Introduction
Baleen whales are the largest animals on Earth, thanks to their ability to filter small prey from seawater using baleen (Pivorunas Reference Pivorunas1979, Werth Reference Werth and Schwenk2000). In contrast to their living relatives, ancient mysticetes were relatively small: at a total body length of 3–4 m, archaic toothed species were diminutive (Fitzgerald Reference Fitzgerald2010, Marx et al. Reference Marx, Tsai and Fordyce2015, Lambert et al. Reference Lambert, Martínez-Cáceres, Bianucci, Di Celma, Salas-Gismondi and Steurbaut2017), and even their baleen-bearing descendants generally stayed below 6 m until the Late Miocene (Slater et al. Reference Slater, Goldbogen and Pyenson2017). The single exception to this pattern is Llanocetus denticrenatus Mitchell 1989 from the latest Eocene of Antarctica, which is estimated to have reached a length of 8 m as early as 34 Ma – possibly, as a result of its Southern Ocean habitat (Fordyce & Marx Reference Fordyce and Marx2018). The present paper shows that L. denticrenatus was neither exceptional, nor the largest of its kind. Three isolated premolar teeth from the Eocene of Antarctica, now housed at the Instituto Antártico Argentino and the Museo de La Plata (Argentina), hint at the existence of a second, substantially larger species of Llanocetus rivalling living baleen whales in size. Together with L. denticrenatus, this new material suggests at least two independent origins of gigantism in mysticete history, and reveals considerable size disparity arising from an early phase of morphological experimentation.
Material and methods
Anatomical descriptions and body size
Dental terminology follows Marx et al. (Reference Marx, Tsai and Fordyce2015), with each tooth considered to have a main denticle (md) flanked by anterior (ad) and posterior (pd) accessory denticles. Denticles are numbered away from md. In the absence of cranial remains, body size was estimated by comparing the size of the upper third premolar with the bizygomatic width of the skull across a variety of archaeocetes and archaic mysticetes. Total body length was then calculated based on bizygomatic width, using the equations of Pyenson & Sponberg (Reference Pyenson and Sponberg2011) and Lambert et al. (Reference Lambert, Bianucci, Post, de Muizon, Salas-Gismondi, Urbina and Reumer2010).
Tooth sharpness measurements
The relative sharpness of the most complete tooth (IAA Pv731) was determined following the method of Hocking et al. (Reference Hocking, Marx, Fitzgerald and Evans2017), which involves a series of individual sharpness measurements of the main denticle and first interdenticular notch (Supplementary Table S1). This is then followed by principal component and discriminant function analyses, both of which compare the new specimen to other archaic mysticetes, archaeocetes, the extinct odontocete Squalodon, and a range of extant terrestrial carnivorans with known feeding strategies (raptorial vs filter feeding).
The tooth was surface scanned using a Go!Scan 20 (Creaform Inc., Canada) with a point spacing of 0.1 mm, and the resulting data assembled into a high resolution 3D model (.ply file format) in Meshlab (Istituto di Scienza e Tecnologie dell'Informazione “A. Faedo” and Consiglio Nazionale delle Ricerche, Italy). Minor cracks in the first posterior interdenticular notch were reconstructed in Geomagic Wrap (Geomagic Inc., North Carolina, USA), using the “curvature” setting of the fill-holes function, which provides a reconstruction based on the curvature of the surrounding undamaged surface mesh. Reconstructions were conservative and underestimate actual sharpness.
Institutional abbreviations
IAA, Instituto Antártico Argentino, San Martín, Argentina; MLP, Museo de La Plata, La Plata, Argentina; OU, Geology Museum, University of Otago, Dunedin, New Zealand; USNM, National Museum of Natural History, Smithsonian Institution, Washington DC, USA.
Results
Systematic palaeontology
Cetacea Brisson, 1762
Mysticeti Gray, 1864
Llanocetidae Mitchell, 1989
Llanocetus Mitchell, 1989
Type species
Llanocetus denticrenatus Mitchell, 1989
Emended diagnosis
Large-sized llanocetid sharing with other members of the family the presence of elongated nasals, low, elongate premolar crowns bearing strong labial and lingual enamel ornaments, and a broad sagittal trough on the parietals lacking a distinct sagittal crest. Differs from Mystacodon in its larger size, and from OU GS10897 in having apically curved accessory denticles and an abruptly depressed anterior entocingulum on the upper premolars.
Llanocetus sp.
Referred material
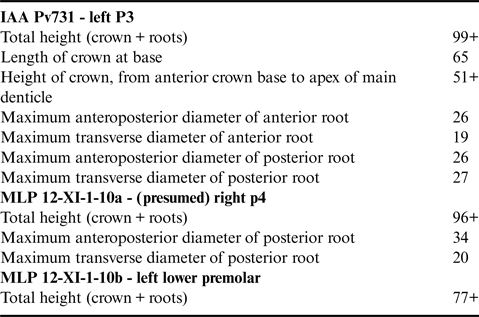
One complete upper third premolar (IAA Pv731) and two fragmentary lower premolars (MLP 12-XI-1-10a,b).
Locality and horizon
The new specimens were recovered from the Submeseta Formation of Seymour Island, Antarctic Peninsula. The La Meseta Formation was originally divided into seven stratigraphic levels, TELMs 1–7 (= Tertiary Eocene La Meseta of Sadler (Reference Sadler1988)), ranging from the upper Ypresian (Early Eocene) to the late Priabonian (Late Eocene). Subsequently, the unit was redefined into the Submeseta and the La Meseta formations (Montes et al. Reference Montes, Nozal, Santillana, Marenssi and Olivero2013).
The highly fossiliferous sediments of the c. 230 m thick Submeseta Formation represent the uppermost part of the infill of the James Ross Basin, a back-arc basin developed on the eastern flank of the Antarctic Peninsula (Del Valle et al. Reference Del Valle, Elliot and Macdonald2004, Marenssi Reference Marenssi2006). This formation comprises mostly poorly consolidated clastic fine-grained sediments, which were deposited in deltaic, estuarine and shallow marine environments (Marenssi et al. Reference Marenssi, Santillana and Rinaldi1998). The Submeseta Formation is characterized by a uniform sandy lithology representing a storm-influenced tidal shelf. It includes three allomembers: Submeseta I (equivalent to TELMs 6 and 7 in partem), Submeseta II (equivalent to TELM 7 in partem), and Submeseta III (equivalent to upper TELM 7). MLP 12-XI-1-10 was recovered from Submeseta II (level 38 of Montes et al. Reference Montes, Nozal, Santillana, Marenssi and Olivero2013), while IAA Pv731 came from the Submeseta III (level 39 of Montes et al. Reference Montes, Nozal, Santillana, Marenssi and Olivero2013).
Magnetostratigraphically calibrated dinocyst biostratigraphy suggests a latest Eocene age (Priabonian) for middle and upper TELM 7 (Douglas et al. Reference Douglas, Affek, Ivany, Houben, Sijp and Sluijs2014), consistent with a mollusc-based 87Sr/86Sr date of 34.2 ± 0.87 Ma for the top of the same unit (Fordyce Reference Fordyce, Prothero, Ivany and Nesbitt2003).
Remarks
The new specimens closely match the archaic mysticete Llanocetus denticrenatus in having low, elongate, palmate crowns with apically curved accessory denticles; an abruptly depressed anterior portion of the entocingulum; strong, elongate to anastomosing enamel ridges both lingually and labially; completely unfused roots, with a broad interradicular space invading the base of the crown; and, especially on the nearly complete upper tooth, well-developed ecto- and entocingula (Fig. 1a & b). They consistently differ from L. denticrenatus in their much larger size (maximum length of P3: 65 vs 42 mm) and greater number of accessory denticles, with four posterior denticles on P3 and six posterior denticles on p4 of Llanocetus sp. being matched by just three and five denticles in L. denticrenatus.
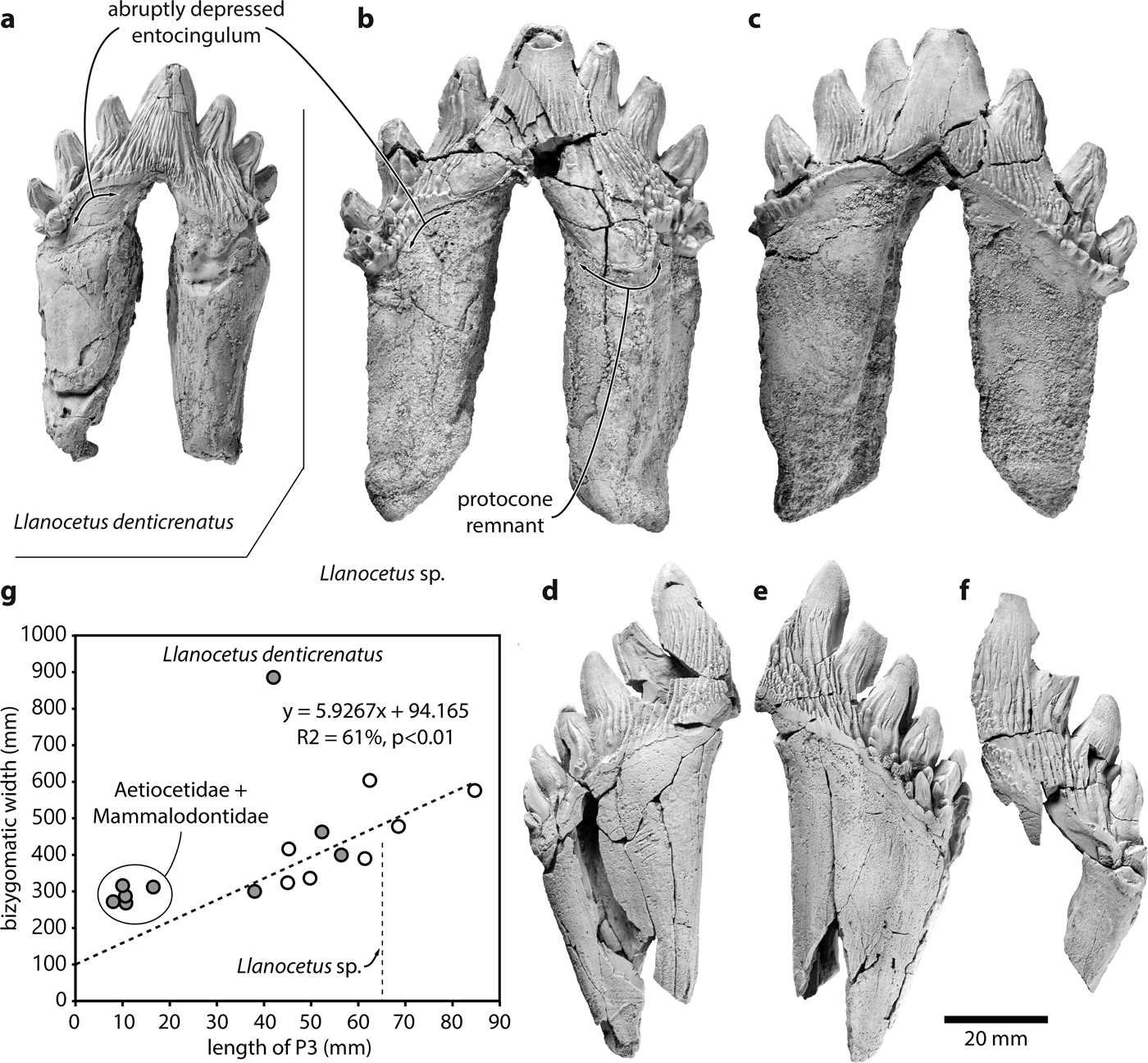
Fig. 1. Teeth of the large Eocene whale Llanocetus sp., and relationship between body and tooth size. Comparison of the left P3 of a. Llanocetus denticrenatus (USNM 183022) and Llanocetus sp. (IAA Pv731) in a., b. lingual and c. labial view; presumed right p4 (MLP 12-XI-1-10a) of Llanocetus sp. in d. labial and e. lingual view; f. left lower premolar (MLP 12-XI-1-10b) of Llanocetus sp. in labial view; g. length of P3 plotted against bizygomatic width (as a proxy for body length); empty circles represent basilosaurids, filled circles archaic mysticetes; the regression line is based on basilosaurids, Coronodon, Mystacodon, and OU GS10897.
Description
IAA Pv731 (Fig. 1b & c; Table I) is nearly complete, and here interpreted as a left P3 based on the presence of a moderately developed protocone remnant and the marked lingual curvature of the crown in anterior or posterior view. The crown consists of a main denticle flanked by three anterior and four posterior denticles, with pd4 inferred from the presence of a large fracture surface posterior to pd3. The roots are robust, elongate, and markedly curved inwards. The posterior root bears well-defined longitudinal troughs both anteriorly and posteriorly. Both the ecto- and the entocingula are well developed, with a generally nodular rim and large cingular denticles on both sides of ad2 and ad3, as well as lingual to pd4.
Table I. Measurements (in mm) of Llanocetus sp.
Enamel ornament on both sides of the crown consists of dorsoventral ridges rising from the cingulum on to each denticle. On ad3 in particular, the ridges are tall and sharp. Especially lingually, but also labial to ad2 and pd3, some of these ridges give rise to a series of denticles near the crown base. All of the major denticles bear anterior and posterior carinae. There is moderate apical abrasion forming windows in the enamel on ad1–pd2 (Fig. 2b). A similar degree of abrasion also seems to occur on three of the anterior cingular denticles, but fracturing of the enamel in this case prevents a clear assessment. As in the P3 of Llanocetus denticrenatus, there is no sign of attrition.
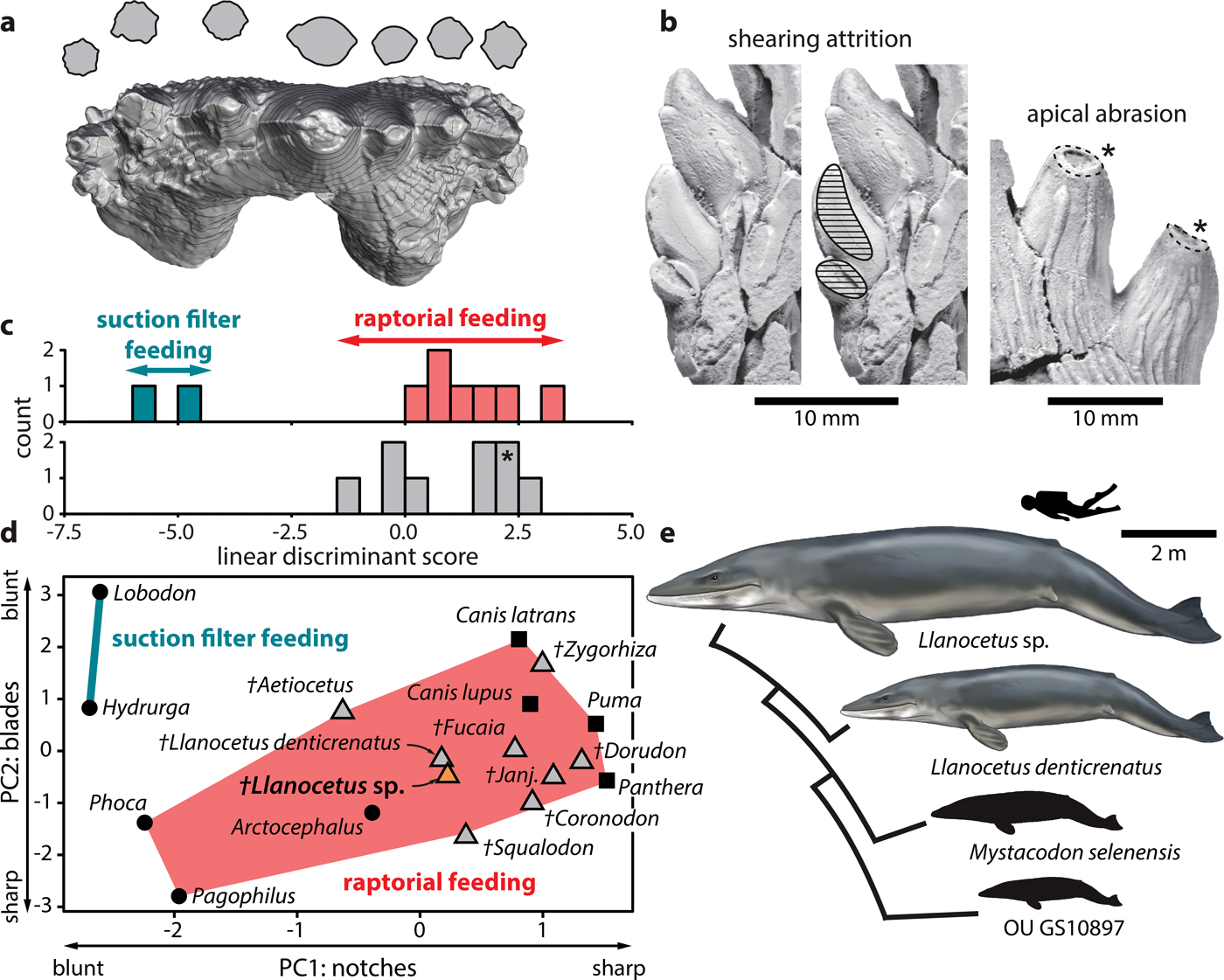
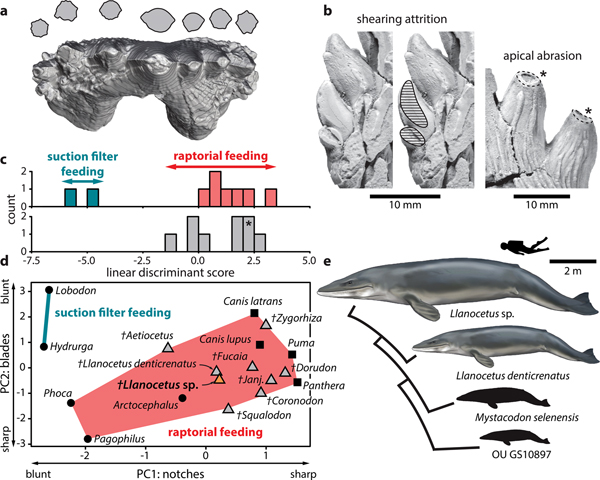
Fig. 2. Feeding strategy of Llanocetus sp. a. three-dimensional reconstruction of the left P3 of Llanocetus sp., with cross sections of the major denticles (at approximately 50% of their reconstructed heights); b. enlarged views of attritional (on MLP 12-XI-1-10a) and abrasive wear (on IAA Pv731); results of the c. discriminant function and d. principal component analyses of tooth sharpness in archaic mysticetes, based on the earlier analysis of Hocking et al. (Reference Hocking, Marx, Fitzgerald and Evans2017); asterisk in c. marks the position of Llanocetus sp.; e. size disparity within Llanocetidae. Life reconstructions of whales by Carl Buell.
MLP 12-XI-1-10a (Fig. 1d & e), here tentatively interpreted as a right p4 based on its size, slender crown, and presence of labial attrition, consists of the posterior half of a tooth bearing six accessory denticles. The root is robust, straight in anterior view, and subdivided into two halves by a longitudinal trough running along its anterior surface. There is no protocone remnant. The ecto-and entocingula are indistinct near the centre of the crown, but extremely well developed posteriorly. As on P3, the enamel ornament consists of sharp, dorsoventrally oriented ridges rising from the cingulum on to the accessory denticles. Lingual to pd3–pd5, denticles arising from some of these ridges merge with cingular denticles to form a ‘forest’ covering the entire surface of the crown. Apical abrasion is present but mild, with no windows in the enamel. The labial surfaces of pd6 and the posteriormost cingular denticle bear small attritional facets.
MLP 12-XI-1-10b (Fig. 1f) is the least complete specimen, preserving only a partial root and the labial side of a fragmentary crown. The tooth is here interpreted as a left lower premolar based on its size and slender crown. There at least four denticles (uncertainly including the main denticle), with the anterior two being badly damaged. Posteriorly, the base of the third denticle gives rise to a notably smaller secondary denticle that partly occludes the space between the third and fourth denticles. The entocingulum is well developed posteriorly, but indistinct along the centre of the crown. Apical abrasion of the two posterior denticles is mild, with no windows in the enamel. There is no obvious sign of attrition.
Body size estimation
Plotting tooth length against bizygomatic width for a sample of archaeocetes and archaic mysticetes reveals a relatively complex pattern (Fig. 1g). The width of the cranium increases linearly with the length of P3 in basilosaurid archaeocetes, Coronodon, Mystacodon and OU GS10897. By contrast, aetiocetids and mammalodontids have somewhat smaller teeth than expected for their size, probably reflecting incipient homodonty and the presence of variably sized diastemata. The picture is further complicated by Llanocetus denticrenatus, which forms an extreme outlier characterized by large body size yet small teeth. This pattern allows for two potential interpretations of the new Llanocetus specimens from Antarctica:
a) Llanocetus denticrenatus is an isolated case, and the new material represents a related species with both absolutely and relatively larger teeth, and little or no diastemata (e.g. Mystacodon). Assuming this species follows the basilosaurid pattern would result in an estimated bizygomatic width of approximately 47.9 cm, and thus a total body length of 4.4–4.6 m.
b) The new Llanocetus specimens are morphologically close to L. denticrenatus, and thus share the peculiar anatomy of its feeding apparatus. This view is supported by the obvious similarity of the teeth (Fig. 1a & b), the geographical proximity of the localities where Llanocetus sp. and L. denticrenatus were found (both Seymour Island, Antarctica), and the absence of the pronounced dental wear characteristic of Mystacodon. In the absence of further comparative data that could inform the relationship between tooth and body size in Llanocetus, the simplest and least assumption-laden estimate is provided by isometric scaling. The latter puts Llanocetus sp. at roughly 1.55 times the length of L. denticrenatus (crown length of P3 = 65 mm vs 42 mm), suggesting a total body length of up to 12 m.
Pending the discovery of better-preserved specimens, this paper argues that Llanocetus sp. and L. denticrenatus are most parsimoniously interpreted as sharing similar overall morphologies, and thus also comparable body proportions.
Tooth sharpness
Significant damage to the tip of the main denticle of IAA Pv731 made it difficult to create an accurate reconstruction, requiring us to take the sagittal and transverse measurements of tip sharpness from the well-preserved third posterior denticle. Visual examination of the main denticle reveals similarly developed anterior and posterior carinae, and suggests a tip shape broadly comparable to that of Llanocetus denticrenatus.
Principal component analysis shows the teeth of Llanocetus sp. to be remarkably sharp. Specifically, the results group IAA Pv731 with Llanocetus denticrenatus, and place both well within the morphospace defined by extant raptorial feeding carnivorans, such as lions, pumas and most pinnipeds – see Hocking et al. (Reference Hocking, Marx, Fitzgerald and Evans2017) for details. Discriminate function analysis corroborates this result by classifying Llanocetus sp. as a raptorial feeder, rather than as a filter feeder.
Discussion
At 12 m, the estimated body length of Llanocetus sp. rivals that of living Bryde's and Omura's whales, and far exceeds that of any other archaic mysticete (Slater et al. Reference Slater, Goldbogen and Pyenson2017, Fordyce & Marx Reference Fordyce and Marx2018). Together, Llanocetus sp. and L. denticrenatus reveal an independent origin of gigantism early in mysticete evolution, predating the rise of large (> 10 m) modern whales by roughly 25 million years (Tsai & Kohno Reference Tsai and Kohno2016, Slater et al. Reference Slater, Goldbogen and Pyenson2017, Fordyce & Marx Reference Fordyce and Marx2018).
The large size of Llanocetus may relate to its polar habitat, wide foraging area, or simply its feeding strategy. Large body size in whales is generally thought to be facilitated by their filter feeding habit (Werth Reference Werth and Schwenk2000), especially in the context of a Pliocene shift towards dense but patchily distributed prey aggregations (Goldbogen & Madsen Reference Goldbogen and Madsen2018). Llanocetus is an exception, with the morphology and wear of its teeth instead hinting at (suction-assisted) raptorial feeding (Fordyce & Marx Reference Fordyce and Marx2018). The new material corroborates this idea, with apical abrasion on the major denticles suggesting biting and direct tooth-on-food contact. In addition, incipient attrition on one of the lower teeth implies an occluding posterior dentition capable of slicing and processing prey (Fig. 2b).
Well-developed carinae traverse the anterior and posterior faces of each denticle, creating bladed edges that probably would cut food as it was forced into the interdenticular notches during jaw closure (Fig. 2a). As demonstrated by principal component and discriminant function analyses of functional shape characteristics, such a morphology is consistent with extant terrestrial carnivorans and piscivorous pinnipeds, but absent in tooth-assisted filter feeding seals like Hydrurga and Lobodon (Hocking et al. Reference Hocking, Marx, Fitzgerald and Evans2017) (Fig. 2c & d). It is therefore inferred that Llanocetus sp., like its close relative L. denticrenatus, fed mostly raptorially.
Our new fossils firmly establish Llanocetus as one of the largest predators of its time. The size of its skull, as judged from a bizygomatic width of 886 mm in L. denticrenatus (Fordyce & Marx Reference Fordyce and Marx2018), and an isometrically scaled width of 1,370 mm in Llanocetus sp., far exceeded that of the largest contemporary archaeocetes, including Cynthiacetus (478 mm) (Martínez Cáceres et al. Reference Martínez Cáceres, Lambert and Muizon2017) and Basilosaurus (576–622 mm) (Kellogg Reference Kellogg1936). The sparseness of available material unfortunately prevents insights into likely prey types, although observations on extant killer whales suggest that moderate apical abrasion is more consistent with a diet of teleost fish than sharks (Ford et al. Reference Ford, Ellis, Matkin, Wetklo, Barrett-Lennard and Withler2011). This interpretation assumes, of course, that moderate abrasion in this case does not simply reflect a relatively young individual.
Llanocetus sp. belongs to the still poorly understood, archaic mysticete family Llanocetidae, which also includes L. denticrenatus, Mystacodon, and an undescribed specimen from New Zealand (OU GS10897) (Fordyce & Marx Reference Fordyce and Marx2018; but see Lambert et al. Reference Lambert, Martínez-Cáceres, Bianucci, Di Celma, Salas-Gismondi and Steurbaut2017 for a different interpretation). A previous analysis partially diagnosed this clade based on the presence of a sagittal trough formed by the parietals (Fordyce & Marx Reference Fordyce and Marx2018). This diagnosis requires clarification, as a parietal trough also occurs in certain basilosaurids, such as Cynthiacetus and Dorudon. In the latter, however, the trough is narrow and cleft-like, as opposed to the more open, broader depression in llanocetids.
Additional features distinguishing the family are its greatly elongated nasals (Fordyce & Marx Reference Fordyce and Marx2018); low, elongate premolar crowns, contrasting with the much higher, more triangular premolars of basilosaurids, mammalodontids and aetiocetids (Emlong Reference Emlong1966, Barnes et al. Reference Barnes, Kimura, Furusawa and Sawamura1995, Fitzgerald Reference Fitzgerald2006, Reference Fitzgerald2010, Marx et al. Reference Marx, Tsai and Fordyce2015, Peredo & Pyenson Reference Peredo and Pyenson2018); strong lingual and labial enamel ornaments (shared with mammalodontids) (Fitzgerald Reference Fitzgerald2010); and the absence of a sagittal crest on the parietals, a feature shared with Mammalodon and, to varying degrees, aetiocetids, but not Coronodon, Janjucetus, eomysticetids and basilosaurids (Deméré & Berta Reference Deméré and Berta2008, Fitzgerald Reference Fitzgerald2010, Snively et al. Reference Snively, Fahlke and Welsh2015, Boessenecker & Fordyce Reference Boessenecker and Fordyce2016, Geisler et al. Reference Geisler, Boessenecker, Brown and Beatty2017).
The lack of a sagittal crest in llanocetids is especially noteworthy, since it implies a weaker (superficial) temporal muscle (see Carpenter & White Reference Carpenter and White1986). Along with the relatively flat rostrum and widely spaced teeth of L. denticrenatus, this may suggest that llanocetids had a less powerful bite than other archaic cetacean raptorial feeders, such as basilosaurids (Snively et al. Reference Snively, Fahlke and Welsh2015, Fordyce & Marx Reference Fordyce and Marx2018). To compensate, prey capture and/or transport may have been facilitated by other means, such as suction (Lambert et al. Reference Lambert, Martínez-Cáceres, Bianucci, Di Celma, Salas-Gismondi and Steurbaut2017).
Despite – or perhaps because of – their early origin, llanocetids are notably disparate in terms of their inferred body size and, presumably, feeding style (Fig. 2e). Unlike Llanocetus, Mystacodon only reaches about 4 m, and is characterized by relatively closely spaced teeth with crowns obliterated by wear (Lambert et al. Reference Lambert, Martínez-Cáceres, Bianucci, Di Celma, Salas-Gismondi and Steurbaut2017). At about 3 m, as inferred from its bizygomatic width (Lambert et al. Reference Lambert, Bianucci, Post, de Muizon, Salas-Gismondi, Urbina and Reumer2010, Pyenson & Sponberg Reference Pyenson and Sponberg2011), OU GS10897 is just one quarter of the length of Llanocetus sp., yet has robust teeth bearing attritional shear facets. Such pronounced intrafamilial disparity is consistent with comparable variation in mammalodontids (macroraptorial vs suction feeding) (Fitzgerald Reference Fitzgerald2010) and aetiocetids (variable degree of homodonty, suction vs raptorial feeding, wide range of body sizes) (Marx et al. Reference Marx, Tsai and Fordyce2015, Tsai & Ando Reference Tsai and Ando2015, Marx et al. Reference Marx, Hocking, Park, Ziegler, Evans and Fitzgerald2016), and supports previous suggestions of a phase of morphological and behavioural ‘experimentation’ early in mysticete evolution (Marx & Fordyce Reference Marx and Fordyce2015).
Acknowledgements
Thanks are due to the Instituto Antártico Argentino and Fuerza Aérea Argentina for logistical support, Guillermo López and Sergio Santillana who found the specimens studied here, Juan Jose Moly and Santiago Bessone for the preparation of the specimens, Pablo Navarro and Erich M.G. Fitzgerald for help with data acquisition, Carl Buell for providing life reconstructions of Llanocetus, and Alberto Collareta and Olivier Lambert for their constructive reviews. F.G.M. was funded by an EU Marie Skłodowska-Curie Global Postdoctoral fellowship (656010/ MYSTICETI), and M.R. by the Instituto Antártico Argentino (PICTA and PICTO 2010-0093).
Author contribution
F.G.M and M.R.B. conceived and organised the project. D.P.H. and A.R.E. carried out the tooth sharpness analyses. F.G.M., M.R.B. and R.E.F. contributed data and conducted the morphological analysis. M.R. coordinated the collection and study of the material. All authors discussed and wrote the paper.
Supplemental Material
Supplemental Table S1 can be found at http://doi.org/10.1017/S095410201800055X