INTRODUCTION
Leishmaniasis is a neglected vector-borne tropical infection found in 98 countries in tropical and subtropical regions of the world. About 2 million new cases of leishmaniasis occur each year worldwide (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). The disease is clinically complex with two major syndromes, visceral and cutaneous leishmaniasis (VL and CL). Additionally, other clinical presentations do occur, including the mucocutaneous, diffuse and disseminated forms of CL and post-kala-azar dermal leishmaniasis (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012).
There is a limited number of proven treatment options for CL. Pentavalent antimonials are still the first line drugs, although reported therapeutic efficacy of standard schemes (20 mg kg−1 of antimony daily for 20 days) is now as low as 55% in some studies conducted in Brazil or 40% in Iran (Machado et al. Reference Machado, Ampuero, Guimaraes, Villasboas, Rocha, Schriefer, Sousa, Talhari, Penna and Carvalho2010; Chrusciak-Talhari et al. Reference Chrusciak-Talhari, Dietze, Chrusciak Talhari, da Silva, Gadelha Yamashita, de Oliveira Penna, Lima Machado and Talhari2011; Neves et al. Reference Neves, Talhari, Gadelha, Silva Junior, Guerra, Ferreira and Talhari2011; Dastgheib et al. Reference Dastgheib, Naseri and Mirashe2012).
Pentamidine and amphotericin B represent second line drugs, which are of limited use due to their toxicity and cost (Alvar et al. Reference Alvar, Croft and Olliaro2006). Oral miltefosine shows species-dependent efficacy against CL and treatment failures have been reported (Soto et al. Reference Soto, Arana, Toledo, Rizzo, Vega, Diaz, Luz, Gutierrez, Arboleda, Berman, Junge, Engel and Sindermann2004; Yardley et al. Reference Yardley, Croft, De Doncker, Dujardin, Koirala, Rijal, Miranda, Llanos-Cuentas and Chappuis2005). Overall, chemotherapeutic agents available for leishmaniasis treatment are limited by problems such as toxicity, variable efficacy and cost but are still used due to the lack of other options. Therefore, the development of new therapeutic options for CL treatment remains essential.
Tamoxifen, an anticancer agent, has proven to be active in vitro against all Old and New World Leishmania species tested so far. Oral and parenteral tamoxifen are effective in experimental models of CL and VL (Miguel et al. Reference Miguel, Yokoyama-Yasunaka and Uliana2008, Reference Miguel, Zauli-Nascimento, Yokoyama-Yasunaka, Katz, Barbieri and Uliana2009; Eissa et al. Reference Eissa, Amer and El Sawy2011). Tamoxifen has been shown to display additive interactions with amphotericin B (Trinconi et al. Reference Trinconi, Reimao, Yokoyama-Yasunaka, Miguel and Uliana2014) and miltefosine (Trinconi et al. Reference Trinconi, Reimao, Coelho and Uliana2016).
Topical therapy for CL has been recommended due to its theoretical local potentiation, low cost and ease of administration, without the requirements for a complex health system infrastructure (Modabber et al. Reference Modabber, Buffet, Torreele, Milon and Croft2007). On the other hand, adoption of drug combination schemes in leishmaniasis has been also recommended with the aim of increasing therapeutic efficacy and preventing resistance emergence. Ideally, the reduction in dose and time of treatment would also result in lower toxicity and greater patient's compliance (Modabber et al. Reference Modabber, Buffet, Torreele, Milon and Croft2007; Meheus et al. Reference Meheus, Balasegaram, Olliaro, Sundar, Rijal, Faiz and Boelaert2010).
With these elements in mind, we set out to investigate the effect of tamoxifen administered by the topical route and its effect in co-administration schemes with pentavalent antimonial in vitro and in vivo, using a model of infection with Leishmania amazonensis.
MATERIALS AND METHODS
Parasites and macrophages
Leishmania (Leishmania) amazonensis (La-WT) (MHOM/BR/1973/M2269) promastigotes were grown in M-199 medium supplemented with 10% heat inactivated-fetal calf serum (Gibco™, Invitrogen Corporation, NY, USA), 25 mm HEPES (pH 6·9), 12 mm NaHCO3, 7·6 mm haemin, 50 U mL−1 penicillin and 50 µg mL−1 streptomycin at 25 °C. Promastigotes of a L. amazonensis transgenic line expressing luciferase (La-LUC) (Reimao et al. Reference Reimao, Trinconi, Yokoyama-Yasunaka, Miguel, Kalil and Uliana2013) were grown in the same medium supplemented with 32 µg mL−1 G418 (Geneticin™, Sigma-Aldrich, St. Louis, MO, USA). Bone marrow-derived macrophages (BMDM) were obtained from BALB⁄c mice as described (Reimao et al. Reference Reimao, Trinconi, Yokoyama-Yasunaka, Miguel, Kalil and Uliana2013).
Drugs
Tamoxifen and tamoxifen citrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of tamoxifen (10 mm) were prepared in dimethyl sulfoxide (DMSO) and kept at −20 °C. Subsequent dilutions were done in culture media. For in vivo topical experiments, 1% tamoxifen was prepared in ethanol (w/v) or formulated as a cream with 0·1% tamoxifen citrate using an oil-free base (Drogaderma, São Paulo, Brazil).
Meglumine antimoniate (Glucantime™, 300 mg mL−1, Aventis-Pharma, Brazil) was kindly donated by the Brazilian Ministry of Health. Glucantime™ solution contained 300 mg mL−1 meglumine antimoniate corresponding to 81 mg mL−1 pentavalent antimonial (SbV). Antimonial doses mentioned henceforth refer to the concentration of SbV. For in vivo experiments, stock solutions of Glucantime™ were prepared in sterile saline (0·9% NaCl).
Infection and treatment of mice
Animal experiments were performed in strict accordance with the recommendations and guidelines of the Sociedade Brasileira de Ciência de Animais de Laboratório (SBCAL) and of the Conselho Nacional de Controle da Experimentação Animal (CONCEA) and were approved by the Ethics Committee for Animal Experimentation (Protocol: 178/2012) of the Biomedical Sciences Institute, University of São Paulo.
Female BALB/c mice (60 days old) were inoculated with 106 stationary-phase La-LUC promastigotes at the base of the tail. Treatment was initiated when lesions were apparent (3–8 weeks). Mice were assigned into experimental groups (n = 4–8), so that the average lesion size was equivalent in all groups.
For the topical administration, 44 µL of 1% tamoxifen in ethanol (0·44 µg or 20 mg kg−1) were applied over the lesion daily. The ethanolic solution was applied dropwise, slowly, until completely dried, once a day during 15 consecutive days. Control groups included untreated mice and a group treated with ethanol.
Treatment efficacy was evaluated by clinical parameters. Lesion size was recorded weekly as the mean of tail base diameters (in horizontal and vertical directions). Lesion growth was considered as the lesion size on the day of observation subtracted from the lesion size at day 0 (the day before starting the treatment) for the same animal. Measurements were taken with a calliper (Mitutoyo Corp., Japan).
In vivo evaluation of topical tamoxifen as a single drug or co-administered with parenteral meglumine antimoniate
Animal infection, distribution into experimental groups and topical treatment of tamoxifen in ethanol were performed as described in the previous section, except for the duration. Topical treatment performed with the 0·1% citrate tamoxifen cream was performed in animals kept individualized in cages covered with filter paper to avoid mechanical removal of the drug. Topical treatment with both drug formulations was applied daily for 30 consecutive days and compared with treated control groups receiving the same vehicle used to formulate tamoxifen.
Treatment with SbV 40 or 80 mg kg−1 day−1, corresponding to twice or four times the SbV dose recommended for human therapy, was given by the intraperitoneal route in 200 µL final volume during 20 consecutive days as monotherapy or in combination with topical tamoxifen.
Clinical evaluation of treated and untreated animals was obtained as described above. Parasite burden was determined at the end of treatment and 4 weeks after the end of treatment through luciferase detection by bioimaging as described (Reimao et al. Reference Reimao, Trinconi, Yokoyama-Yasunaka, Miguel, Kalil and Uliana2013; Trinconi et al. Reference Trinconi, Reimao, Yokoyama-Yasunaka, Miguel and Uliana2014). In brief, the luminescence intensity of a fixed-size region of interest was determined 20 min after luciferin administration (75 mg kg−1, i.p.) (VivoGlo™ Luciferin, Promega, Madison, WI, USA). Images were collected through high-resolution mode with 2 min of exposure time (IVIS Spectrum, Caliper Life Sciences, Inc. MA/USA). Results were quantified with Living Image software version 4.3·1 (Caliper Life Sciences, Inc. MA/USA) and expressed as the number of photons s−1 cm−2 sr−1.
Assessment of tamoxifen and SbV interaction in intracellular amastigotes
BMDM were infected with La-LUC stationary phase promastigotes as described previously (Trinconi et al. Reference Trinconi, Reimao, Yokoyama-Yasunaka, Miguel and Uliana2014). Infected macrophages were treated with tamoxifen (0·19–12 µ m) and SbV (18·75–1200 µg mL−1) for 120 h. The viability of intracellular amastigotes was determined using the One Glo™ Luciferase Assay System (Promega Corporation, Madison, WI, USA), as previously described (Trinconi et al. Reference Trinconi, Reimao, Yokoyama-Yasunaka, Miguel and Uliana2014). Briefly, at the end of the experiment the medium was replaced with 100 µL of PBS plus 20 µL of OneGlo™ reagent at room temperature followed by homogenization. Luminescence was recorded in a microplate reader (Polarstar Omega, BMG Labtech, Ortenberg, Germany). The luminescence reading from treated wells was used to calculate sigmoidal regression curves using untreated infected macrophages as controls. Each point was tested in triplicate. In some experiments, the morphology of treated cells was evaluated through optical microscopy after panoptical staining, as described (Reimao et al. Reference Reimao, Trinconi, Yokoyama-Yasunaka, Miguel, Kalil and Uliana2013).
The interactions between drugs were evaluated by a modified isobologram method (Fivelman et al. Reference Fivelman, Adagu and Warhurst2004; Seifert and Croft, Reference Seifert and Croft2006), as described (Trinconi et al. Reference Trinconi, Reimao, Yokoyama-Yasunaka, Miguel and Uliana2014). Briefly, the highest concentrations of drugs were prepared in ratios of 5:0, 4:1, 3:2, 2:3, 1:4 and 0:5 (tamoxifen: SbV) and a dose–response curve was constructed for each drug combination. The EC50 of tamoxifen and SbV were determined for each ratio. The fractional inhibitory concentration index (FICI), the sum of FICI (∑FICI) and the mean sum of FICI (x∑FICI) were determined as described (Fivelman et al. Reference Fivelman, Adagu and Warhurst2004; Seifert and Croft, Reference Seifert and Croft2006). Isobolograms were built plotting the FICI values for each drug ratio. The ∑FICI was used to classify the drug interactions as follows: x∑FICI ⩽ 0·5 indicates synergism, 0·5 > x∑FICI ⩽ 4 indicates indifferent/additive interaction and x∑FICI > 4 indicates antagonism (Odds, Reference Odds2003). Two independent experiments were performed in duplicate for each drug combination assay.
Statistical analysis
Data were analysed for statistical significance by the t-Student's test or one-way ANOVA, followed by the Tukey post-test, using GraphPad Prism 5 software. P values ⩽ 0·05 were considered statistically significant.
RESULTS
In vivo efficacy of topical tamoxifen
The efficacy of topically administered tamoxifen was investigated in L. amazonensis infected BALB/c mice. As an initial proof-of-concept, an experiment was performed applying 1% tamoxifen solutions prepared in ethanol onto the tail lesions of L. amazonensis infected mice, daily, for 15 days. The outcome in the vehicle control group treated with ethanol was indistinguishable from untreated animals (data not shown). At the end of treatment, lesion size was significantly reduced in animals treated with topical tamoxifen as compared with control animals, untreated and treated with vehicle (Fig. 1A and data not shown). This effect was sustained since a significant reduction in lesion size was observed 4 weeks after the interruption of treatment (Fig. 1B).
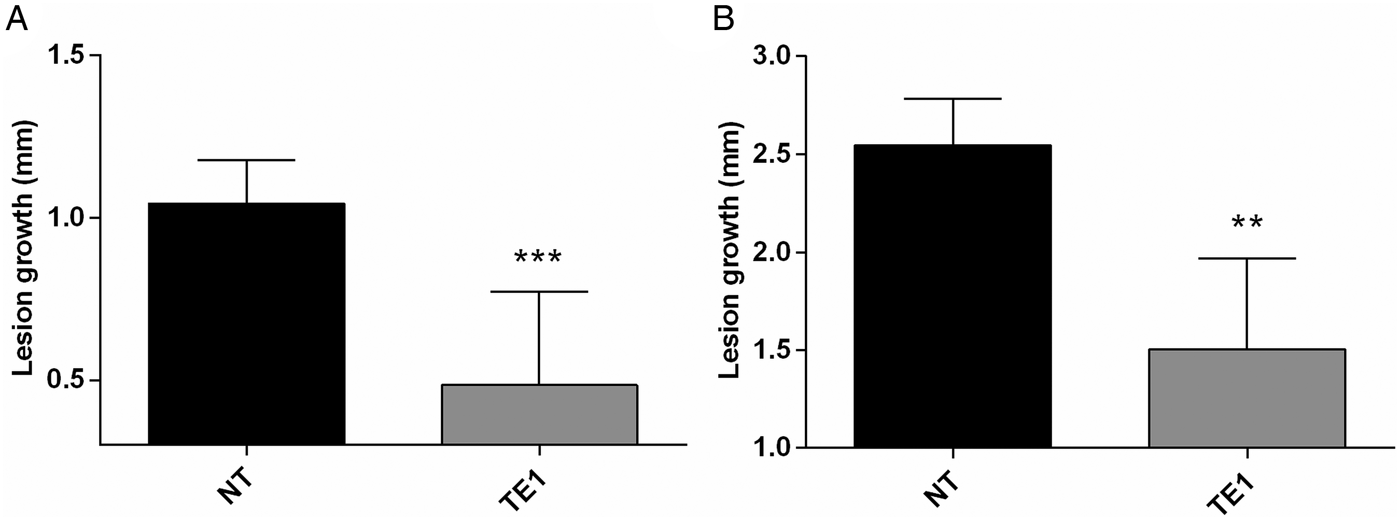
Fig. 1. Efficacy of topical tamoxifen in the treatment of L. amazonensis-infected mice. Mice were treated topically with 1% tamoxifen dissolved in ethanol. Treatment started 8 weeks post-infection and was given for 15 days. Data represent the mean size of lesions and standard deviation at the end of treatment (A) and 4 weeks after the end of treatment (B). NT, untreated group; TE1, treated with 1% tamoxifen. **P = 0·005; ***P = 0·0005.
We then searched for a tamoxifen formulation of potential clinical relevance. A cream formulation of 0·1% citrate tamoxifen in oil-free cream was chosen given its previous use in the long-term therapy of cheloids (Macena et al. Reference Macena, Ávila, Mattar, Daguer, Ruiz and Filho2006; Pasquetti et al. Reference Pasquetti, Mota, Santos and Brito2010). Starting 3 weeks post-infection, mice were treated with 1% tamoxifen in ethanol or 0·1% tamoxifen citrate in oil-free cream for 30 days (Fig. 2). Control groups were left untreated or were treated with the oil-free cream vehicle. A reduction in lesion size at the end of the treatment was observed in the vehicle group, probably due the moisturizing effect of the cream. Mice treated with 0·1% tamoxifen cream displayed a discrete skin reaction at the lesion site with erythema and oedema at the end of treatment. The localized oedema disappeared with the interruption of treatment and was undetectable 1 week later (59th day post-infection). The follow-up of tamoxifen treated mice showed a sustained and significant reduction in lesion size, when compared with the control groups (Fig. 2). Interestingly, 0·1% tamoxifen formulated as a cream was more effective than 1% tamoxifen applied as an ethanolic solution, although neither resulted in full lesion healing.
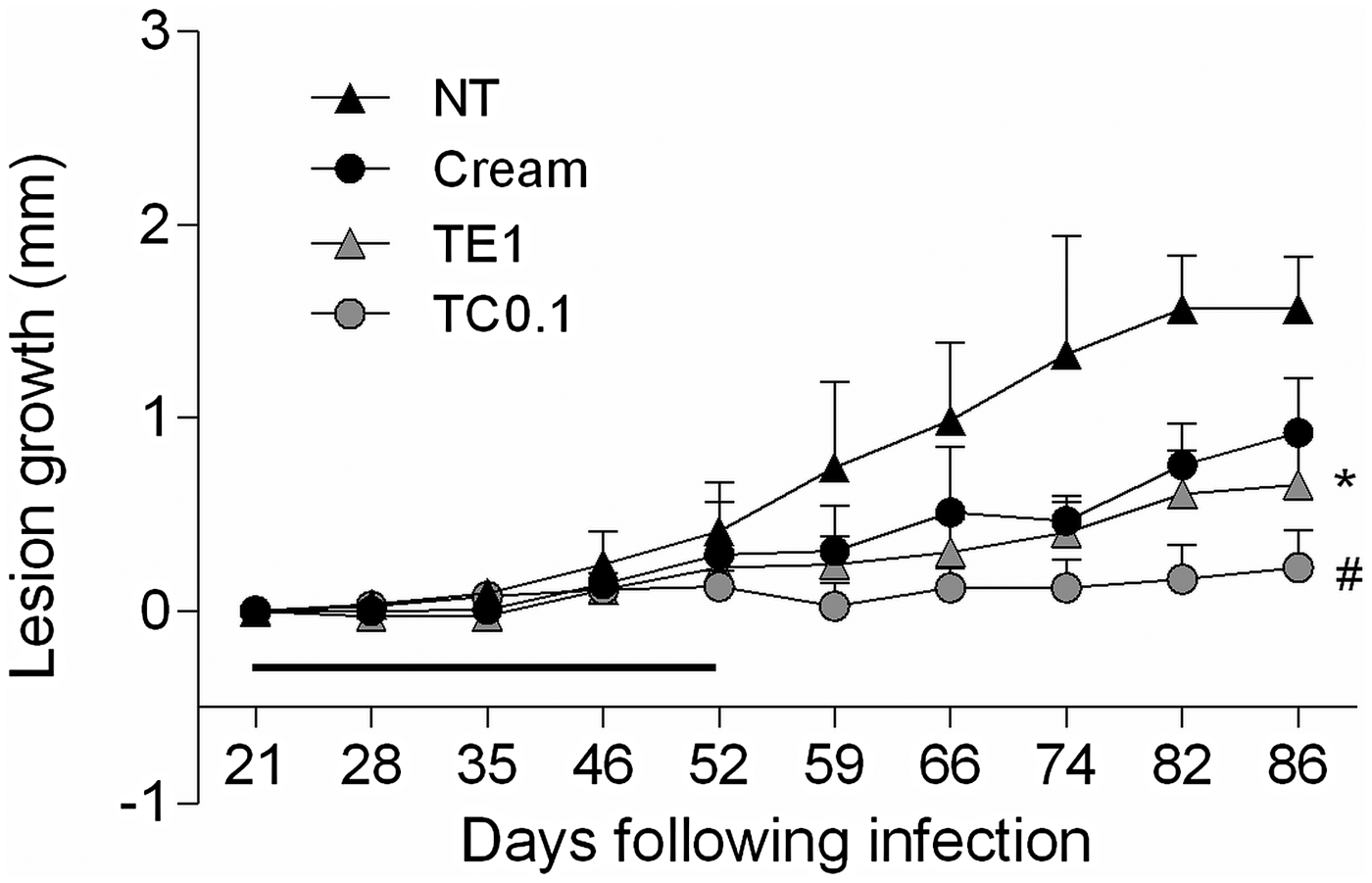
Fig. 2. Follow up of L. amazonensis infection in BALB/c mice treated with topical tamoxifen. Progression of lesion size (mean ± s.d.) in L. amazonensis-infected mice untreated (NT) or treated with 1% tamoxifen diluted in ethanol (TE1), 0·1% citrate tamoxifen formulated as a cream (TC0·1) and the control vehicle without drug (Cream). The horizontal black bar indicates the period of topical treatment, which was initiated 3 weeks post-infection. *P < 0·01, #P < 0·0001.
Efficacy of tamoxifen administered topically as single therapy or associated with systemic SbV
We were then interested in testing the efficacy of topical tamoxifen as part of a combination scheme with the first choice drug for CL treatment in Brazil, meglumine antimoniate.
In vitro interactions between tamoxifen and SbV were tested in L. amazonensis intracellular amastigotes. The analysis of EC50 determinations in various drug combinations indicated a sum of FICI of 1·05 (Table 1) indicating an additive interaction between the two drugs. This was confirmed by isobologram analysis (Supplementary Fig. 1), justifying the investigation of co-administration schemes of tamoxifen and SbV in an in vivo model.
Table 1. EC50 and FICI of tamoxifen and Sbv combinations against L. amazonensis intracellular amastigotes
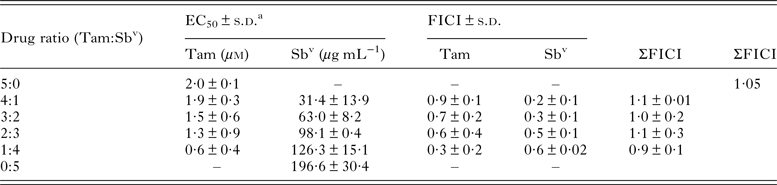
a 50% effective concentration ± standard deviation of two independent experiments. Values were determined against intracellular amastigotes in infected macrophages by luminescence.
Tam, tamoxifen; SbV: pentavalent antimonial; FICI, fractional inhibitory concentration ± s.d. of two independent experiments; ΣFICI, sum of FICIs; xΣFICI, mean sum of FICIs.
Three weeks after infection, groups of mice were assigned to be treated with topical tamoxifen alone (1% diluted in ethanol or 0·1% formulated as a cream), SbV alone in two different doses (40 or 80 mg kg−1 day−1) or with the combination of the two drugs. Topical tamoxifen was applied for 30 days and SbV was given for 20 days.
Figure 3 shows the clinical and parasitological evaluation at the end of treatment and 4 weeks after the interruption of treatment. At the end of treatment, all treated groups had significant reductions in lesion size compared with the untreated group, with the exception of 40 mg Kg−1 day−1 SbV monotherapy (Fig. 3A). Co-administration of tamoxifen (1% in ethanol or 0·1% as a cream) and SbV resulted in a more pronounced reduction in lesion size and parasite load than 40 or 80 mg kg−1 day−1 SbV single therapy (Fig. 3A and B). There was no significant difference between groups treated with 0·1% tamoxifen alone or combined with antimonial. The parasite burden of representative animals from each group at the end of the treatment is shown in Fig. 4A.
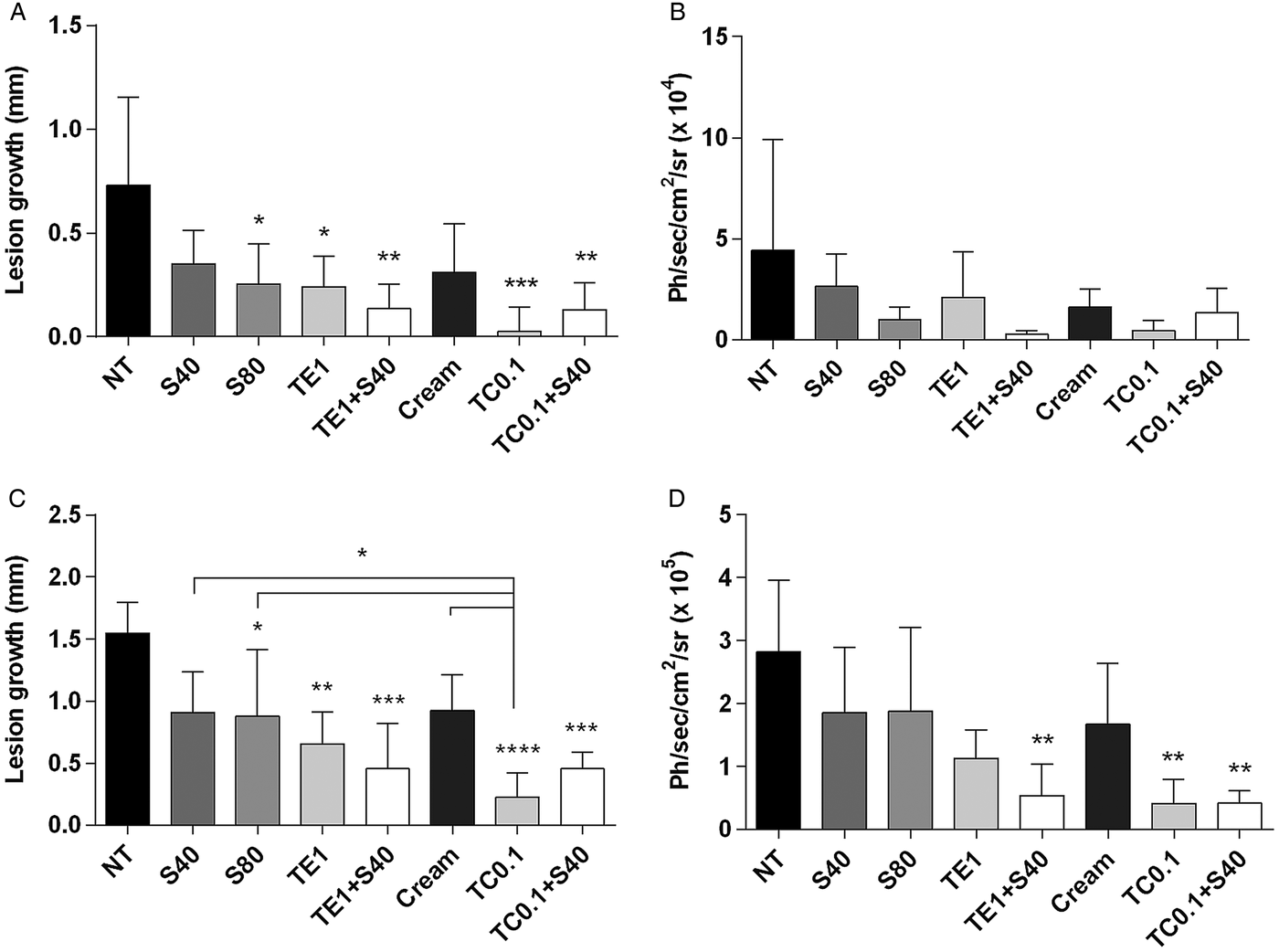
Fig. 3. Efficacy of topical tamoxifen alone or in combination with pentavalent antimonial in the treatment of L. amazonensis-infected mice. (A–D) Efficacy of topical tamoxifen as single therapy or in combination with SbV: evaluation of lesion size (A, C) and parasite burden (B, D) in mice at the end of treatment (A, B) and 4 weeks after the end of treatment (C, D). Mice were treated with topical tamoxifen for 30 days and/or Sbv for 20 days. The mean (n = 5) and standard deviation of the data are indicated. NT, untreated; Cream, treated with oil-free cream (vehicle control group); S40, S80, treated with 40 or 80 mg kg−1 day−1 Sbv i.p.; TE1, treated with 1% tamoxifen in ethanol; TC0·1, treated with 0·1% tamoxifen citrate in oil-free cream. *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001. Ph s−1 cm−2sr−1: photons per second per square centimetre per steradian.
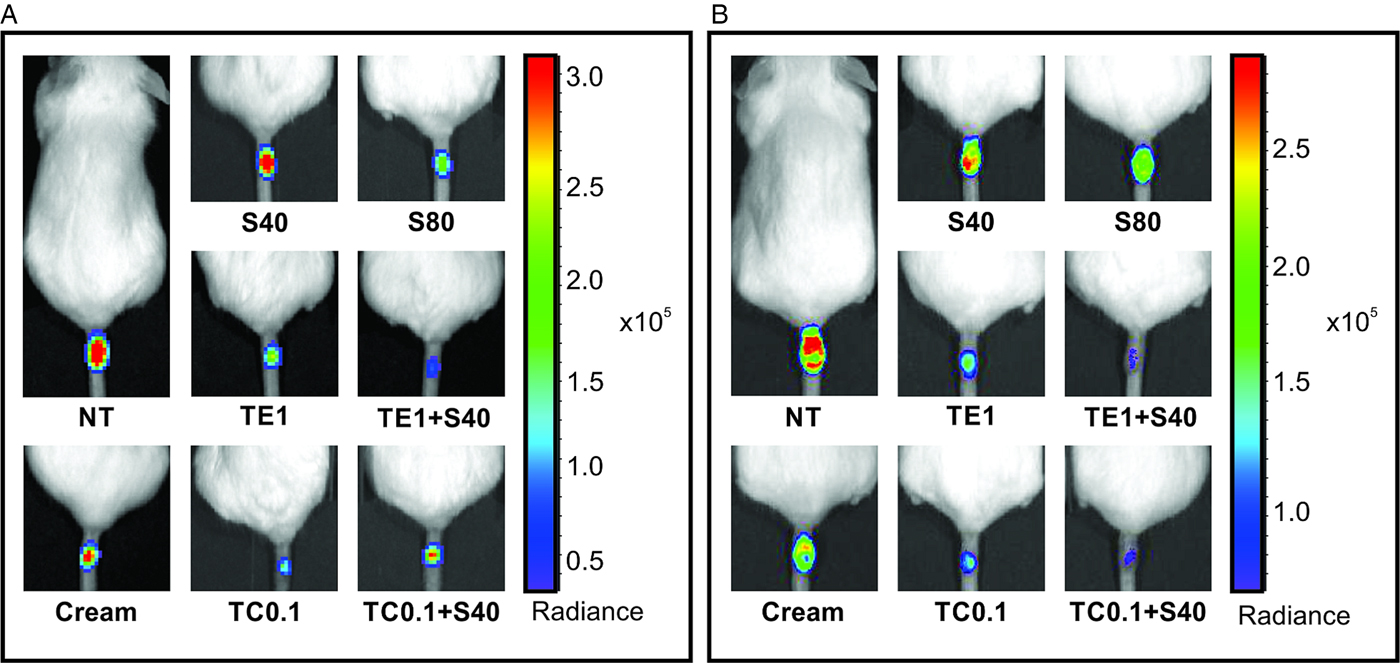
Fig. 4. Parasite burden in L. amazonensis-infected mice treated with tamoxifen topical formulations and/or pentavalent antimonial. Representative animals treated with single or combined tamoxifen topical formulations (1% ethanolic solution or 0·1% oil-free cream) and pentavalent antimonial (40 or 80 mg kg−1 day−1, i.p.) at the end of treatment (A) and 4 weeks later (B). NT, untreated; Cream, treated with oil-free cream (vehicle control group), TE1, treated with 1% tamoxifen in ethanolic solution; TC0·1, treated with 0·1% tamoxifen citrate oil-free cream, S40, S80, treated with 40 or 80 mg kg−1 day−1 Sbv. Ph s−1 cm−2 sr−1: photons per second per square centimetre per steradian. The bar on the right shows a pseudo-colour scale representing light intensities.
Four weeks after the end of the treatment, significant reduction in lesion size and parasite burden was observed in animals treated with 0·1% tamoxifen as compared with the control animals (Figs 3C, D and 4B). Tamoxifen 0·1% formulated as a cream resulted in significant clinical and parasitological responses, when compared with SbV monotherapy schemes. Groups treated with the combination of topical tamoxifen and SbV had improved outcome, both clinical and parasitological, as compared with SbV monotherapy groups. On the other hand, the outcome of the combination was not significantly different from topical tamoxifen alone. Bioimaging of representative animals from each group at this late time point is shown in Fig. 4B.
DISCUSSION
Drug delivery through the skin as a tool for CL treatment may be advantageous if used cautiously: administration is easy, the risk of systemic side-effects is reduced and hepatic first-pass metabolism is avoided, resulting in reduction in the amount of drug to be administered (Paudel et al. Reference Paudel, Milewski, Swadley, Brogden, Ghosh and Stinchcomb2010).
Here we investigated the leishmanicidal activity of tamoxifen when given by the topical route. Initial experiments were performed with ethanol as a dilutant since it has been widely used in pharmaceutical preparations and cosmetic products as a transdermal delivery system (Lachenmeier, Reference Lachenmeier2008) and is capable of increasing drug partitioning into the stratum corneum. The efficacy of this formulation was evaluated in L. amazonensis infections, a very aggressive CL experimental model. The significant improvement of lesion size in treated animals compared to the control group at the end of the treatment and 4 weeks later demonstrated the activity and clinical efficacy of tamoxifen administered by the topical route.
Tamoxifen has been previously applied topically to the treatment and prevention of human hypertrophic scars and keloids (Hu et al. Reference Hu, Hughes and Cherry1998; Gragnani et al. Reference Gragnani, Warde, Furtado and Ferreira2010; Mousavi et al. Reference Mousavi, Raaiszadeh, Aminseresht and Behjoo2010). A formulation containing 0·1% tamoxifen citrate in oil-free cream applied twice a day for 4–12 months to post-burn keloid scars was reported to be effective, with minimal side-effects (Macena et al. Reference Macena, Ávila, Mattar, Daguer, Ruiz and Filho2006; Pasquetti et al. Reference Pasquetti, Mota, Santos and Brito2010). Topical treatment with 0·1% tamoxifen citrate cream formulation controlled the lesion growth in a sustained manner, as evaluated 4 weeks after the end of treatment. The parasite burden was also significantly reduced when compared with untreated groups at this late time point. Interestingly, the 0·1% cream formulation was more effective than the 1% ethanolic solution, both clinically and parasitologically, probably due to a more adequate formulation.
Although topical treatment for CL may have various appealing advantages, in various instances it could be considered unsafe if applied as monotherapy. That would be the case in infections with Leishmania braziliensis or Leishmania guyanensis, where mucocutaneous or disseminated disease may develop. The same considerations would apply to other New World species. In these circumstances, the use of a second, systemic drug may be instrumental. Considering that the treatment success of one course of meglumine antimoniate treatment in L. braziliensis and L. guyanensis infections may be as low as 53–55% (Machado et al. Reference Machado, Ampuero, Guimaraes, Villasboas, Rocha, Schriefer, Sousa, Talhari, Penna and Carvalho2010; Chrusciak-Talhari et al. Reference Chrusciak-Talhari, Dietze, Chrusciak Talhari, da Silva, Gadelha Yamashita, de Oliveira Penna, Lima Machado and Talhari2011; Neves et al. Reference Neves, Talhari, Gadelha, Silva Junior, Guerra, Ferreira and Talhari2011), a new associated tool may prove of great value.
Based on these factors, topical efficacy of tamoxifen was also evaluated in combination with meglumine antimoniate, the main pentavalent antimonial used in Latin America for the treatment of leishmaniasis. In vitro studies with fixed-ratio dilutions and isobologram analysis demonstrated an additive interaction between tamoxifen and SbV against intracellular amastigotes with the absence of toxicity to the murine BMDM. The interaction between tamoxifen and SbV was tested in vivo using both topical formulations of tamoxifen (formulated in ethanol and cream) and SbV given by the parenteral route. The efficacy of the combination was superior to the response observed in groups treated with antimonial alone and equivalent to topical tamoxifen administered as a cream.
CL can cause extensive lesions and result in large scars mostly in exposed parts of the body, as hands and face. The impact of scarring may be extensive leading to social exclusion (Reithinger et al. Reference Reithinger, Aadil, Kolaczinski, Mohsen and Hami2005; Kassi et al. Reference Kassi, Kassi, Afghan, Rehman and Kasi2008). Collagen remodelling observed upon treatment of human keloids and hypertrophic scars with tamoxifen has clinical impact, measured as improved skin texture, reduced pruritus and reduced scarred area (Macena et al. Reference Macena, Ávila, Mattar, Daguer, Ruiz and Filho2006; Gragnani et al. Reference Gragnani, Warde, Furtado and Ferreira2010; Pasquetti et al. Reference Pasquetti, Mota, Santos and Brito2010). These aspects are potentially beneficial for the treatment of CL as well.
In conclusion, topical use of tamoxifen is effective for the treatment of CL in this experimental model. The co-administration of topical tamoxifen with SbV resulted in improved and sustained efficacy in comparison with SbV monotherapy, even for groups treated with high doses of SbV, illustrating the advantages of combining the two drugs. The tamoxifen oil-free cream is a low-cost formulation, safe to humans and can be easily administered, representing an alternative to be tested for the treatment of human CL.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017000130
ACKNOWLEDGEMENTS
The use of bioimaging equipment, provided by CEFAP-ICB was instrumental to this work.
FINANCIAL SUPPORT
This work was supported by the São Paulo Research Foundation (FAPESP) (grant numbers 2011/20484-7 and 2015/09080-2); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number 473343/2012-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). DCM, CTT, JQR and CRE were supported by FAPESP fellowships (grant numbers 2005/59881-0, 2011/18858-6, 2011/21970-2 and 2015/05130-5, respectively). JQR and VB received support from PNPD-CAPES and JKUYY was supported by a CNPq fellowship (grant number 473343/2012-6). SRBU is the recipient of a senior researcher scholarship from CNPq.








