Type 1 diabetes (T1D) is a chronic autoimmune disease typically characterised by the progressive destruction of insulin-producing β-cells in the islets of Langerhans within the pancreas that leads to the loss of glucose homeostasis( Reference Anderson and Bluestone 1 ). T1D is diagnosed when β-cells are almost completely destroyed, and patients need insulin replacement therapy in order to survive. T1D is initiated as a consequence of a collapse in immune regulation, i.e. infiltration of pathogenic macrophages and dendritic cells into pancreatic islets is followed by the recruitment of autoreactive CD4+ and CD8+ T lymphocytes( Reference van Belle, Coppieters and von Herrath 2 ). Since β-cells possess very low antioxidant protection, they easily succumb to the detrimental effect of macrophage-derived cytokines, reactive oxygen species (ROS) and reactive nitrogen species (RNS) or to the action of T cell-secreted pro-inflammatory cytokines such as interferon-γ (IFN-γ) and IL-17( Reference Haskins, Bradley and Powers 3 – Reference Emamaullee, Davis and Merani 6 ). Furthermore, during T1D initiation, there is a breach in T helper (Th) 2 and regulatory T cell (Treg) responses and overall inability to control the development of autoimmunity( Reference Tang, Adams and Penaranda 7 , Reference Gallichan, Balasa and Davies 8 ).
Despite the growing knowledge about T1D aetiology, therapies that can change the course of immune-mediated destruction and preserve or even regenerate pancreatic β-cells seem to be promising in pre-clinical trials, but, unfortunately, so far, they are unsuccessful in human studies. This provides the need for the development of new therapeutic strategies. One of the potential approaches would be exploring traditional phytotherapies as alternative and/or supplementary therapy for diabetes treatment. Extensive studies have linked the consumption of food and natural products of plant origin rich in biophenols with protection against chronic diseases( Reference Del Rio, Rodriguez-Mateos and Spencer 9 ). Origanum vulgare L. ssp. hirtum (Greek oregano) is a native plant rich in phenolic and ester compounds, especially in rosmarinic acid( Reference Yang and Shetty 10 ). This plant has been used as an antiseptic and for stomach and respiratory ailments( Reference Eddouks, Maghrani and Lemhadri 11 ). Also, it was tested for the treatment of non-immune ‘toxic’ diabetes induced by a high dose of streptozotocin (STZ)( Reference Lemhadri, Zeggwagh and Maghrani 12 ). The main medicinal activities of oregano are anti-bacterial( Reference Burt and Reinders 13 ), antioxidant( Reference Zhang, Guo and Wang 14 ) and antithrombotic( Reference Goun, Cunningham and Solodnikov 15 ) activities.
Since oxidative stress is one of the contributors to T1D development, the goal of the present study was to evaluate the effect of oregano leaf extracts on diabetes induction in the multiple low doses of STZ (MLDS) model of diabetes in C57BL/6 mice, and to determine the mode of action by testing the plausible antioxidant, immunomodulatory and cytoprotective effects.
Experimental methods
Plant material, reagents and standards
O. vulgare L. ssp. hirtum (Greek oregano) was a commercial sample (voucher accession no. UOI100912, University of Ioannina obtained from a biological cultivator (Maria Komini) from the region of Epirus, northwestern Greece (biological cultivation)). All solvents were of appropriate purity and were purchased from various suppliers. Formic acid was of analytical grade from Merck. Folin–Ciocalteu phenol reagent was obtained from Fluka, 2,2-diphenyl-1-picrylhydrazyl (DPPH) (approximately 90 %), carvacrol (98 %) and naringenin (95 %) were from Sigma-Aldrich. Rosmarinic acid (95 %) and eriodictyol (95 %) were from Fluka.
Sample preparation
The dried plant material was pulverised into a fine powder. In order to prepare methanolic oregano extract (MOE) for preliminary experiments, ground oregano leaves (5 g) were extracted sequentially with 200 ml of four solvents of gradually increasing polarity in a Soxhlet apparatus for 6 h with each solvent, except for methanol (12 h). The sequence of the solvents was as follows: hexane (to remove chlorophylls); ethyl acetate; dichloromethane; methanol. MOE was concentrated using a rotary evaporator and kept in sealed dark flasks after a few minutes of N2 flushing. For all the other experiments, MOE was prepared from 32·7 g of plant material that were placed in a different Soxhlet apparatus and were extracted with 200 ml of organic solvents, and then the extracts were combined. For preliminary experiments with aqueous oregano extract (AOE), ground oregano leaves (5 g) were placed in 100 ml of boiling distilled water, kept in it for 1 h (out of fire) and then filtered. Water was removed with a freeze-dryer. For all the subsequent experiments, 15·0 g of plant material were extracted with 300 ml of distilled water and combined together. The yield of MOE was 15 % and of AOE 9 %.
Liquid chromotography–MS analysis
All liquid chromotography–MS n experiments were performed on a quadruple ion-trap mass analyser (model MSD trap SL; Agilent Technologies) retrofitted to a 1100 binary HPLC system equipped with a degasser, autosampler, diode array detector and electrospray ionisation source (Agilent Technologies). All hardware components were controlled by Agilent Chemstation Software.
AOE was dissolved in H2O–MeCN (50–50 %, v/v) to the desired concentration of 1 mg of dry extract/ml, and MOE was dissolved in methanol to the same concentration. A 10 μl aliquot was filtered (0·45 μm) and injected into the liquid chromotography–MS instrument. Separation was achieved on a 25 cm × 4·6 mm inner diameter, 5 μm Zorbax Eclipse XDB-C18 analytical column (Agilent Technologies), at a flow rate of 0·6 ml/min, using solvent A (water–formic acid, 99·9:0·1, v/v) and solvent B (acetonitrile). The gradient used for the analysis of oregano extracts was: 0–25 min, 90–30 % A; 25–29 min, 30 % A; 29–30 min, 30–0 % A; 30–35 min, 0–90 % A. UV–vis spectra were recorded in the range of 200–400 nm, and chromatograms were acquired at 254, 280 and 330 nm.
Both precursor and product (MS2 and MS3) ions scanning of the phenolic compounds were monitored between m/z 50 and m/z 1·000 in negative polarity. The ionisation source conditions were as follows: capillary voltage, 3·5 kV; drying gas temperature, 350°C; N2 flow and pressure, 11 litres/min and 50 psi, respectively. The maximum accumulation time of the ion trap and the number of MS repetitions to obtain the MS average spectra were set at 30 and 3 ms, respectively.
Determination of the total phenolic content
The total phenolic content of the extracts was measured using the Folin–Ciocalteu method described in detail in our previous work( Reference Kontogianni, Tomic and Nikolic 16 ). All measurements were performed in duplicate.
Antioxidant activity: 2,2-diphenyl-1-picrylhydrazyl radical-scavenging assay
The DPPH radical-scavenging effect was evaluated according to the method described previously( Reference Kontogianni, Tomic and Nikolic 16 ). Results are presented as the values of scavenging concentration 50% (SC50). SC50 is the concentration of the extract that is sufficient to obtain 50 % of a maximum scavenging capacity of the stable DPPH radical. Thus, the SC50 value is negatively related to antioxidant activity.
Multiple low-dose streptozotocin-induced diabetes
C57BL/6 mice were bred and kept in the animal facility at the Institute for Biological Research ‘Sinisa Stankovic’ (University of Belgrade, Belgrade, Serbia). Both institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Ethics Committee at the Institute for Biological Research ‘Sinisa Stankovic’, University of Belgrade, Belgrade, Serbia (approval number 2-27/10-01-189) in compliance with the Directive 2010/63/EU.
In order to investigate the efficacy of the extracts and rosmarinic acid in the treatment of diabetes, a mouse model of diabetes that closely resembles inflammatory changes within the pancreas in humans was used. Diabetes was induced in 8- to 12-week-old male C57BL/6 mice with MLDS (40 mg/kg per d, intraperitoneally for five consecutive days; Sigma-Aldrich). Each experimental group comprised ten animals. MOE or AOE was administered for ten consecutive days by intraperitoneal injections at a dose of 5 mg/kg per d, starting from the day of MLDS induction (‘prophylactic’ regimen) or starting 1 d after the last STZ injection (‘early therapeutic’). Rosmarinic acid was administered intraperitoneally for ten consecutive days (2·5 mg/kg per d), starting from the day of diabetes induction. Glucose concentration was measured from the blood drawn from the tail vein using a glucometer (Sensimac; IMACO GmbH). Clinical diabetes was defined by hyperglycaemia in non-fasted animals (blood glucose >11 mm). MOE was also administered to healthy mice for 10 d, and general metabolic and immune parameters, and antioxidant enzymes were evaluated 14 d from the beginning of the experiment. The control mice received the vehicle (PBS+dimethyl sulfoxide (DMSO)).
Metabolic parameters and leucocyte and erythrocyte blood counts
Urine was tested for the presence of proteins, glucose and blood by using semi-quantitative Bayer Multistix® 10 SG Urine Test Strips (Bayer). Blood was diluted in Türk's solution (Sigma-Aldrich) and leucocytes were counted in the haemocytometer, while blood was diluted in saline for counting erythrocytes. Glutathione S-transferase (as a measure of liver toxicity) was determined by enzymatic reaction. Briefly, glutathione S-transferase catalyses the reaction of 1-chloro-2,4-dinitro benzene with the glutathione sulfhydryl group. Newly created 1-chloro-2,4-dinitro benzene-S-glutathione has maximum absorption at 340 nm that was detected using a Shimadzu UV-160 spectrophotometer (Shimadzu Scientific Instruments, Shimadzu Corporation).
Assessment of insulin
Insulin concentration in the sera of non-fasted mice was determined using an ELISA kit (Mercodia).
Cell preparation and culture
Ex vivo analysis was performed on MLDS-induced C57BL/6 mice after ‘prophylactic’ administration of MOE on day 10 of post-diabetes induction. In vitro analysis was performed on cells isolated from male C57BL/6 mice. Mice were killed by cervical dislocation. To obtain spleen cells (SC) and pancreatic lymph node cells (PLNC), organs were mechanically disrupted by gentle teasing through the cell strainer (BD Bioscience), and cells were collected by centrifugation. Peritoneal cells (PC) were collected from the peritoneal cavity of mice in cold PBS. Erythrocytes were lysed using lysis buffer (eBioscience). Samples of conditioned medium, used for ex vivo detection of cytokines or NO, were obtained by seeding the cells (5 × 106 SC/ml per well, 3 × 106 PLNC/ml per well, or 1 × 106 PC/ml per well) for 48 h in twenty-four-well culture plates (Sarstedt) in Roswell Park Memorial Institute-1640 medium (with 25 mm-HEPES and 2 mm-l-glutamine) supplemented with 5 % (v/v) fetal calf serum (PAA Chemicals), penicillin/streptomycin (Sigma-Aldrich), and 5 μm-β-mercaptoethanol (Sigma-Aldrich) at 37°C in a 5 % CO2 incubator and in the presence of 1 μg/ml of concanavalin A (Sigma-Aldrich) for SC and PLNC, and 5 ng/ml of lipopolysaccharide for PC.
Isolation of pancreatic-infiltrating mononuclear cells
To obtain pancreatic-infiltrating mononuclear cells, pancreas samples were processed from individual mice by collagenase type V digestion( Reference Rydgren and Sandler 17 ), and digests were passed through a 20 μm cell strainer, centrifuged on Histopaque®-1077 gradient (Sigma-Aldrich) at 800 g for 20 min. After centrifugation, mononuclear cells were found between Ficoll and medium, collected, washed and used for flow cytometric analysis.
Immunofluorescence analysis
The phenotype of PC, SC, PLNC and pancreatic-infiltrating mononuclear cells was assessed by flow cytometry using the following antibodies: rat anti-mouse IgG2bκ CD3-PE, CD4-PE, IgG2bκ CD4-FITC, IgG2aκ CD8-PE, IgG1 CD25-PE, IgG2aκ F4/80-FITC; Armenian hamster anti-mouse IgM CD40 APC (eBioscience); goat anti-mouse IgG CD206-PE (R&D). Treg were detected by the Mouse Regulatory T cell Staining Kit, according to the manufacturer's instructions (eBioscience). For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate (100 ng/ml) and ionomycin (400 ng/ml) in the presence of Brefeldin A (5 μm) (eBioscience) for 4 h. Cells were fixed in 2 % paraformaldehyde, permeabilised and stained for intracellular cytokines with the following antibodies: rat anti-mouse IgG1κ IFN-γ-PE, IL-4-PE, RORγt-PE; rat anti-mouse IgG2b GATA3-APC (eBioscience); IgG1κ IL-17-PE (BD Biosciences). Isotype-matched controls were included in all experiments (eBioscience). Stained cells were detected on Partec CyFlow Space and analysed by FlowMax software (Partec).
Determination of cytokine and nitrite levels
Cytokine concentration in cell-culture supernatants was determined by sandwich ELISA using MaxiSorp plates (Nunck) and anti-mouse paired antibodies, according to the manufacturer's instructions. Samples were analysed in duplicate for murine IL-17, IL-1β, TNF (BD Biosciences), IL-10, IL-2 (eBioscience), IFN-γ and IL-4 (R&D Systems).
Nitrite accumulation, an indicator of NO production, was measured in cell-culture supernatants using the Griess reagent( Reference Stojanovic, Saksida and Nikolic 18 ).
Immunoblot analysis
SC (5 × 106) were disrupted with 500 μl of TRI Reagent® Solution (Applied Biosystems), mixed with chloroform (350 μl) and centrifuged at 12 000 g at 4°C for 20 min. The organic phase was used for the isolation of proteins, according to the manufacturer's instruction. The amount of protein was determined by the Lowry method( Reference Lowry, Rosenbroudh and Ferr 19 ). For immunoblot analysis, the following antibodies were employed: rabbit anti-mouse phosphorylated-p38 (1:1000; Cell Signaling Technology); phosphorylated-signal transducer and activator of transcription 3 (STAT3) and STAT3 (1:1000; Santa Cruz Biotechnology); rabbit anti-mouse IgG β-actin (1:1000; Abcam) followed by incubation with secondary donkey anti-rabbit horseradish peroxidase-linked antibody at 1:10 000 dilution (GE Healthcare). Detection was performed by chemiluminescence (ECL; GE Healthcare) and photographs were made by X-ray films (Kodak). Densitometry was performed with Scion Image Alpha 4.0.3.2 (Scion Corporation).
RNA isolation, reverse transcription and PCR
Total RNA was isolated from SC (5 × 106) with TRI Reagent® Solution (Applied Biosystems). RNA (1 μg) was reverse transcribed using RevertAid™ M-MuLV Reverse Transcriptase and random hexamer primers (Fermentas). PCR amplification of complementary DNA was carried out in a real-time PCR machine using the SYBR Green PCR master mix (Applied Biosystems), as described previously(
Reference Stojanovic, Saksida and Nikolic
18
). Primer pair sequences for β-actin were 5′-GACCTGACAGACTACC-3′ and 5′-GGCATAGAGGTCTTTACGG-3′ (NM_007393.2), for IL-17 5′-GGAGAGCTTCATCTGT-3′ and 5′-GACCCTGAAAGTGAAGGG-3′ (NM_010552.3), for RAR-related orphan receptor γt (RORγt) 5′-CCGCTGAGAGGGCTTCAC-3′ and 5′-TGCAGGAGTAGGCCACATTACA-3′ (NM_011281.1), and for IL-4 5′-ATCCTGCTCTTCTTTCTCG-3′ and 5′-GATGCTCTTTAGGCTTTCC-3′ (NM_021283.2). Gene expression was calculated as
![]() $$2^{ - ( C _{ti} - C _{ta})} $$
(C
ti, cycle threshold of the gene of interest; C
ta, β-actin cycle threshold) and displayed as mean values and standard deviations.
$$2^{ - ( C _{ti} - C _{ta})} $$
(C
ti, cycle threshold of the gene of interest; C
ta, β-actin cycle threshold) and displayed as mean values and standard deviations.
Histology and immunohistochemistry
Aseptically removed pancreas was fixed in neutral buffered formalin and then embedded in paraffin, sectioned (5 μm thick) and stained with Mayer's haematoxylin (Bio-Optica) to assess the incidence and degree of inflammatory changes. Insulitis scoring was performed by examining at least fifteen islets per mouse and graded in a blinded fashion as follows: grade 0, intact islet; grade 1, peri-islet infiltrate; grade 2, heavy intra-islet infiltrate. Results are expressed as a percentage of graded islets out of the total number of islets. For immunohistochemical staining, tissues were incubated with 0·1 % trypsin for 30 min at 37°C for antigen retrieval, incubated with 3 % H2O2 for the blockade of endogenous peroxidase and then probed with rabbit anti-nitrotyrosine antibody (1:500; Sigma-Aldrich) or rabbit anti-mouse insulin antibody (1:100; Santa-Cruz Biotechnology) followed by the Rabbit ExtrAvidin Peroxidase Staining Kit (Sigma-Aldrich), according to the manufacturer's instructions. Staining was developed with diaminobenzidine (DakoCytomation), and sections were counterstained with haematoxylin. Representative islets were photographed using a Leica photomicroscope (Leitz) at × 400 magnification.
Culture of murine insulinoma cell lines and primary pancreatic islets
Rat insulinoma RINm5F cells (ATCC® CRL 11 605™) (1 × 104/well) were cultured in the presence of recombinant mouse or rat cytokines (TNF+IFN-γ+IL-1β) (R&D Systems), 10 ng/ml each, with or without MOE (25–200 μg/ml).
Primary pancreatic islets were isolated from C57BL/6 mice. Groups of thirty islets were incubated in the presence or absence of TNF+IFN-γ+IL-1β (10 ng/ml), with or without MOE (25–100 μg/ml).
Caspase 3 assay
Caspase 3 activity was measured using an assay kit (Biotium), according to the manufacturer's instructions, and quantified fluorometrically (excitation at 485 nm and emission at 535 nm) on a Chameleon plate reader (Hidex).
Cell viability assays
DNA–histone complexes present in the cytoplasmic fraction of apoptotic islets were detected using Cell Death Detection ELISA (Roche).
Mitochondrial activity of treated RINm5F or PLNC in the presence or absence of concanavalin A was measured using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma-Aldrich) assay, as described previously( Reference Stojanovic, Saksida and Nikolic 18 ). Cell viability was expressed as a percentage of the control value (untreated cells) that was arbitrarily set to 100 %. The proliferative index for PLNC was calculated as a ratio between absorbance values of concanavalin A-stimulated v. unstimulated cells after 48 h incubation.
Determination of antioxidant enzyme activity
Thawed pancreas was homogenised and sonicated in 0·25 m-sucrose, 1 mm-EDTA and 0·05 m-Tris–HCl buffer pH 7·4 (all from Sigma-Aldrich) before centrifugation for 90 min at 105 000 g . The supernatant was used to determine enzyme activities using a Shimadzu UV-160 spectrophotometer (Shimadzu Scientific Instruments; Shimadzu Corporation). Blood samples were processed as described previously( Reference Nikolic-Kokic, Stevic and Blagojevic 20 ). All antioxidant enzyme activities were determined as described previously( Reference Nikolic-Kokic, Stevic and Blagojevic 20 ). Briefly, superoxide dismutase (SOD) activity was determined by the adrenaline method after the removal of Hb. One unit of SOD activity is defined as the amount of enzyme necessary to decrease by 50 % the rate of adrenalin auto-oxidation at pH 10·2. Catalase activity was determined by the rate of H2O2 disappearance measured at 230 nm. Glutathione peroxidase (GSHPx) activity was determined by the glutathione-dependent reduction of t-butyl hydroperoxide (Fluka). One unit of GSHPx activity is defined as the amount needed to oxidise 1 nm-NADPH/min (Sigma-Aldrich) at 25°C and pH 7·0. Glutathione reductase activity was determined by the assay based on NADPH oxidation concomitant with GSHPx reduction. One unit of glutathione reductase activity is defined as the oxidation of 1 nm-NADPH/min at 25°C and pH 7·4. All enzyme activities were expressed as units/mg protein. The amount of proteins was determined by the Lowry method.
Statistical analysis
Data are presented as means and standard deviations for each experimental protocol. Statistical analysis of differences was made using one-way ANOVA, followed by the Student–Newman–Keuls post hoc test and, finally, Student's t test or Mann–Whitney U test, where appropriate. Comparison of diabetes incidence was made using the χ2 test. Statistica 6.0 (StatSoft, Inc.) software was used. P <0·05 was considered to be statistically significant.
Results
Phytochemical composition of oregano extracts
The liquid chromotography/diode array detection/electrospray ionisation–MS n analysis of the specific extract led to the separation and identification of the majority of the constituents. Since negative ionisation gave higher sensitivity than positive ionisation, the negative ionisation mode was used for the analysis (see online supplementary Fig. S1). Both MOE and AOE presented a similar profile with rosmarinic acid (caffeic acid dimer) as the main compound. The samples also contained other caffeic acid derivatives, such as caffeic acid trimers (salvianolic acid C) and tetramers (dimers of rosmarinic acid (salvianolic acid B)) identified according to Lu & Foo( Reference Lu and Foo 21 ), Nuengchamnong et al. ( Reference Nuengchamnong, Krittaslip and Ingkanina 22 ) and Zeng et al. ( Reference Zeng, Xiao and Liu 23 ), comparing both their UV spectra and their fragmentation pattern. For the quantitative analysis of these derivatives, the rosmarinic acid calibration curve was utilised in accordance with Barros et al. ( Reference Barros, Dueñas and Dias 24 ). Detailed analysis of oregano extract composition is given in online supplementary Results and Table S1.
Quantitative measurements of the main compounds in oregano extracts
Quantitative analysis was performed by the HPLC method using a diode array detector. Since reference compounds for caffeic acid derivatives were not available, they were quantified as rosmarinic acid (at 330 nm). Flavonoids, eriodictyol and naringenin, along with carvacrol, were quantified at 280 nm. The calibration curves were proved to be linear for rosmarinic acid in the range of 5–150 mg/l with R 2 0·995, for eriodictyol in the range of 2–20 mg/l with R 2 0·996, for naringenin in the range of 2–20 mg/l with R 2 0·988 and for carvacrol in the range of 5–100 mg/l with R 2 0·999. In total, in MOE, five caffeic acid derivatives along with rosmarinic acid were quantified, and in AOE, three caffeic acid derivatives along with rosmarinic acid were quantified. The quantitatively dominating compounds in both extracts were rosmarinic acid followed by salvianolic acid B–lithospermic acid B. However, the amount of rosmarinic acid in MOE was 2-fold higher than that in AOE (Table 1).
Table 1 Concentrations of compounds in aqueous oregano extract (AOE) and methanolic oregano extract (MOE, mg/g dry extract) (Mean values and standard deviations)

R t, retention time.
* These compounds were found in trace amounts in extracts so it was not possible to quantify them.
† These compounds were measured as rosmarinic acid.
‡ Identification was confirmed using commercial standards.
Total phenolic content and antioxidant activity
The total phenolic content in MOE was 240·0 (sd 15·3) μg caffeic acid/mg of dry extract (mg/g) and 244·2 (sd 4·9) μg caffeic acid/mg of dry extract (mg/g). Antioxidant activity evaluated by the DPPH radical-scavenging assay was 1·5-fold lower in MOE than in AOE (SC50= 38·7 (sd 1·2) μg/ml v. SC50= 26·1 (sd 2·8) μg/ml).
Assessment of oregano extracts for the treatment of multiple low doses of streptozotocin-induced diabetes
To investigate the effect of oregano extracts on T1D development, MLDS-challenged C57BL/6 mice were prophylactically treated either with MOE or AOE. The absence of hyperglycaemia development was observed only in MOE-treated mice (Fig. 1(a)). However, when MOE was applied after diabetes induction as an ‘early therapeutic’ treatment, it only transiently protected mice from diabetes propagation. After treatment interruption, all mice developed hyperglycaemia (Fig. 1(b)). Therefore, we focused on MOE ‘prophylactic’ treatment and found that treated mice had higher serum insulin levels (Fig. 1(c)) and significant number of well-preserved β-cells as determined by immunohistochemical insulin staining (Fig. 1(d)). Also, MOE-treated mice had a lower percentage of islets with mononuclear cell infiltrations (insulitis) compared with diabetic mice, but the same number of islets with benign insulitis (grade 1) (Fig. 1(e)). Importantly, the MOE treatment was well tolerated and had no visible side effects judging by animal behaviour, general appearance and body weight gain (data not shown). In addition, administration of MOE to healthy mice had no impact on their glycaemia, no apparent effect on kidney function (increased concentration of proteins, glucose or erythrocytes in the urine), and the change in leucocyte or erythrocyte concentration in blood was not observed (Table 2). Also, MOE did not alter the activity of glutathione S-transferase (a key enzyme involved in the detoxification of xenobiotics in the liver) compared with diluent-treated healthy mice (Table 2).

Fig. 1 Methanolic oregano extract (MOE) treatment ameliorates diabetes induction in C57BL/6 mice. (a) C57BL/6 mice were subjected to diabetes induction (multiple low doses of streptozotocin, MLDS) and treated either with MOE (MLDS+MOE) or aqueous oregano extract (AOE; MLDS+AOE) for 10 d (prophylactic treatment). Diabetes incidence was calculated as the percentage of hyperglycaemic animals (blood glucose ≥ 11 mm). ![]() , MLDS;
, MLDS; ![]() , MLDS+MOE;
, MLDS+MOE; ![]() , MLDS+AOE. (b) Diabetes incidence in mice that received MOE under ‘early therapeutic’ regimen (MOE treatment lasted for 10 d).
, MLDS+AOE. (b) Diabetes incidence in mice that received MOE under ‘early therapeutic’ regimen (MOE treatment lasted for 10 d). ![]() , MLDS;
, MLDS; ![]() , MLDS+MOE therapeutic. (c) Insulin was measured by ELISA 14 d after the MOE prophylactic treatment (ANOVA followed by the Mann–Whitney U test). (d) Representative pancreatic islet sections stained for insulin. (e) Percentage of pancreatic islets without infiltration (intact): grade 0, mononuclear cells surrounding the islets (peri-insulitis); grade 1, mononuclear cell infiltrations (insulitis); grade 2, representative pancreatic islet sections from diabetic (MLDS) and MOE-treated mice (MLDS+MOE).
, MLDS+MOE therapeutic. (c) Insulin was measured by ELISA 14 d after the MOE prophylactic treatment (ANOVA followed by the Mann–Whitney U test). (d) Representative pancreatic islet sections stained for insulin. (e) Percentage of pancreatic islets without infiltration (intact): grade 0, mononuclear cells surrounding the islets (peri-insulitis); grade 1, mononuclear cell infiltrations (insulitis); grade 2, representative pancreatic islet sections from diabetic (MLDS) and MOE-treated mice (MLDS+MOE). ![]() , MLDS;
, MLDS; ![]() , MLDS+MOE. Values are means (n 10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of MLDS-treated mice (P <0·05; χ2 test). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn.
, MLDS+MOE. Values are means (n 10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of MLDS-treated mice (P <0·05; χ2 test). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn.
Table 2 Effect of methanolic oregano extract (MOE) on metabolic parameters in healthy mice (Mean values and standard deviations, n 7 mice per group)
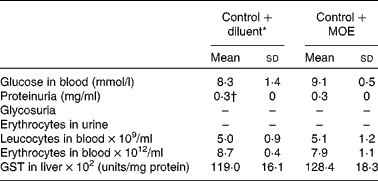
GST, glutathione S-transferase.
* Diluent is PBS+DMSO.
† Normal concentration of proteins in the urine of C57BL/6 mice.
Antioxidant status of methanolic oregano extract-treated mice
MLDS application generally results in increased ROS and RNS activities that initially destroy β-cells of the pancreas( Reference Szkudelski 25 ). Diabetic mice exhibited systemically higher activity of SOD and catalase than mice treated with MOE diluent (DMSO+PBS). MOE itself had no impact on the activity of these enzymes in the blood, while MOE+MLDS-treated mice expressed lower activity of total SOD and catalase, indicating lower levels of superoxide radicals and peroxides, respectively (Fig. 2(a)). However, MLDS-triggered systemic up-regulation of antioxidant defence was not evident within the pancreatic tissue. Interestingly, MOE itself down-regulated GSHPx activity and activated glutathione reductase activity, indicating its antioxidant potential especially at the level of lipid peroxides and rapid turnover of glutathione (Fig. 2(b)). The percentage of pancreatic islets with nitrosylated proteins, a marker of nitrosative stress, was significantly higher in diabetic than in MOE-treated mice (Fig. 2(c)).

Fig. 2 Effect of methanolic oregano extract (MOE) treatment on the antioxidant status of diabetic mice. (a) Activity of superoxide dismutase (SOD, × 1000) and catalase (CAT, × 1000) within the erythrocytes of C57BL/6 mice receiving PBS+DMSO (diluent, control, ![]() ), MOE-treated control mice (control+MOE,
), MOE-treated control mice (control+MOE, ![]() ), diabetic mice (multiple low doses of streptozotocin (MLDS),
), diabetic mice (multiple low doses of streptozotocin (MLDS), ![]() ) and MOE-treated mice (MLDS+MOE,
) and MOE-treated mice (MLDS+MOE, ![]() ) 14 d post-diabetes induction. (b) Activities of antioxidant enzymes within the total pancreas homogenate 14 d post-diabetes induction. (c) Percentage of pancreatic islets stained with anti-nitrotyrosine antibody in MLDS and MLDS+MOE-treated mice. Representative pancreatic islet sections are shown. Values are means (n 7–10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of MLDS-treated mice (P <0·05; ANOVA followed by the Mann–Whitney U test). † Mean value was significantly different from that of the control group (P <0·05; ANOVA followed by the Mann–Whitney U test). GR, glutathione reductase; GSHPx, glutathione peroxidase. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn.
) 14 d post-diabetes induction. (b) Activities of antioxidant enzymes within the total pancreas homogenate 14 d post-diabetes induction. (c) Percentage of pancreatic islets stained with anti-nitrotyrosine antibody in MLDS and MLDS+MOE-treated mice. Representative pancreatic islet sections are shown. Values are means (n 7–10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of MLDS-treated mice (P <0·05; ANOVA followed by the Mann–Whitney U test). † Mean value was significantly different from that of the control group (P <0·05; ANOVA followed by the Mann–Whitney U test). GR, glutathione reductase; GSHPx, glutathione peroxidase. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn.
Methanolic oregano extract specifically targets T-cell-mediated immune response
Although macrophages are the first cells to infiltrate pancreatic islets, MOE did not affect their infiltration, polarisation, cytokine or NO production (see online supplementary Results and Fig. S2). However, MOE successfully suppressed the T-cell-mediated immune response in MLDS-challenged mice judging by lower proliferative capacity of splenocytes (Fig. 3(a)) and the reduced number of activated CD4+CD25+ cells in both peripheral and pancreatic tissues (Fig. 3(b)). Importantly, MOE reduced the number of T lymphocytes (CD3+), i.e. it specifically affected the proportion of Th (CD4+) cells without an impact on cytotoxic CD8+ lymphocytes within the spleen (Fig. 3(c)), pancreatic lymph nodes (Fig. 3(d)) or pancreatic infiltrates (Fig. 3(e)). Furthermore, the proportion of Treg was moderately increased within the spleen (Fig. 3(c)), but significantly enhanced in the pancreatic infiltrates (Fig. 3(e)) after the MOE treatment. Therefore, the immunosuppressive effect of MOE was probably mediated through the reduction in the Th population.

Fig. 3 Effect of methanolic oregano extract (MOE) on lymphocytes. (a) Proliferation of splenocytes isolated from diabetic and MOE-treated mice and cultured with or without concanavalin A (ConA) for 48 h was determined by the MTT assay. Results are presented as the ratio between ConA-treated cells v. untreated cells. (b) Percentage of activated CD4+CD25+T lymphocytes within splenocytes (SC), pancreatic lymph node cells (PLNC) and pancreatic mononuclear cell infiltrates (PMC) measured by flow cytometry. Distribution of T lymphocytes within the spleen (c), pancreatic lymph node (d) or pancreatic infiltrates (e) measured by flow cytometry. (f) In vitro cytokine secretion from SC isolated from untreated mice and stimulated with ConA in the presence or absence of MOE (measured by ELISA). (g) ConA-stimulated ex vivo secretion of cytokines from SC isolated from diabetic (multiple low doses of streptozotocin, MLDS) or MOE-treated mice (MLDS+MOE). Distribution of three major subsets of T helper cells within the PLNC (h) or PMC (i) measured by flow cytometry. Representative dot plots are shown (depicted cells are already gated on the CD4+ population). ![]() , MLDS-treated;
, MLDS-treated; ![]() , MLDS+MOE-treated;
, MLDS+MOE-treated; ![]() , Con-A-stimulated;
, Con-A-stimulated; ![]() , Con-A-stimulated+MOE-treated. Values are means (n 7 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the MLDS-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test). † Mean value was significantly different from that of the ConA-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test). IFN, interferon; FoxP3, forkhead box P3; SSC, side scatter.
, Con-A-stimulated+MOE-treated. Values are means (n 7 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the MLDS-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test). † Mean value was significantly different from that of the ConA-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test). IFN, interferon; FoxP3, forkhead box P3; SSC, side scatter.
To determine which Th-cell population is affected by MOE, we examined the proportion of Th1, Th17 and Th2 cells by measuring their signature cytokines, IFN-γ, IL-17 and IL-4, respectively. The results indicated that the suppressive effect of MOE on diabetes development was probably mediated by the inhibition of IL-17 and the up-regulation of IL-4 production in splenocytes detected both in vitro (Fig. 3(f)) and ex vivo (Fig. 3(g)). The proportion of Th17 cells upon MOE treatment in vivo was reduced in pancreatic lymph nodes (Fig. 3(h)), while their homing to the pancreatic islets was similar as in MLDS-challenged mice (Fig. 3(i)). However, MOE seemed to promote an anti-inflammatory response judging by its significant effect on Th2 cells both at the periphery (Fig. 3(h)) as well as within the pancreas (Fig. 3(i)). Pro-inflammatory Th1 cells and their production of IFN-γ remained unaffected by the MOE treatment (Fig. 3(g–i)).
Decoding the mechanism of the methanolic oregano extract-mediated effect on T helper 17, T helper 2 and T regulatory cell differentiation
To investigate the mechanism of MOE action on Th17 differentiation, we first measured the levels of cytokines that are either involved in the differentiation (IL-6 and transforming growth factor-β (TGF-β)) or propagation (IL-23) of Th17 cells. Interestingly, MOE increased only the SC-derived production of TGF-β, without any impact on the other cytokines tested (Table 3). However, it targeted down-stream signalling mediators mandatory for IL-17 expression and production. Namely, the phosphorylation of p38 mitogen-activated protein kinase and the activation of transcription factor STAT3 were significantly reduced (Table 3). This probably led to the marked reduction in the number of RORγt-containing cells (Fig. 5(c)) as well as the down-regulation of RORγt mRNA expression. All of these events resulted in the down-regulation of mRNA IL-17 expression (Table 3).
Table 3 Mechanism of the effect of methanolic oregano extract (MOE) on T helper (Th)17, Th2 and regulatory T cells (Mean values and standard deviations, n 5–7 mice per group)
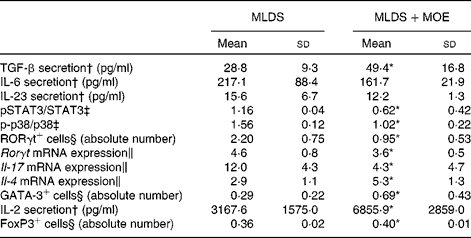
MLDS, multiple low doses of streptozotocin; TGF-β, transforming growth factor-β; p, phosphorylated; STAT3, signal transducer and activator of transcription 3; RORγt, RAR-related orphan receptor γt; FoxP3, forkhead box P3.
* Mean value was significantly different from that of MLDS-treated mice (P< 0·05).
† Concanavalin A-stimulated secretion of cytokines from spleen cells (SC) was measured by ELISA.
‡ Protein expression was measured by immunoblot in SC.
§ Flow cytometric analysis.
∥ mRNA expression was measured by real-time PCR in SC.
The up-regulation of Th2 proportion coincided with MOE-mediated increase in IL-4 mRNA expression in SC that was probably mediated by the potentiating effect of MOE on GATA binding protein 3 (GATA3) transcription factor (Table 3).
As for the mechanism of Treg induction, it was found that MOE enhanced the production of IL-2, a mandatory cytokine for Treg development. Also, the mean fluorescence intensity for forkhead box P3 (FoxP3) transcription factor was increased in MOE-treated SC (Table 3).
Methanolic oregano extract protects β-cells from cytokine insult in vitro
In addition to its effect on the immune system, MOE exerted a cytoprotective function. In vitro administration of MOE protected RINm5F (rat insulinoma cells) from destructive effect of cytokines (Fig. 4(a)). Also, MOE protected the pancreatic islets isolated from C57BL/6 mice from apoptosis induction by cytokines in vitro (Fig. 4(b)). The mechanism of the cytoprotective action of MOE was related to the suppression of cytokine-driven caspase 3 activity in the pancreatic islets (Fig. 4(c)).

Fig. 4 Methanolic oregano extract (MOE) exerts a cytoprotective effect towards pancreatic β-cells. (a) Viability of RINm5F cells after 48 h-long treatment with interferon (IFN)-γ+TNF+IL-1β alone (point 0) or combination of cytokines and increasing MOE concentrations was measured by the MTT assay. (b) Pancreatic islets were treated with MOE in the absence (![]() ) or presence of cytokines (
) or presence of cytokines (![]() ; IFN-γ, TNF and IL-1β) for 24 h and apoptosis was measured by DNA-Histone ELISA. (c) Caspase 3 activity was measured fluorimetrically after 24 h of islets incubation with cytokines (
; IFN-γ, TNF and IL-1β) for 24 h and apoptosis was measured by DNA-Histone ELISA. (c) Caspase 3 activity was measured fluorimetrically after 24 h of islets incubation with cytokines (![]() ; IFN-γ, TNF and IL-1β) or cytokines in the presence of 50 μg/ml of MOE (
; IFN-γ, TNF and IL-1β) or cytokines in the presence of 50 μg/ml of MOE (![]() ). Values are means, with standard deviations represented by vertical bars. Representative out of three experiments is shown. * Mean value was significantly different from that of the cytokine-only treated group (P< 0·05; ANOVA followed by Mann–Whitney U test). † Mean value was significantly different from that of the MOE-only-treated group (P< 0·05; ANOVA followed by Mann–Whitney U test).
). Values are means, with standard deviations represented by vertical bars. Representative out of three experiments is shown. * Mean value was significantly different from that of the cytokine-only treated group (P< 0·05; ANOVA followed by Mann–Whitney U test). † Mean value was significantly different from that of the MOE-only-treated group (P< 0·05; ANOVA followed by Mann–Whitney U test).
Effect of rosmarinic acid on diabetes induction in mice
One of the major differences between the investigated extracts is a 2-fold higher quantity of rosmarinic acid in MOE in comparison with AOE. Therefore, the aim of the present study was to investigate whether rosmarinic acid is the active phytochemical of MOE. In vitro studies have revealed that rosmarinic acid affected both macrophages and lymphocytes. It inhibited macrophage function judging by reduced NO production (Fig. 5(a)) and IL-1β secretion (Fig. 5(b)). However, rosmarinic acid stimulated T lymphocyte proliferation (Fig. 5(c)) and Th2 response (Fig. 5(d)). Also, rosmarinic acid protected β-cells from cytokine-induced apoptosis (Fig. 5(e)). In contrast to MOE that significantly ameliorated MLDS-induced diabetes progression, rosmarinic acid only partially protected mice from diabetes induction (diabetes incidence 50 %, four out of eight mice) (Fig. 5(f)).

Fig. 5 Rosmarinic acid (RA) only partly protects mice from diabetes induction. Peritoneal cells (1 × 106/ml per well) were stimulated in vitro by lipopolysaccharide (LPS) in the presence (![]() ) or absence (
) or absence (![]() ) of 50 μg/ml of RA and the secretion of nitric oxide (a) or cytokines (b) was measured after 48 h of incubation. (c) Spleen cells (SC, 5 × 106/ml per well) were stimulated with concanavalin A (ConA) in the presence (
) of 50 μg/ml of RA and the secretion of nitric oxide (a) or cytokines (b) was measured after 48 h of incubation. (c) Spleen cells (SC, 5 × 106/ml per well) were stimulated with concanavalin A (ConA) in the presence (![]() ) or absence (
) or absence (![]() ) of 50 μg/ml of RA and cell proliferation was determined by the MTT assay. (d) In vitro secretion of cytokines from SC stimulated with ConA in the presence (
) of 50 μg/ml of RA and cell proliferation was determined by the MTT assay. (d) In vitro secretion of cytokines from SC stimulated with ConA in the presence (![]() ) or absence (
) or absence (![]() ) of 50 μg/ml of RA, measured by ELISA. (e) Viability of RINm5F cells after 48 h-long treatment with cytokines alone interferon (IFN)-γ+TNF+IL-1β (Cyt) or in combination with 50 μg/ml of RA (Cyt+RA) was measured by the MTT assay. (f) Mice were challenged prophylactically with multiple low doses of streptozotocin (STZ) and were untreated (
) of 50 μg/ml of RA, measured by ELISA. (e) Viability of RINm5F cells after 48 h-long treatment with cytokines alone interferon (IFN)-γ+TNF+IL-1β (Cyt) or in combination with 50 μg/ml of RA (Cyt+RA) was measured by the MTT assay. (f) Mice were challenged prophylactically with multiple low doses of streptozotocin (STZ) and were untreated (![]() ) or treated with RA (
) or treated with RA (![]() ). Diabetes incidence was calculated as a percentage of animals that developed hyperglycaemia ( ≥ 11 mm glucose). Representative out of three in vitro experiments is shown. Values are means (n 10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the RA-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test).
). Diabetes incidence was calculated as a percentage of animals that developed hyperglycaemia ( ≥ 11 mm glucose). Representative out of three in vitro experiments is shown. Values are means (n 10 mice per group), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the RA-treated mice (P< 0·05; ANOVA followed by Mann–Whitney U test).
Discussion
In the present study, we have shown that the beneficial effect of MOE on the diabetes development in mice is mediated by its innate antioxidant capacity, the ability to suppress T-cell-related pro-inflammatory immune response and to block β-cell apoptosis. Since rosmarinic acid alone was not able to ameliorate diabetes completely, it is reasonable to assume that the active component of MOE is not a single compound, yet it could be rosmarinic acid complemented with some other active component(s) of MOE.
T1D develops as a consequence of T-cell-mediated autoimmune attack that is accompanied by macrophages responsible for ROS- and RNS-mediated damage of β-cells( Reference Nicolls, Haskins and Flores 4 , Reference Padgett, Broniowska and Hansen 5 ). Since MOE possesses antioxidant properties, the MLDS model of diabetes that consolidates nitrosative damage of β-cells and consecutive β-cell-directed immune response was chosen for the present study( Reference Friesen, Büchau and Schott-Ohly 26 ). This animal model of diabetes does not closely resemble the disease in humans, but covers several aspects of the autoimmune disease( Reference Weide and Lacy 27 ). STZ specifically enters β-cells, causing the generation of superoxide radicals, H2O2 and hydroxyl radicals( Reference Szkudelski 25 ). However, it seems that the increased level of superoxide and peroxides within the pancreas was not the case in our system at least judging by the absence of SOD and GSHPx activation. However, nitrosylated proteins within the islets prove that the oxidative stress has already occurred in MLDS-treated animals. This discrepancy might be attributed to the fact that the activity of antioxidant enzymes was determined within the whole homogenate of the pancreas, not specifically in the islets. However, it is evident that MLDS+MOE-treated animals had lower systemic activity of antioxidant enzymes compared with diabetic ones. It is plausible that MOE neutralised the generation of ROS and RNS and, therefore, alleviated the need for the up-regulation of antioxidant machinery. The strong antioxidant activity of the extract is mainly attributed to its key phenolic compounds such as rosmarinic acid, salvianolic acid B-lithospermic acid B and caffeic acid that exhibit high radical-scavenging capacity( Reference Zhang, Guo and Wang 14 , Reference Chen, Li and Yuan 28 ). It has been shown that caffeic acid dynamically and synergistically interacts with ascorbic acid and α-tocopherol and amplifies their antioxidant potential and the protection of cells along mitotic phases( Reference Laranjinha and Cadenas 29 ). Also, the down-regulating effect of MOE on GSHPx in the pancreas of both healthy and MLDS-induced mice can be explained by the known efficacy of its constituent salvianolic acid B to inhibit lipid peroxidation, thus resulting in the redundancy of GSHPx. In addition to the effect mediated by STZ itself, elevated glucose levels contribute to the systemic increase in ROS activity( Reference El-Bahr 30 ). Therefore, along with MOE-mediated antioxidant properties, the absence of hyperglycaemia in treated mice could be an additional reason for the lower activity of antioxidant enzymes.
Several reports have described that extracts rich in biophenols can act directly on glucose metabolism to accelerate glucose transfer from the blood into the cells( Reference Lemhadri, Zeggwagh and Maghrani 12 ). Having in mind that MOE indeed possesses biophenols, its beneficial effect on diabetes development could be related to its plausible glucose-lowering function. However, MOE administration to healthy mice did not alter glycaemia, suggesting that MOE does not affect glucose metabolism in mice.
Apart from its antioxidant action, MOE successfully suppresses the pathogenic immune response during diabetes development. The fact that mice treated with MOE after the induction of diabetes maintained normoglycaemia only during the course of treatment suggests that once the immune response towards β-cells is established, short-term treatment with MOE is not able to revert it. Also, these results suggest that MOE is not able to induce β-cell regeneration. However, if long-term treatment is applied, this could provide additional aid in reverting T1D symptoms. There are only a few studies considering the effect of oregano extracts or its oil on the immune response. The present results indicate that MOE action is T cell-specific. The absence of the effect on macrophages is somewhat surprising since oregano oils have been shown to primarily affect macrophage pro-inflammatory cytokine production( Reference Ocaña-Fuentes, Arranz-Gutiérrez and Señorans 31 ). This discrepancy could be attributed to the different preparations of oregano extracts.
The effect of MOE on Th-cell differentiation is probably crucial for the prevention of diabetes development. T1D is associated with infiltration of pro-inflammatory CD4+ Th cells and cytotoxic CD8+ lymphocytes( Reference Padgett, Broniowska and Hansen 5 ). We have found that MOE strictly acts on CD4+ Th17 cells. Although the function of Th17 is directed towards anti-microbial defence, there are several studies that implicate Th17 in the autoimmune attack during diabetes pathogenesis( Reference Emamaullee, Davis and Merani 6 , Reference Mensah-Brown, Shahin and Al-Shamisi 32 , Reference Korn, Bettelli and Oukka 33 ). Th17 cell differentiation is initiated by T cell receptor (TCR) stimulation and the action of TGF-β+IL-6/IL-21( Reference Mangan, Harrington and O'Quinn 34 , Reference Zhou, Ivanov and Spolski 35 ). These cytokines stimulate the expression of RORγt, a transcription factor mandatory for IL-17 expression( Reference Korn, Bettelli and Oukka 33 ). In contrast, IL-23 is a cytokine important for the stabilisation of the Th17 phenotype( Reference Aggarwal, Ghilardi and Xie 36 ). According to the results of this study, MOE-mediated interference with Th17 cells is not at the level of their differentiation or propagation, rather it is generated by the down-regulating effect on p38 mitogen-activated protein kinase and STAT3 phosphorylation that results in RORγt suppression. Both p38 and STAT3 are redox-dependent molecules, i.e. both are up-regulated in the presence of peroxides( Reference Usatyuk, Vepa and Watkins 37 , Reference Carballo, Conde and El Bekay 38 ). Therefore, the reason for their lower activation in MOE-treated mice could actually be a ROS and RNS-free environment.
An important notion is that one naive T lymphocyte could either develop into Th17 or Treg depending upon a cytokine milieu( Reference Komatsu, Okamoto and Sawa 39 ). Therefore, the observed up-regulation of the Treg population could imply that MOE performs a switch from the pro-Th17 signalling pathway towards the pro-Treg pathway within the cell. Having in mind the plasticity of Th17 cell, i.e. their ability of re-differentiating into Th1 or Th1/Th17 cells( Reference Chen, Li and Yuan 28 ), it could be speculated that the lower percentage of Th17 in MOE-treated animals is the result of their transformation. However, the percentage of Th1/Th17 (data not shown) and Th1 cells did not increase after MOE treatment compared with diabetic animals.
The up-regulation of IL-2 (a Treg growth factor) and TGF-β confirms that MOE promotes Treg proliferation( Reference Chen, Jin and Hardegen 40 , Reference Laurence, Tato and Davidson 41 ). Of note, a high number of islets from MOE-treated mice exhibited peri-insulitis, and these mice still maintained normoglycaemia. This could be a consequence of the composition of the infiltrating population in the islets of MOE-treated animals. Although a similar number of Th1 and Th17 cells compared with diabetic mice was found, the presence of higher Th2 and Treg numbers in pancreatic islets infiltrates could account for preventing switch from benign to destructive insulitis in MOE-treated mice. Also, MOE directly protects β-cells by interfering with apoptosis induced by cytokines. The β-cells succumb to apoptosis either via ROS and RNS production or by cytokine-mediated mitochondrial damage that eventually leads to caspase-induced cleavage of proteins( Reference Padgett, Broniowska and Hansen 5 , Reference Vieira, Escargueil-Blanc and Meilhac 42 ). The observed cytoprotective effect of MOE could either relate to its innate property for scavenging ROS or to the direct interruption of apoptotic signalling pathways. For several components of MOE has been shown to inhibit apoptosis induction, for example caffeic acid significantly prevented LDL-induced apoptosis in endothelial cells( Reference Vieira, Escargueil-Blanc and Meilhac 42 ), while rosmarinic acid inhibited adriamycin-induced apoptosis cardiac muscle cells( Reference Kim, Kim and Woo 43 ). Furthermore, salvianolic acid protected PC12 neuronal cells from H2O2-induced apoptosis by inhibiting caspase 3 activity( Reference Liu, Chen and Zhang 44 ). Similarly, MOE rescued islets from cytokine-driven apoptosis through the down-regulation of caspase 3, an enzyme that cleaves vital cellular proteins at the final stage of the apoptotic process.
Since rosmarinic acid was shown to be beneficial in several inflammatory diseases, and it is a major component of MOE, it was presumed that it could be its active component. Although in vitro results regarding the production of Th signature cytokines and β-cell cytoprotection are in accordance with the results obtained when using MOE, in vivo treatment was significantly less favourable compared with MOE. Therefore, the presence of other active components could be assumed. Total content of salvianolic acids, for example, is significantly higher in MOE, and this compound proved beneficial in preventing hyperglycaemia induction and oxidative injury in rats( Reference Wang, Yang and Tian 45 ). Therefore, combination of rosmarinic and salvianolic acids could exert the observed protection from diabetes induction.
MOE is a rich source of biophenols that probably act additively, thus achieving antioxidant, anti-inflammatory and cytoprotective action observed in this model of autoimmune disease (Fig. 6). Future studies should chart the bioactive compound formulation of the extract and investigate a possible translation for human therapy of autoimmune or inflammatory diseases.

Fig. 6 Mechanism of the anti-diabetogenic action of methanolic oregano extract (MOE). MOE acts as an antioxidant, immunomodulator and in the anti-apoptotic manner. Its innate antioxidant features alleviate the need for the up-regulation of systemic antioxidant machinery (SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; GSPHx, glutathione peroxidase). MOE exerts no effect on macrophages and T helper (Th)1 cells (upper left panel), but specifically down-regulates p-p38, phosphor-signal transducer and activator of transcription 3 (p-STAT3) and RAR-related orphan receptor γt (RORγt)-mediated IL-17 production and the number of Th17 cells, up-regulates GATA3-driven IL-4 production and the number of Th2 cells as well as forkhead box P3-mediated regulatory T cells (Treg) (lower left). By increasing the infiltration of Th2 cells and Treg within the pancreatic islets (upper right), MOE exerts anti-inflammatory action and preserves normal β-cell function (insulin secretion). Also, MOE blocks protein nytrosilation and displays cytoprotective properties through the inhibition of caspase 3-driven apoptosis (in vitro data; lower right). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514004048
Acknowledgements
The authors are grateful to Karsten Buschard (Bartholin Institutet, Copenhagen, Denmark) for the donation of the RINm5F cells.
The present study was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (grant no. 173013). It was co-financed by the European Union (European Regional Development Fund) and Greek National Funds Through the Operational Program ‘THESSALY–MAINLAND GREECE AND EPIRUS-2007–2013’ of the National Strategic Reference Framework (NSRF 2007–2013) and by the European Union (European Social Fund-ESF) and Greek national funds through the Operational Program ‘Education and Lifelong Learning’ of the National Strategic Reference Framework (NSRF)-Research Funding Program: ARISTEIA II, Investing in knowledge society through the European Social Fund.
The authors' contributions were as follows: M. V., S. S.-G., A. G. T. and I. S. designed the research; M. V., I. N., V. G. K., T. S., P. C., Z. O.-D., S. S.-G. and I. S. acquired the data; D. B., A. G. T. and I. S. analysed the data; V. G. K., Z. O.-D. and I. S. drafted the article. All authors revised the manuscript critically and made a final approval of the version to be published.
There are no conflicts of interest to declare.












