado, Outram and Orlando2018) before concentrating them using Agencourt bead purification, quantifying them on a TapeStation 4200 instrument (Agilent technologies), and pooling them together with other triple-indexed DNA libraries for sequencing on the Illumina MiniSeq. Sequencing reads were demultiplexed based on their internal adapter indexes using AdapterRemoval 2, allowing for a single nucleotide mismatch at best on each internal index, before they were parsed through PALEOMIX v1.2.13 with default parameters, except that seeding was disabled for BWA (Li and Durbin Reference Li and Durbin2009) mapping (version 0.7.17-r1194) in order to identify high-quality reads mapping uniquely against the horse nuclear reference genome EquCab2 and the horse mitochondrial genome (Accession # NC_001640). The resulting read alignment files were then processed with the Zonkey package (Schubert et al. Reference Schubert, Mashkour, Gaunitz, Fages, Seguin-Orlando, Sheikhi, Alfarhan, Alquraishi, Al-Rasheid, Chuang, Erminia, Gamba, Weinstock, Vedat and Orlando2017) to identify first-generation equine hybrids and determine the molecular sex, merging the sequence data from both experiments (Supplemental Text 2).
Results
Radiocarbon Dating
AMS radiocarbon dating of dentin from the Lehi horse lower-right first molar returned a calibrated 2σ range of AD 1681–1939 (127 ± 18 14C YBP, OxA-38552), with a median date of AD 1838. The dentine C/N ratio (3.2) and yield (9.5%) fell within standard quality-control parameters, providing good support for the radiocarbon date. These results demonstrate that the Lehi horse is historic, not Pleistocene in age. Although narrowing the timing of the burial is challenging because of the multimodal calibrated probability distribution and shape of the calibration curve over this time period, the co-occurrence of the burial with a modified stone tool suggests that it predates permanent Mormon settlement in the region (ca. 1850) and may fall within the earlier half of this calibrated range.
Osteological Study
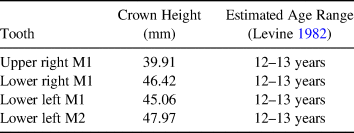
The morphology of the incisor grinding table (which lacks enamel spots on the lower but not the upper incisors), the fusion of all identified epiphyseal surfaces, and the crown height measurements of available cheek teeth suggest that the Lehi horse was approximately 12–13 years old at the time of death (Table 1), but it is important to note that the reliability of wear-based age estimates can be influenced by factors such as environment and diet (Allen Reference Allen2008). Unfortunately, no relevant portions of the cranium or premolars were available for study. Although we identified no canines, the destruction of the cranium makes it impossible to ascribe the absence of these teeth in the recovered assemblage to sex. The presence of marked enamel hypoplasia on the available cheek teeth is far from uncommon in wild or domestic equids, but it indicates some degree of nutritional stress during the animal's lifetime.
Table 1. Crown Height Measurements Used to Assess Age of the Lehi Horse.
We identified extreme levels of pathological bone formation on the vertebrae. This includes pathologic growth along the articular facets and ventral surface of vertebrae, particularly those of the lower back area most impacted by a seated rider (T16–18 and L1–L2), as well as irregular, undulating, transverse fractures of the caudal articular surface on T17 and T18 (Figure 4). Although much of the vertebral column was destroyed prior to excavation, we found impinging spinous processes on the T13 at the contact with both the T12 and T14.
The specimen exhibits extreme arthritis of the lower limbs and other pathologies that severely impacted its mobility (Holson Reference Holson2018). The Lehi horse exhibits such severe arthritis of the right rear tibia and tarsal joint that the surface of the astragalus is deeply pitted, and most of the tarsals have fused into a single bony mass (Figure 6). In addition to this rear limb issue, the right radius/metacarpal joint of the forelimb is also impacted by arthritis, and the horse exhibits laminitis of the front left distal phalanx and a fracture to the medial splint bone (the left second metacarpal). These features may be related in that the immobility of the right rear joint may have caused conformation issues (laminitis) and compensation in the forelimbs, adding stress that caused a fracture of the splint bone. Pathological features identified through osteological analysis are summarized in Table 2.

Figure 6. Examples of arthritic bone from the right hind leg. Severe arthritic bone formation and pitting of the posterior face of tibia (left) and the astragalus and tarsals (right), resulting in the complete immobility and fusion of many of the tarsal bones. (Color online)
Table 2. Pathological Features Identified in the Lehi Horse.
Isotope Analysis
Sequential sampling of M1 δ13C (n = 10) yielded an average value of −10.4 ± 0.6‰ with a 1.9‰ range of −9.9 to −11.8‰ (Table 3). Assuming a fractionation constant of +13.8‰, as noted above (Cerling and Harris Reference Cerling and Harris1999), dietary forage δ13C ranged between −25.6 and −23.7‰ with an average of –24.2‰. Given a fractionation constant of approximately +14, a protohistoric horse consuming 100% C3 vegetation would exhibit an enamel δ13C value of c. −11‰, whereas a 100% C4 diet would yield a carbonate δ13C value of approximately +4‰ (Lee-Thorp Reference Lee-Thorp1989; Lee-Thorp et al. Reference Lee-Thorp, Sealy and van der Merwe1989; Levin et al. Reference Levin, Simpson, Quade, Cerling and Frost2008). Estimates of reliance on C3 forage for the Lehi horse thus range between 100% and 91%, averaging 94.6% C3 intake. Average δ18O is −11.7 ± 0.4‰ (n = 10) with a 1.3‰ range of −11.2 to −12.5‰. δ13C and δ18O are inversely related, and both datasets suggest seasonal shifts in local forage and the temperature of imbibed water, respectively, across the period of enamel formation (Figure 7; Table 3). Strontium isotope values (n = 3; Figure 7) were consistent to the fourth decimal place (0.710526–0.710570) across the 18 mm sampled region (Figure 8), falling within an expected range for the greater Salt Lake region (Tipple et al. Reference Tipple, Valenzuela and Ehleringer2018) and in agreement with modern measured values from water sources in the Utah Lake region (Figure 1).

Figure 7. Normalized stable isotope measurements for stable oxygen (closed circle), carbon (open circle), and strontium (closed triangle) for the Lehi horse, as sampled from the lingual surface of the lower-right first molar.

Figure 8. Lehi horse's lower right Molar 1, showing sample locations and location of crown height measurement. (Color online)
Table 3. Normalized Stable Isotope Measurements and Standard Deviation for the Lehi Horse, as Sampled from the Lingual Surface of the Lower-Right First Molar.

Notes: Fractionation between enamel and dietary C3 inputs calculated using a value of +13.8‰. Plant CO2 concentrations in the Lehi region during the late prehistoric period were ~2.0% more positive than today (Dombrosky Reference Dombrosky2020).
DNA Analysis
Combined, our two DNA extractions from the Lehi horse successfully yielded a total of 7,292 reads uniquely mapping to the reference mitochondrial and nuclear genomes of the domestic horse (E. caballus). The specimen yielded low but sufficient endogenous DNA to assess the sex, species, and first-generation hybrid status at virtually zero false-positive rates using the Zonkey pipeline (Schubert et al. Reference Schubert, Mashkour, Gaunitz, Fages, Seguin-Orlando, Sheikhi, Alfarhan, Alquraishi, Al-Rasheid, Chuang, Erminia, Gamba, Weinstock, Vedat and Orlando2017). These results provide clear indication that the animal is a domestic horse, E. caballus, and that the horse is female (Table 4).
Table 4. Genetic Identification of Species and Sex of the Lehi Horse.

a Prior to removal of PCR duplicates.
Discussion
Early Historic Indigenous Horse Riding and Herding in the Great Basin
Our results provide conclusive evidence that the Lehi horse is early historic rather than Ice Age in origin, and that this animal was used for mounted horseback riding, likely by Native peoples during the eighteenth or early nineteenth centuries. Intact horse teeth have proven to be the most reliable and contamination-resistant biological material for dating archaeological horse remains (Zazzo et al. Reference Zazzo, Lepetz, Magail and Gantulga2019), and our results place the Lehi horse burial between approximately 1680 and the early twentieth century. The recovery of flaked stone within the excavation points to an association with Indigenous cultures for the Lehi horse, and the deep overburden of sediment may have been generated by Euro-American farming and land-clearing activities after about 1850 (Morris and Rowe Reference Morris and Rowe2014). Based on ethnohistoric reconstructions, the area from which the Lehi horse was recovered would have straddled the boundary between Ute and Shoshone territory (Stewart Reference Stewart1970:209). The Northern Utah Ute acquired the horse as early as the seventeenth century, and they relied on it for their wide-ranging seasonal movements (Duncan Reference Duncan and Cuch2003). The Shoshone obtained horses by the year 1700, likely from the Ute, and they played a major role in funneling horses to the northern Plains and Plateau (Mitchell Reference Mitchell2015). Although our data do not allow us to determine whether this particular horse belonged to the Ute, the Shoshone, or both, our results do articulate with the ethnohistory of the region, given that the horse had become critically important to both Ute and Shoshone societies by the eighteenth century.
Analysis of the animal's skeleton demonstrates that the horse was ridden, and it reveals health issues that imply a degree of human protection or care. Damage to the vertebrae of the lower back—osteophytes, impinging spinous processes, and transverse fractures of the caudal articular surface—is characteristically associated with mounted riding, and it is generally absent from wild animals (Levine Reference Levine, Levine, Rassamakin, Kislenko and Tatarintseva1999; Levine et al. Reference Levine, Whitwell and Jeffcott2005). Although this type of damage is found in many ancient assemblages linked to horseback riding, it is particularly pronounced among archaeological horses predating the innovation of the frame saddle because riding bareback entails more direct trauma to the vertebral column (Levine et al. Reference Levine, Whitwell and Jeffcott2005). Among those vertebrae that yielded osteophytes and new bone formation (T5, T13, and T16-L2), this bone growth was universally asymmetric, in favor of more developed bone spurs on the animal's left side (Figure 9). We have identified similarly pronounced asymmetry in ridden-horse assemblages from Eurasia (Li et al. Reference Li, Zhang, Taylor, Chen, Flad, Boivin, Wang, Liu, You, Ren, Xi, Han, Wen and Ma2020). Asymmetric bias in equine skeletal damage may be related to rider handedness or riding technique (Taylor and Tuvshinjargal Reference Taylor, Tuvshinjargal, Bartosiewicz and Gal2018), but it could also be influenced by consistent mounting from one side. Previously identified vertebral asymmetry in East Asian assemblages is clearly left biased (Levine et al. Reference Levine, Whitwell and Jeffcott2005; Li et al. Reference Li, Zhang, Taylor, Chen, Flad, Boivin, Wang, Liu, You, Ren, Xi, Han, Wen and Ma2020). Although many Native American horsemen are known historically to have mounted from the right side, it may be that this difference in weight bearing is caused by the handedness of the rider and the dynamics of mounted activities.

Figure 9. Arrows indicate bony exostoses on the ventrolateral surfaces of vertebrae from the Lehi horse, showing asymmetric bone formation on the animal's left side in all cases. Thoracic vertebrae 5 (left), 13 (center), and 16 (right). All in ventrocranial view. (Color online)
The pattern of pathological damage to the lower back observed in the Lehi horse clearly indicates that the animal was used for riding. Archaeological assemblages of animals used only for chariot traction, for example, show increased pathology in the forelimbs and neck region but little damage to the thoracic or lumbar vertebrae (Levine Reference Levine2005; Weber Reference Weber, Vila, Gourichon, Choyke and Buitenhui2008). This observation does not rule out the animal's use for other purposes, however. Many Native American groups in western North America employed horses for pack transport (Ewers Reference Ewers1955), either with or without a travois (a pole-based sledge system adapted from existing technology used for dog transport). This system could be, and often was, used in tandem with a saddle and rider, which could have produced an osteological signature similar to that observed in the Lehi horse.
Extreme arthritis and other bone pathologies in the animal's limbs provide further evidence of its use in transport. The fusion of most of the bones of the lower joint of the right rear limb would have given this horse extreme difficulty in movement. Any horse—wild or domestic—can suffer from these maladies, which are likely to limit an animal's mobility severely. In the Lehi horse, these pathologies are severe, and they imply that the horse must have been cared for, or at least intentionally retained, even after reaching levels of impaired mobility. The issues with this joint may have radiated throughout the body as the animal experienced continued use. The horse also developed laminitis in a front hoof and fractured a splint bone, both potentially caused by compensation for the impaired hoof. These features suggest that, despite lameness, the Lehi horse continued to be ridden (or perhaps used in transport in other ways, such as pulling loads in a travois). Indeed, the age of 12 seems remarkably young for a horse to have developed the observed level of pathology through natural aging processes. We argue that one of the only ways an animal with such severe mobility issues could have survived without dying from predation or starvation would be through human management or care.
The genomic identification of the Lehi horse as the domestic E. caballus and its identification as a female support its identification as a pastorally managed horse. Although the animal's injuries would have drastically reduced its usefulness in transport, female horses continue to produce young annually until around 15 years of age (Garrott et al. Reference Garrott, Eagle and Plotka1991). As a result, archaeological assemblages related to pastorally managed breeding herds of horses rarely yield breeding-age females. Instead, they consist largely of elderly females (along with young males, who are culled before reaching breeding age; Levine Reference Levine, Levine, Rassamakin, Kislenko and Tatarintseva1999; Taylor Reference Taylor2017). The advanced age of the Lehi horse, and the co-occurrence of mobility-restricting pathological issues in the limbs suggest that the mare was either retained as a breeding animal until she died or that she was culled/sacrificed after reaching the end of her reproductive viability.
Mobility and Movement
Our lower stable oxygen isotope values likely correspond to a single, late-spring snow melt event, bracketed by slightly elevated δ18O values characteristic of lower-elevation precipitation, during the Lehi horse's second year of life (Figures 4 and 7; Table 3). The lowest Lehi horse δ18O reading is paired with the highest δ13C value, and δ13C is loosely inversely related with δ18O across all but the last two samples taken at 16 mm and 18 mm from the enamel base. These data indicate a slight shift in the type of forage relied upon through winter into late spring—perhaps an increase in consumption of C4 forbs beginning to green up in mid-spring, incrementally increasing the stable carbon isotope values of the Lehi horse. De Winter and colleagues (Reference de Winter, Snoeck and Claeys2016) also found a similar pattern of higher δ13C values with winter δ18O values in modern horses in Europe, which may be attributable to changes in plant water use efficiency or slight variations in plant choices during the winter season. Maize, also a C4 domesticate, was farmed by prehistoric Fremont groups along the eastern rim of the Great Basin, but maize agriculture was abandoned by approximately AD 1150 in the Utah and Great Salt Lake Valleys, coincident with the end of the Medieval Climate Anomaly (Coltrain and Leavitt Reference Coltrain and Leavitt2002). Prior to pioneer settlement in 1847, maize was not cultivated by indigenous Ute or Shoshone populations. Thus, foddering with maize is unlikely to have made a significant contribution to the Lehi horse's very slightly elevated spring δ13C values.
Historically, the Ute are known to have made fall and winter seasonal movements between low-elevation wintering grounds and high-elevation summer hunting grounds, as well as between territories to the south and east, ranging as far as eastern Colorado and western Kansas, and hunting in New Mexico, Arizona, and the Texas and Oklahoma panhandles. It is possible that Indigenous pastoralism of equids included moving herds south from Utah Valley during winter months, but neither strontium nor stable oxygen isotope values for the Lehi horse support this interpretation for this particular individual. Strontium signatures do not vary seasonally, but they are consistent at the fourth decimal place (0.710526–0.710570). Moreover, all observed values fall within an expected range for the greater Salt Lake region (Tipple et al. Reference Tipple, Valenzuela and Ehleringer2018), providing no evidence that carbon and oxygen isotope patterning was the consequence of nonlocal movement.
Conclusion
Our results demonstrate that the Lehi horse is indeed an early historic domestic horse, likely managed and used by Indigenous people. Osteological pathologies indicate that the Lehi horse was ridden, perhaps bareback or using a soft pad saddle. The animal's use continued even after it developed issues with the right rear limb, which caused ancillary effects in other limbs—including arthritis, a fracture, and laminitis. Pathological bone growths on the vertebrae suggest that the horse experienced consistently higher loads on its left-hand side, a pattern that is known from other archaeological assemblages and is characteristic of ridden horses. DNA confirms the identification of the Lehi horse as Equus caballus, not an Ice Age wild equid, and reveals that the animal was a mare. This is consistent with expectations for a pastorally managed herd animal, and it explains the apparent care for the horse into advanced age despite severe mobility issues. Stable isotopes sampled from the horse's enamel point to seasonal shifts in the isotope chemistry of imbibed water and available vegetation, which—in tandem with minimal variance in strontium isotope—suggests that the horse was raised and tended locally within the Wasatch Front near where it was found.
This study provides an example of how to engage data quality in zooarchaeology (e.g., Wolverton Reference Wolverton2013) as well as a template for the integration of morphological and biomolecular approaches (Steele Reference Steele2015)—subjects that have been a key focus of discussion in archaeozoological circles in recent years (e.g., Jones and Gabe Reference Jones and Gabe2015; Kansa et al. Reference Kansa, Atici, Kansa and Meadow2019; LeFebvre et al. Reference LeFebvre, Brenskelle, Wieczorek, Kansa, Kansa, Wallis, King, Emery and Guralnick2019; Nims and Butler Reference Nims and Butler2019). Our identification of the Lehi horse as an early domestic rather than an Ice Age horse suggests that prior misclassifications may have influenced museum collection practices and the interpretation of archaeological and paleontological assemblages, leading to gaps in the faunal record of Native horsemanship. Consequently, the reevaluation of horse skeletons in natural history collections, focusing on both osteological markers of human activity and biomolecular analyses, appears warranted—and such studies may significantly change our understanding of the timing and nature of early Indigenous horse use in the Americas.
Acknowledgments
This research was funded by an award from the National Science Foundation (Horses and Human Societies in the American West, Award #1949305). Additional osteological and isotopic research was funded by the Max Planck Institute for the Science of Human History, with supplementary funding by the Haus der Kulturen der Welt “Mississippi: An Anthropocene River” project. Special thanks to the Hill Family for reporting the discovery of the specimen and to Dr. Juan Bautista Belardi for translating the Spanish language version of the abstract. This project also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 681605).
Data Availability Statement
All data collected during this research are provided in the manuscript and supplemental materials.
Supplemental Materials
For supplemental material accompanying this article, visit https://doi.org/10.1017/aaq.2020.109.
Supplemental Text 1. Lehi Horse Stratigraphic Description.
Supplemental Text 2. Zonkey Analysis Report.
Supplemental Figure 1. Horse burial from the early seventeenth-century Saxman site in Kansas, informally excavated and disposed of during the 1970s, on the assumption that it was modern.
Supplemental Figure 2. Still photo from a video recorded during excavation; photos of the subsurface stratigraphy during excavation; photos of the subsurface stratigraphy directly adjacent to the horse remains during excavation.















