Introduction
Invasive meningococcal disease (IMD) is characterised by high mortality and morbidity, particularly in children [Reference Burman1, Reference Nadel and Ninis2]. The incidence of IMD peaks in children under 5 years of age and in adolescents and young adults, with the greatest mortality in the elderly [Reference Martinón-Torres3, 4]. In Europe, the incidence rate of IMD has slowly declined over the period 2000–2016 [Reference Maiden5], with an age-standardised rate of 0.64 confirmed cases per 100,000 and a case fatality rate of 10.4% in 2016 [6]. Six meningococcal serogroups are responsible for the majority of IMD, serogroups A, B, C, W, X and Y. In 2017, 96% of confirmed cases of IMD in Europe were caused by serogroups B, C, W and Y, with serogroup B causing 51% of confirmed cases [4], however, the contribution of each serogroup varies across the individual European countries. Recent increases have been seen in cases of IMD due to serogroups W and Y in several European countries [4, Reference Booy7–Reference Knol10]; in 2016 these accounted for 17% and 12% of cases, respectively, with the highest case-fatality rates observed for serogroup W (14%) [4]. As within Europe, the global patterns of IMD vary; in North America, the predominant serogroups are B and Y; in South America, serogroups B and C are the most prevalent; while in Australia, serogroup B contributes the most to IMD [Reference Shaker, Fayad and Dbaibo11]. Increases in IMD due to serogroup W are also being seen in other regions, such as Australia and Canada [Reference Martin12–Reference Borrow14].
Vaccination remains the best strategy to control meningococcal disease [Reference Bosis, Mayer and Esposito15]; however, prevention of IMD is challenging given its dynamic and unpredictable epidemiology [Reference Booy7]. Meningococcal polysaccharide protein-conjugated vaccines (MCVs) using carrier proteins (diphtheria toxoid (DT), diphtheria CRM197 (CRM) or tetanus toxoid (TT)) are available against single meningococcal serogroups (PsA-TT (MenAfriVac™; Serum Institute of India, Pune, India) against serogroup A for 1–29-year-olds in sub-Saharan Africa; MenC-CRM (Menjugate®; GlaxoSmithKline Vaccines Srl, Siena, Italy) and MenC-TT (NeisVac-C™; Pfizer Ltd, Kent, UK) against serogroup C, for children from 2 months of age, adolescents and adults in Europe and North America) and against multiple serogroups (quadrivalent vaccines (MCV4) against A, C, W and Y: MCV4-DT (Menactra®, Sanofi Pasteur, USA) for individuals ≥9 months to 55 years of age in the USA; MCV4-TT (Nimenrix®, Pfizer Europe, Belgium) for individuals ≥6 weeks of age in the European Union; and MCV4-CRM (Menveo®, GlaxoSmithKline, Italy) for individuals 2 months to 55 years of age in the USA and ≥2 years in the European Union).
The meningococcal vaccination schedule varies across the individual European countries [16], with differences between countries only partly accounted for by differences in local IMD epidemiology. Modification of the vaccination schedule was seen with the introduction of monovalent MenC conjugate (MCC) vaccines, firstly in the UK in 1999, followed by several other countries in Europe, which led to a significant decrease in IMD due to MenC [Reference Shaker, Fayad and Dbaibo11, Reference Trotter17–Reference Jafri19]. Vaccination with MCC vaccines has now been introduced into the national routine childhood immunisation programmes of 15 EU/EEA countries [16], where MenC-TT vaccine is more widely used than MenC-CRM. More recently, in response to the increasing incidence of cases due to serogroup W in Europe, there has been a progressive switch from MCC to MCV4 vaccines in childhood; thus, among those countries that recommend meningococcal vaccine during childhood, some use a mixture of MCC and MCV4 vaccines according to age group (e.g. Italy, Spain and the UK) while others offer exclusively MCC (e.g. Belgium, France and Germany) or exclusively MCV4 (e.g. the Netherlands) [4, 16, Reference Presa20].
MenACYW-TT vaccine (Sanofi Pasteur, USA) is an investigational tetanus toxoid-conjugate quadrivalent meningococcal vaccine developed for use in individuals 6 weeks of age and older. Previous studies have demonstrated its immunogenicity and safety in toddlers, children, adolescents and adults (including those >65 years) [Reference Anez21–Reference Kirstein24]. This study was conducted in Europe to evaluate the immunogenicity and safety of a single dose of MenACYW-TT compared to a single dose of MCV4-TT in toddlers aged 12–23 months who were either meningococcal vaccine-naïve or who had received a MCC vaccine in the first year of life.
Methods
Study design and participants
This was a Phase III, modified double-blind, randomised, parallel-group, an active-controlled study conducted from 24 February 2017 to 26 October 2017 (study period) at 34 sites over four countries; Germany, Finland, Hungary and Spain (NCT02955797). Healthy toddlers aged 12–23 months were recruited if they were either meningococcal vaccine-naïve or if they had received at least one dose of MCC vaccine prior to 12 months of age (MCC-primed). Participating countries were selected according to their national immunisation programmes/recommendations, in order to include both meningococcal vaccine-naïve and MCC-primed participants: in Germany, MCC vaccine is recommended from only 12 months of age and Finland does not include a meningococcal vaccine in their national immunisation programme; while in Hungary and Spain, MCC vaccines are recommended from infancy. Participants were eligible for inclusion if their parents or guardians had provided signed informed consent prior to the start of the study and they had received all recommended standard-of-care non-meningococcal vaccinations according to their age as per local regulations. Participants were excluded if they had been involved in another clinical trial in the 4 weeks prior to the start of the present study, or had planned simultaneous participation in another trial, receipt of any vaccine in the 28 days preceding the trial vaccination or planned to receive another vaccine prior to Visit 2 (30–44 days after the vaccination at Day 0), except for influenza vaccination, or had previous vaccination with a MCV4 or with a meningococcal B vaccine. Among the other exclusion criteria are a history of an Arthus-like reaction after vaccination with a tetanus toxoid-containing vaccine, a history of Guillain-Barré syndrome (GBS), or meningococcal infection confirmed either clinically, serologically, or microbiologically, or a demonstrable high risk of meningococcal infection during the trial or known systemic hypersensitivity to any of the vaccine's components, or a history of a life-threatening reaction to these components.
Meningococcal vaccine-naïve participants were randomised 1:1 by an Interactive Web Response System to receive a single dose at Day 0 of either MenACYW-TT or MCV4-TT vaccine and MCC-primed participants were randomised 2:1 to receive a single dose at Day 0 of either MenACYW-TT or MCV4-TT vaccine. MCC-primed participants were also stratified by the priming vaccine (MenC-TT (NeisVac-C®) or MenC-CRM (Menjugate®)); to ensure adequate representation of participants with both the MCC vaccines, 25–50% of participants had to be primed with MenC-CRM. Participants, their parents/guardians, investigators and study technicians were unaware of treatment assignments throughout the study. An unblinded vaccine administrator administered the appropriate vaccine but was not involved in the collection of safety data.
MenACYW-TT (Sanofi Pasteur) was supplied as 0.5 ml liquid solution in single-use vials and contained 10 μg of each serogroup (A, C, W and Y) and approximately 55 μg of tetanus toxoid protein carrier per dose. MCV4-TT (Nimenrix®, Pfizer) was supplied as a powder in a vial with the accompanying solvent in a pre-filled syringe for reconstitution to 0.5 ml and contained 5 μg of each serogroup (A, C, W and Y) and 44 μg of tetanus toxoid protein carrier per dose. Both vaccines were administered intramuscularly.
The appropriate independent ethics committees and institutional review boards approved the study prior to its initiation. The conduct of this study was consistent with standards established by the Declaration of Helsinki and compliant with the International Conference on Harmonisation guidelines for Good Clinical Practice, including all local and/or national regulations and directives.
Immunogenicity
All participants were to provide blood samples for immunogenicity assessments at baseline (Day 0; pre-vaccination) and at Day 30 (30–44) post-vaccination. A serum bactericidal antibody assay using human complement (hSBA; Global Clinical Immunology, Sanofi Pasteur, Swiftwater, PA, USA) was used to measure functional antibodies against meningococcal serogroups A, C, W and Y with a lower limit of quantification (LLOQ) of 1:4. In a subset of participants, a serum bactericidal assay with rabbit complement (rSBA; Public Health England, Manchester, UK) was used to measure antibodies against meningococcal serogroups A, C, W and Y with a LLOQ of 1:4. Seroprotection was defined as hSBA titres ≥1:8 at Day 30 and hSBA vaccine seroresponse was defined for a participant with a pre-vaccination titre <1:8 as a post-vaccination titre ≥1:16 and for a participant with a pre-vaccination titre ≥1:8, as at least a 4-fold greater titre than pre-vaccination. rSBA vaccine seroresponse was defined as a post-vaccination rSBA titre ≥1:32 for participants with pre-vaccination rSBA titre <1:8, or a post-vaccination titre ≥4 times the pre-vaccination titre for participants with pre-vaccination rSBA titre ≥1:8 [Reference Findlow, Balmer and Borrow25–Reference Pina27].
The co-primary objectives of this study assessed the non-inferiority of a single-dose of MenACYW-TT vs. MCV4-TT in the proportion of participants with hSBA seroprotection to each of the meningococcal serogroups A, C, W and Y at Day 30 in those who were meningococcal vaccine-naïve and in participants who were either meningococcal vaccine-naïve or MCC-primed. The secondary objectives were the comparisons of antibody responses in terms of geometric mean titres (GMTs) measured by hSBA at Day 0 and Day 30 after MenACYW-TT or MCV4-TT in meningococcal vaccine-naïve participants, MCC-primed participants and in participants who were either meningococcal vaccine-naïve or MCC-primed.
Additional observational objectives described the functional antibody responses to MenACYW-TT and MCV4-TT as measured by rSBA, and also described the antibody vaccine seroresponse to serogroups A, C, W and Y, measured by hSBA at Day 0 and Day 30 in MenACYW-TT or MCV4-TT recipients who were meningococcal vaccine-naïve, MCC-primed and in participants who were either meningococcal vaccine-naïve or MCC-primed.
Safety
All participants were observed for 30 min after vaccination to assess the occurrence of unsolicited systemic adverse events (AEs). Participants' parents/guardians were provided with diary cards and instructed to record information on solicited injection site reactions (tenderness, erythema and swelling) and systemic reactions (fever, vomiting, abnormal crying, drowsiness, appetite loss, irritability) from Day 0 to Day 7 post-vaccination and unsolicited AEs through to Day 30. Serious AEs (SAEs) were recorded throughout the duration of the study for each participant (from vaccination up to Day 30 + up to 14 days permitted window for the final visit). Parents or guardians were asked to inform the Investigators of any potential SAEs immediately. AEs of special interest (AESI; included as a requirement of EU regulators) were generalised seizures (febrile and non-febrile), Kawasaki disease, GBS and idiopathic thrombocytopenic purpura and were reported as SAEs during the study period. The AESI list was developed as guided by the European Medicines Agency driven by generic AEs observed with the currently licensed MCV4s and was not based on any events or signals observed with this investigational vaccine.
Statistical analyses
With the planned enrolment of 510 participants in the MenACYW-TT groups and 408 participants in the MCV4-TT groups, the study would have an overall power of 88% to demonstrate both co-primary objectives: (1) non-inferiority of a single-dose of MenACYW-TT to that of MCV4-TT in participants who were meningococcal vaccine-naïve, with an alpha level of 2.5% (1-sided hypotheses) and under the assumption that approximately 80% of participants were evaluable (n = 243 participants per group), the overall power was over 90% using the Farrington and Manning formula; (2) non-inferiority of a single-dose of MenACYW-TT to that of MCV4-TT in participants who were either meningococcal vaccine-naïve or MCC-primed with an alpha level of 2.5% (1-sided hypotheses) and under the assumption that approximately 80% of participants were evaluable, the overall power was over 98% (based on simulations using the Minimum Risk weights method with the null variance).
All immunogenicity analyses were performed on the per-protocol analysis set (PPAS), which comprised participants who met all eligibility criteria, received the study vaccine according to randomisation, had a valid post-vaccination serology result and had no protocol deviations that affected the participant's immune response. Safety assessments were carried out on the safety analysis set (SafAS), which included all participants who had received at least one dose of study vaccine and had safety data available. Categorical variables were summarised and presented as frequency counts with 95% confidence intervals (CIs) calculated using the normal approximation for quantitative data and the exact binomial distribution (Clopper-Pearson method) for percentages.
For both co-primary objectives, the non-inferiority of the hSBA seroprotection for each of the serogroups, A, C, W and Y, was tested separately. If the lower limit of the 2-sided 95% CI of the difference between the MenACYW-TT and MCV4-TT groups in terms of percentages of participants who achieved an hSBA seroprotection was >–10%, the inferiority assumption was rejected. The overall non-inferiority of each co-primary objective was demonstrated if all four individual null hypotheses were rejected. For the first co-primary endpoint, in meningococcal vaccine-naïve participants, the CI of the difference in proportions was computed using the Wilson Score method without continuity correction. For the second co-primary endpoint, non-inferiority in participants who were meningococcal vaccine-naïve or MCC-primed, the 95% CI of the difference was stratified on the priming status (meningococcal vaccine-naïve or MCC-primed) and calculated using the Wald method (normal approximation). The weighted average of the difference over strata was calculated using the Minimal Risk weights with the null variance method.
For GMTs, the 95% CIs of point estimates were calculated using normal approximation assuming they are log-normally distributed. For the comparison of GMTs in participants who either were meningococcal vaccine-naïve or MCC-primed, the 95% CI of the ratio of post-vaccination GMTs was stratified on the priming vaccination status (meningococcal vaccine-naïve or MCC-primed) and calculated using an analysis of variance (ANOVA) model of log10-transformed titres.
Results
Study participants
The study enrolled 918 participants; meningococcal vaccine-naïve participants were enrolled in Finland (n = 356 (38.8%)) and Germany (n = 256 (27.9%)) and MCC-primed participants were enrolled in Hungary (n = 145 (15.8%)) and Spain (n = 161 (17.5%)). Among those who were meningococcal vaccine-naïve, 306 were randomised to receive MenACYW-TT and 306 to receive MCV4-TT; among MCC-primed participants, 203 were randomised to the MenACYW-TT group and 103 to the MCV4-TT group (Fig. 1). Among MCC-primed participants who received MenACYW-TT, 151 (74.4%) and 52 (25.6%) participants were MenC-TT and MenC-CRM-primed, respectively. Of those who received MCV4-TT, 77 (74.8%) and 26 (25.2%) participants were MenC-TT and MenC-CRM-primed, respectively. Baseline demographic characteristics were well-balanced between the vaccine groups (Table 1).
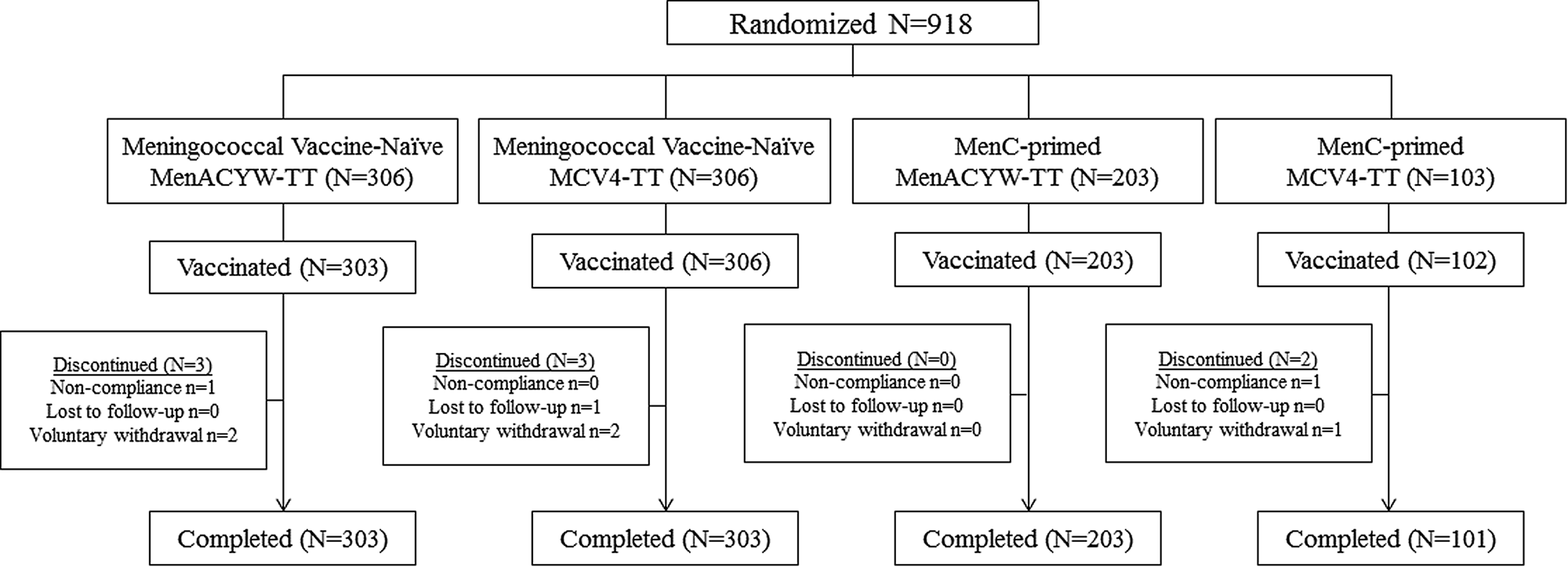
Fig. 1. Participant disposition.
Table 1. Baseline demographics (all randomised participants)
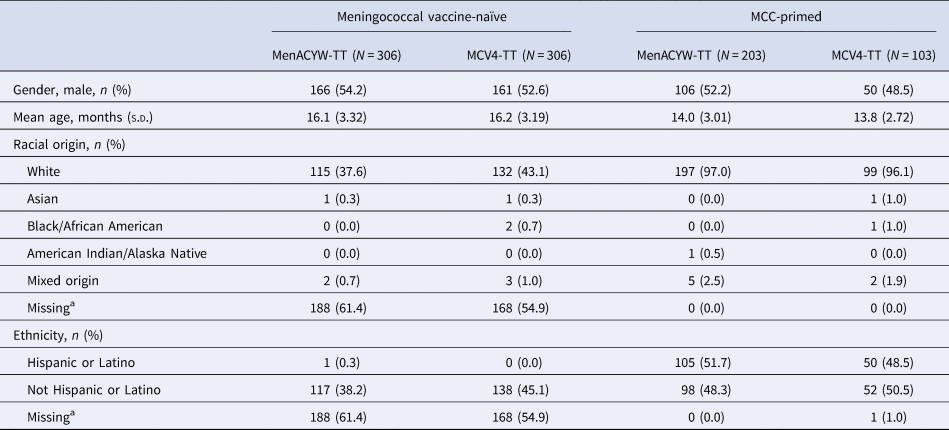
MCC, meningococcal C conjugate vaccine; N, number of participants randomised; n, number of participants fulfilling the item listed; s.d., standard deviation.
a Racial origin and ethnicity were not collected in Finland as per local regulations.
Immunogenicity
The immune response to MenACYW-TT was non-inferior to that induced by MCV4-TT when administered as a single dose in meningococcal vaccine-naïve participants, based on the proportion of participants achieving hSBA seroprotection against all four serogroups at Day 30 (Table 2). The immune response to MenACYW-TT was also non-inferior to that induced by MCV4-TT when administered as a single dose in participants who were either meningococcal vaccine-naïve or MCC-primed, based on the proportion of participants achieving hSBA seroprotection against all four serogroups at Day 30 (Table 3).
Table 2. Non-inferiority of the proportion of meningococcal vaccine-naïve participants who achieved hSBA vaccine seroprotectiona at Day 30 with MenACYW-TT compared with MCV4-TT (PPAS)

CI, confidence interval; n, number of participants with seroprotectiona; N, number of participants with available data for the endpoint.
95% CI of the single percentage calculated from the exact binomial method.
95% CI of the difference calculated from the Wilson Score method without continuity correction.
a Seroprotection defined as hSBA titre ≥1:8.
b The overall non-inferiority will be demonstrated if the lower limit of the 2-sided 95% CI of the difference is >–10% for all four serogroups.
c Data available for 295 participants.
Table 3. Non-inferiority of the hSBA antibody response (seroprotectiona) for MenACYW-TT compared with MCV4-TT at Day 30 in meningococcal vaccine-naïve or MCC-primed participants (PPAS)
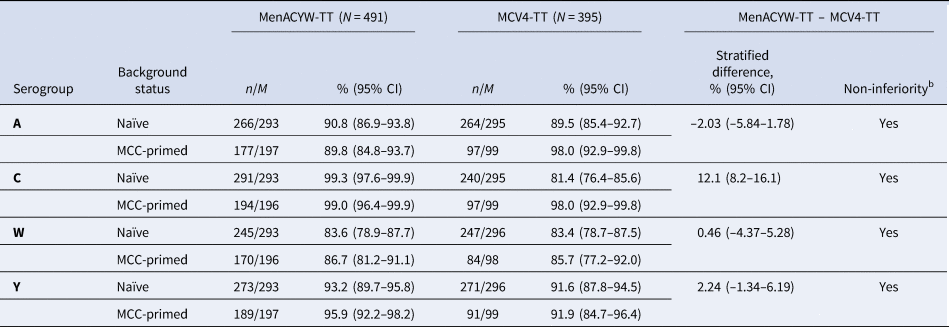
CI, confidence interval; MCC, meningococcal C conjugate vaccine; n, number of participants with seroprotectiona; M, number of participants with available data for the endpoint
95% CI of the single percentage calculated from the exact binomial method.
95% CI stratified on the priming status was calculated using the Wald method (normal approximation)
Weighted average difference over strata calculated using the Minimal Risk weights with the null variance method
a Seroprotection defined as hSBA titre ≥1:8.
b Non-inferiority concluded if the lower limit of the two-sided 95% CI of the overall difference of proportion stratified on the priming status is >–10%.
Following vaccination with either MenACYW-TT or MCV4-TT, the hSBA GMTs increased from baseline for all four serogroups in both those who were meningococcal vaccine-naïve and those who were MCC-primed (Fig. 2). In participants who were meningococcal vaccine-naïve, the hSBA GMTs at Day 30 were higher for MenACYW-TT recipients than MCV4-TT recipients for serogroups C and W, while GMTs for serogroups A and Y were comparable (Fig. 2a). In the participants who were MCC-primed, the hSBA GMTs at Day 30 for serogroup Y were higher in the MenACYW-TT than in the MCV4-TT group, lower for serogroup A and comparable for serogroups C and W (Fig. 2b). Among the MCC-primed participants further sub stratified into relatively small sub groups based on the type of MCC vaccine received at priming, there were some variations in GMTs, particularly for serogroup C where participants in both MenC-TT primed groups had higher GMTs than those in both MenC-CRM primed groups (Supplementary Table 1); however, this variability was not seen in levels of seroprotection which were comparable (Supplementary Table 2). For serogroup A, GMTs at Day 30 were higher for both MCC priming vaccines in the MCV4-TT group than in the MenACYW-TT group, with higher GMTs seen for MenC-TT primed participants (Supplementary Table 1). The seroprotection against serogroup A did not differ between MenACYW-TT and MCV4-TT groups who were primed with MenC-TT. In the MenACYW-TT group, seroprotection rates were lower for participants primed with MenC-CRM than participants primed with MenC-TT (Supplementary Table 2).
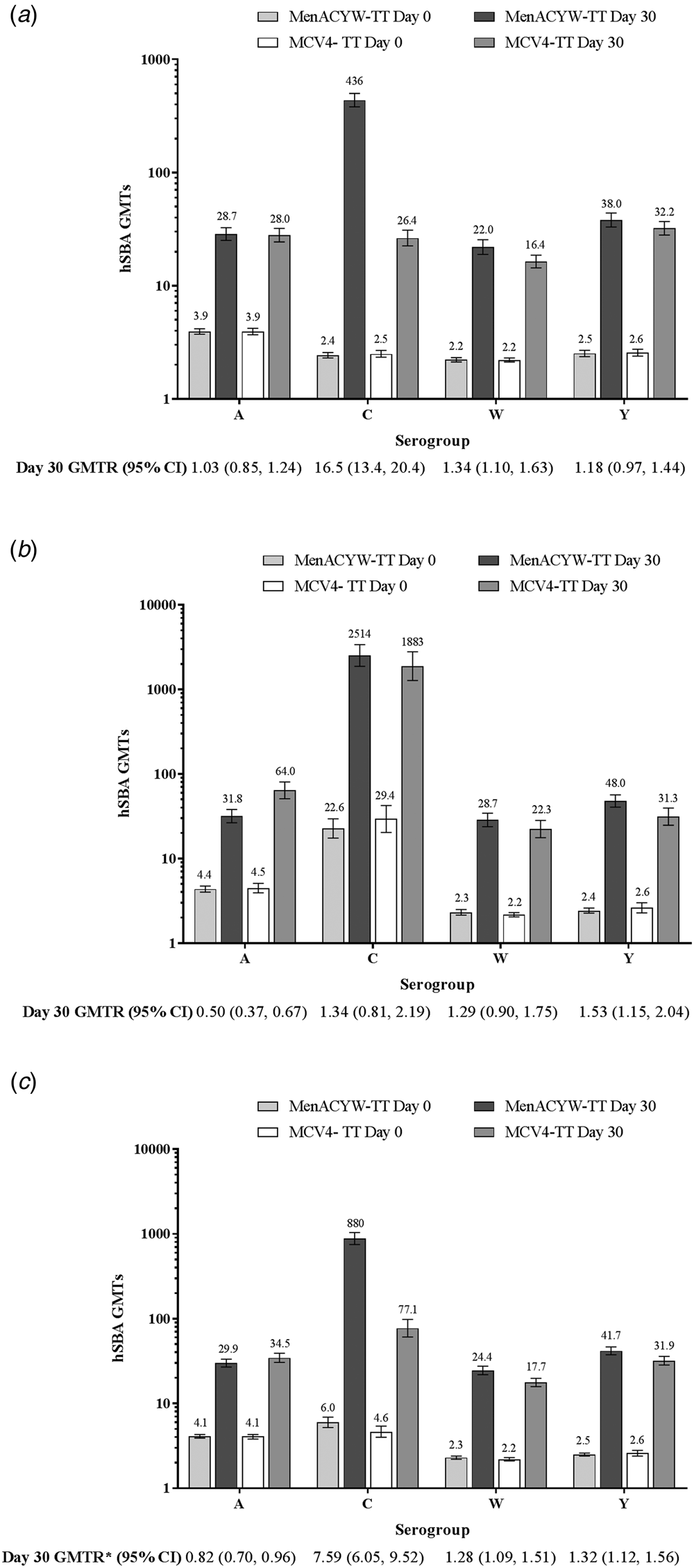
Fig. 2. hSBA GMTs at baseline and Day 30 in the MenACYW-TT and MCV4-TT groups in those who were (a) vaccine-naïve, (b) MCC-primed or (c) vaccine-naïve and MCC-primed combined (PPAS). Error bars indicate 95% CI. GMTR, geometric mean titre ratio (Day 30 MenACYW-TT/MCV4-TT). GMTR*, ratio stratified on priming vaccination background and 95% CI calculated using an analysis of variance model of log10-transformed titres.
Thirty days after vaccination, in the pooled population of both vaccine-naïve and MCC-primed participants, the proportion with an hSBA vaccine seroresponse, was higher in the MenACYW-TT group than in the MCV4-TT group for serogroup C and comparable for serogroups A, W and Y, while hSBA GMTs were comparable for serogroup A and higher for C, W and Y in the MenACYW-TT group (Fig. 2c and Fig. 3).
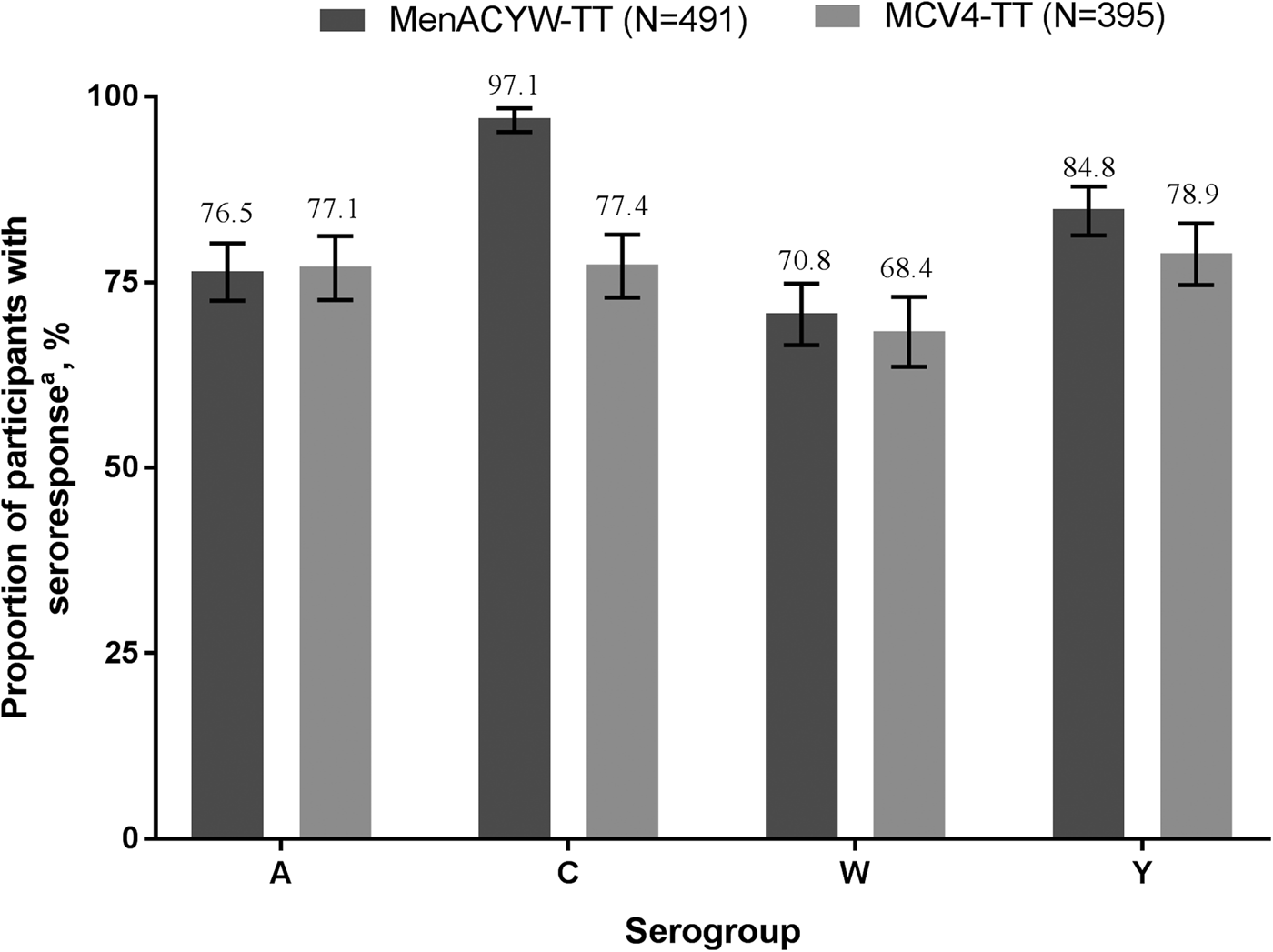
Fig. 3. Proportion of participants with hSBA seroresponsea at Day 30 (PPAS). MenACYW-TT or MCV4-TT recipients who were either meningococcal vaccine-naïve or MCC-primed. *Seroresponse defined as follows: for a participant with a pre-vaccination titre <1:8, the post-vaccination titre must be ≥1:16 and for a participant with a pre-vaccination titre ≥1:8, the post-vaccination titre must be a least 4-fold greater than the pre-vaccination titre. Error bars indicate 95% CI.
In the subgroup of participants with rSBA antibody titres, at Day 30 the proportion with titres ≥1:8 and ≥1:128 were comparable between vaccine groups for the combined population of either meningococcal vaccine-naïve or MCC-primed (Supplementary Table 3), as were the proportions of participants with rSBA vaccine seroresponse (MenACYW-TT A: 91.8% (95% CI 86.8–95.3); C: 97.8% (95% CI 94.5–99.4); W 97.8% (95% CI 94.5–99.4); Y: 98.4 (95% CI 95.3–99.7) and MCV4-TT A: 98.7% (95% CI 95.3–99.8); C: 98.0% (95% CI 94.2–99.6); W 99.3% (95% CI 96.3–100.0); Y: 96.6 (95% CI 92.3–98.9)).
Safety
In the combined groups of those who were meningococcal-naïve or MCC-primed, the safety profiles of MenACYW-TT and MCV4-TT were comparable (Table 4). The proportion of participants reporting at least one solicited systemic reaction and injection site reaction of any grade was similar in both vaccine groups, with the most commonly reported injection-site reaction being injection site tenderness and injection site erythema and the most commonly reported solicited systemic reactions being irritability, abnormal crying and appetite loss. There were no immediate unsolicited AEs or adverse reactions (ARs) in either vaccine group, or any discontinuations from the study or death due to an AE, AR or SAE. Seven participants experienced at least 1 SAE. None of these SAEs was considered as related to the investigational vaccine by the investigator. The percentages of participants who reported at least 1 SAE were less than 1% and comparable between MenACYW-TT and MCV4-TT recipients. Two AESI were reported in the MenACYW-TT group: febrile partial seizure with concomitant viral upper respiratory tract infection occurred in one participant 23 days after vaccination and generalised non-febrile convulsions following accidental trauma occurred in another participant 6 days after vaccination; neither was considered as related to the vaccination by the investigator and the sponsor. The most commonly reported non-serious systemic AEs were infections and infestations, with upper respiratory tract infection the most frequently reported in 12.5% (63/506) and 11.3% (46/408) of participants in the MenACYW-TT and MCV4-TT groups, respectively. The proportion of participants who had reported at least one Grade 3 unsolicited non-serious systemic AE were similar in both groups (6.1% (31/506) vs. 5.4% (22/408) in the MenACYW-TT compared with MCV4-TT group, respectively). The percentages of participants who reported at least one unsolicited non-serious systemic AR were low and comparable between MenACYW-TT and MCV4-TT-recipients: 2.4% (12/506) and 2.7% (11/408), respectively. None of these events was of Grade 3 intensity.
Table 4. Safety overview after vaccine injection (SafAS)
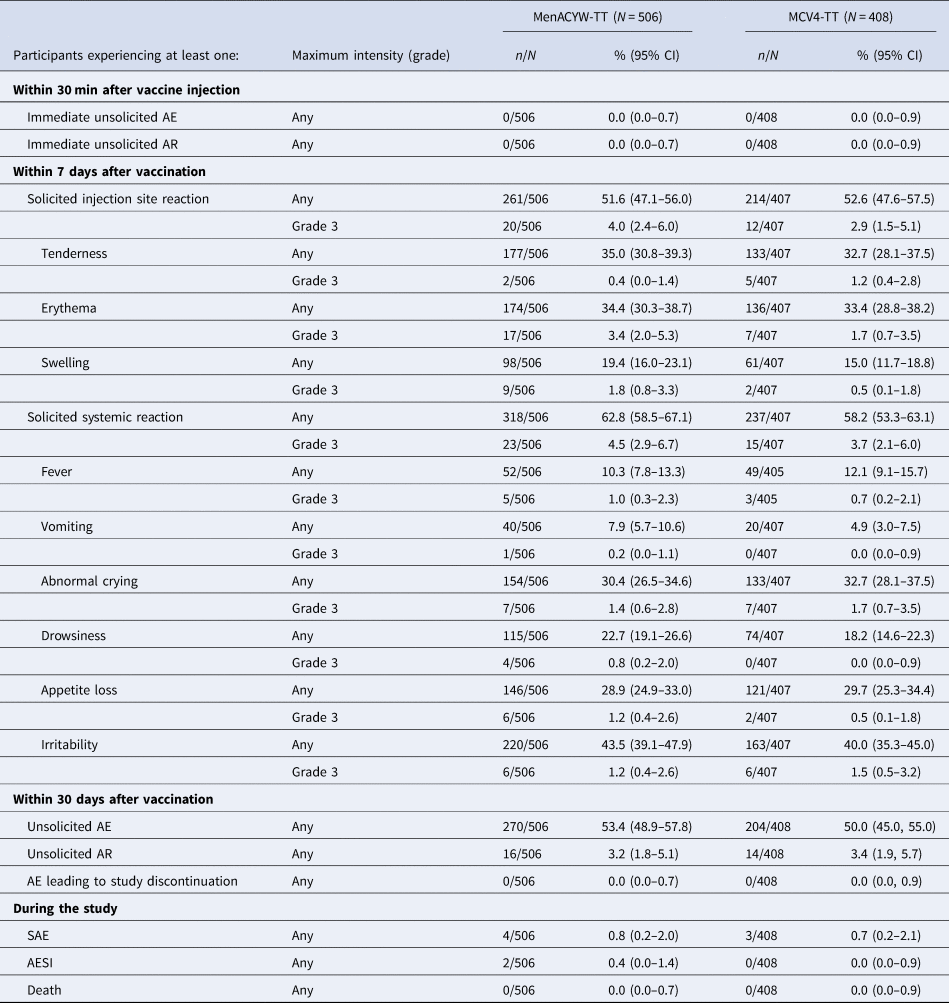
AE, adverse event; n, number of participants who reported an AE; N, number of participants in the safety analysis set.
Solicited reactions tended to be reported with higher frequency in meningococcal vaccine-naïve toddlers than in MCC-primed toddlers in both the MenACYW-TT and MCV4-TT treatment groups, particularly for solicited injection site reactions (Supplementary Table 4). The safety profile of MenACYW-TT was comparable to that of MCV4-TT in meningococcal vaccine-naïve toddlers. In MCC-primed toddlers, solicited reactions and especially solicited injection site reactions, tended to be reported with higher frequency in toddlers vaccinated with MenACYW-TT as compared to toddlers vaccinated with MCV4-TT (albeit with overlapping 95% CIs). Nevertheless, both Grade 3 solicited injection site and Grade 3 systemic reactions were reported in less than 5% of subjects after MenACYW-TT and did not raise any safety concerns.
Discussion
The immune response to MenACYW-TT was non-inferior to that of the licensed MCV4-TT when administered as a single dose in toddlers 12–23 months of age, regardless of their meningococcal vaccine background (either meningococcal vaccine-naïve or had received monovalent MCC vaccination during infancy) and both MenACYW-TT and MCV4-TT were well tolerated. Seroprotection against the four meningococcal serogroups was demonstrated in 81–99% of participants in both vaccine arms, with high antibody titres also observed for all serogroups. Particularly high titres were seen against serogroup C with MenACYW-TT, in both meningococcal vaccine-naïve and MCC-primed toddlers and slightly lower titres were seen against serogroup A with MenACYW-TT compared with MCV4-TT in MCC-primed toddlers.
Previous studies of the effect of subsequent meningococcal vaccines following MCC priming have demonstrated differences in titres based on the protein conjugate of the primary and booster vaccines, with the highest titres for serogroup C seen with MCV4-TT [Reference Ishola28] or MenC-TT [Reference Ladhani29, Reference Pace30] boosting after MenC-TT priming. In this study, we also saw the highest titres for serogroup C in the MenC-TT primed groups, as compared to MenC-CRM primed, following MenACYW-TT or MCV4-TT vaccination. Although differences were observed for serogroup A GMTs between the two vaccines in participants primed with MCC vaccines, the percentages of participants achieving a hSBA seroprotection were high (≥ 89%) in both meningococcal vaccine-naïve and MCC-primed toddlers after both vaccines. The lower immune response to serogroup A after vaccination with MenACYW-TT as compared to MCV4-TT was only observed in MCC-primed toddlers who had received a the MenC-CRM vaccine, which contributed approximately 25% of the MCC-primed population (49 participants who received the MenACYW-TT vaccine and 25 participants who received the MCV4-TT vaccine). Since the corresponding differences were not observed when these study sub groups were evaluated using seroprotection as an endpoint, these results in a very small population should be interpreted with caution.
The robust immune response induced by a single dose of MenACYW-TT in this study indicates that MenACYW-TT would be an effective vaccine against IMD by all four serogroups in those who are meningococcal vaccine-naïve, as well as in those who had previous exposure to a MCC vaccine during the first year of life. Vaccination programmes against IMD are effective, as demonstrated by the introduction of MCC vaccination and the corresponding decrease in the incidence of IMD due to MenC [Reference Shaker, Fayad and Dbaibo11, Reference Trotter17–Reference Jafri19]. A switch from MCC to MCV4 vaccines would not only ensure that the protection to serogroup C is maintained, but provide additional benefits of protection against serogroups A, W and Y; indeed, the prevention of the particularly virulent strain of serogroup W which has high morbidity and mortality rates [Reference Krone31, Reference Tsang32, Reference Booy7] and of serogroup Y which has been reported at an increased frequency over the past decade, is of particular importance in several regions [4, Reference Presa20]. Immunisation against multiple serogroups thus allows for wider coverage against IMD and may also result in a better overall vaccine uptake rate. Furthermore, cost-effectiveness studies of routine monovalent MCC vs. MCV4 vaccinations in Canada have shown potential cost benefits for the use of MCV4 vaccines over monovalent MCC vaccines in vaccination programmes [Reference De Wals and Zhou33, Reference Delea34]. A separate modelling study of MCC and MCV4 vaccination in Canada suggests that routine infant immunisation with MCV4, followed by a booster at 15 years, may have a greater impact on reducing IMD than a MCC vaccine alone [Reference Vickers35].
The first meningococcal vaccines were polysaccharide vaccines, which were immunogenic in adults, but not efficacious during the first 2 years of life and did not elicit long-term memory [Reference Bröker36], consequently, protein conjugated polysaccharide vaccines were developed that triggered T-cell responses in addition to B-cell responses. Several vaccines are often coadministered as part of an immunisation programme, with the potential for both positive and negative immune interference between the protein carriers and vaccines. Current evidence suggests that even where the immune interference is negative, the antibody levels achieved were still above that required for protection [Reference Findlow and Borrow37, Reference Burrage38]. The immune response to the tetanus toxoid conjugate protein component of the two vaccines in this study was not assessed, however previous assessment of MenACYW-TT in a phase II study in toddlers found that the levels of anti-tetanus antibodies increased following vaccination, with no adverse impact [Reference Vesikari23]. This is consistent with that seen with other tetanus toxoid conjugate vaccines [Reference Bröker36] and coadministration of MenACYW-TT with Tdap and HPV4 vaccines in a phase II study of adolescents also demonstrated no negative immune interference [Reference Chang22].
While this study was conducted in countries in Europe in toddlers aged 12–23 months and cannot be generalised to other age groups, the immunogenicity evaluation showed similar trends for immunogenicity endpoints (hSBA and rSBA seroprotection and GMTs), suggesting that MenACYW-TT could be suitable in a broad range of countries regardless of their infant immunisation programme. In those toddlers who had received a MenC vaccination during their first year of life, the numbers who had received previous vaccination with either MenC-TT or MenC-CRM were small and so no inference could be made on any difference in outcomes by priming vaccine type. While both vaccines used in this study are tetanus toxoid conjugates, any similarities or difference in the conjugation chemistry and that may have led to differences in responses between them are unknown; the only known difference is in the quantities of the tetanus toxoid and polysaccharide antigens.
MenACYW-TT was well-tolerated and demonstrated a non-inferior immune response compared with the licensed MCV4-TT vaccine when administered as a single dose to MenC vaccine primed and/or meningococcal vaccine-naïve toddlers aged 12–23 months. The high titres against serogroup C with MenACYW-TT suggest that this quadrivalent vaccine may, in the future, be an alternative to the established monovalent MenC vaccination. The use of quadrivalent vaccines offers the distinct advantage of broadening the protection against other serogroups without requiring further injections to be added to busy paediatric vaccine schedules.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268821000261.
Acknowledgements
The authors would like to thank the participants who volunteered to take part in the study; the investigators (Ildikó Batta, Budapest, Hungary; Éva Horzsa, Budapest, Hungary; Éva Kovács, Szeged, Hungary; István Tokodi, Székesfehérvár, Hungary; Alfonso Carmona Martinez, Sevilla, Spain; Iolanda Jordan Garcia, Barcelona, Spain; Maria Luisa Navarro Gómez, Madrid, Spain; Tiina Karppa, Helsinki, Finland; Anitta Ahonen, Järvenpää, Finland; Satu Kokko, Oulu, Finland; Miia Virta, Tampere, Finland; Marita Paassilta, Espoo, Finland; Ilkka Seppä, Turku, Finland; Matthias Donner, Mönchengladbach, Germany; Rolf Ebert, Tauberbischofheim, Germany; Eivy Franke-Beckmann, Erfurt, Germany; Michael Horn, Schönau, Germany; Roland Knecht, Bretten, Germany; Ralph Köllges, Mönchengladbach, Germany; Thorsten Krause, Goch, Germany; Renate Mangelsdorf-Taxis, Bönnigheim, Germany; Andreas Schmutte, Datteln; Thomas Beck, Bochum, Germany; Friedrich Kaiser, Hamburg, Germany) and their site staff, staff at the Vaccine Evaluation Unit, Public Health England, the NETSTAP-Study Group in Germany (http://www.netstap.de) and the Sanofi Pasteur team which supported the study especially Jennifer Kinsley, Judy Pan, Amy Strickland, Marie-Caroline Guichard and Oscar Borg-Olivier, and the staff of the local Clinical Study Units. Editorial assistance with the preparation of this manuscript was provided by Vicky Hinstridge and Nicola Truss, inScience Communications, Springer Healthcare Ltd, UK and this study was funded by Sanofi Pasteur.
Funding
This study was funded by Sanofi Pasteur.
Author contributions
DvdV, SB'C, EJ and MSD were involved in the study design, TV, BS, FM-T, GM, AF, TA, CDG and RS were involved in data acquisition, all authors contributed to data analysis or interpretation, review and critical revision of the manuscript and final approval for submission.
Conflict of interest
FM-T has received honoraria from GSK, Pfizer, Sanofi Pasteur, MSD, Seqirus and Janssen for taking part in advisory boards and expert meetings, and for acting as speaker in congresses outside the scope of the submitted work. FM-T has also acted as principal investigator in RCTs of the above-mentioned companies as well as Ablynx, Regeneron, Roche, Abbott, Novavax and MedImmune, with honoraria paid to his institution. FM-T research activities received support from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud): Fondo de Investigación Sanitaria (FIS; PI070069/PI1000540/PI1601569/PI1901090) del plan nacional de I + D + I and ‘fondos FEDER’ and 2016-PG071 Consolidación e Estructuración REDES 2016GI-1344 G3VIP (Grupo Gallego de Genética Vacunas Infecciones y Pediatría, ED341D R2016/021). AF, BS, GM, RS, TA and CDG, have no conflicts to declare. TV, received funding to his institute from Sanofi Pasteur for the conduct of this study, has received honoraria from Sanofi Pasteur for advisory boards. DvdV, SB'C, DN, EJ, MSD are employees of Sanofi Pasteur.
Data sharing
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies and process for requesting access can be found at https://www.clinicalstudydatarequest.com
Previous presentation
The data in this study were presented at the 37th Annual Meeting of the European Society of Pediatric Infectious Diseases (ESPID), 6–11 May 2019, Ljubljana, Slovenia.










