From the 99 cases presented in the previous chapters, we have touched on issues related to advance care planning, carer stress in different case scenarios and the support needed, practical tips about disclosing a dementia diagnosis, issues surrounding management, home safety, and dementia-friendly communities – with recommendations for the primary care team. In Section 1.3, we gave an overview of dementia work-up, diagnosis, and management. This chapter is a summary of the key lessons learned from the cases and a discussion of the recommended actions and further reading materials for consolidation and continued studies. These key lessons are grouped into five topics: advance care planning (Section 5.1); Carer Stress and Support (Section 5.2); Formulating and Disclosing the Dementia Diagnosis (Section 5.3); Issues Surrounding Management (Section 5.4); and Dementia-Friendly Communities and Prevention (Section 5.5).
5.1 Advance Care Planning
Advance care planning is a process of communication (Reference Mullick, Martin and Sallnow1). When it is foreseeable that a person may lose the ability to make decisions, advance care planning is important for the person, their families, care providers, and others to communicate appropriate care. In Section 1.5, we allude to the need for advance care planning for people living with dementia. This is especially the case in Alzheimer’s disease: its typical trajectory with relatively predictable functioning and needs allows planning, while the multidimensional needs at different disease stages require coordinated services.
Key Considerations in Primary Care
The process of advance care planning in dementia is far from straightforward. As dementia progresses, the ability to consider future thoughts and actions becomes compromised, affecting decision-making abilities. Family carers may increasingly find themselves in a position where they need to inform, or directly make, decisions on behalf of the person with dementia (Reference Harrison Dening, Sampson and De Vries2).
No high-quality guidelines are currently available for advance care planning in dementia (Reference Piers, Albers, Gilissen, De Lepeleire, Steyaert and Van Mechelen3). Evidence of planning’s effectiveness for people with cognitive impairment/dementia is limited but growing. Although most evidence of how advance care planning can improve outcomes (such as end-of-life outcomes) for people living with dementia and their carers has come from Western cultures (Reference Dixon, Karagiannidou and Knapp4), increasing evidence is becoming available from Asian areas such as Taiwan (Reference Huang, Lu, Liu and Chang5) and Singapore (Reference Tay, Hum, Ali, Leong, Wu and Chin6).
There are, however, key barriers to the uptake of advance care planning in dementia: uncertainties with planning and communication problems could reduce carers’ willingness to engage in active decision-making (Reference Cresp, Lee and Moss7, Reference Sellars, Chung, Nolte, Tong, Pond and Fetherstonhaugh8); providers may have insufficient knowledge about dementia, lack confidence, and be uncertain about when to initiate advance care planning, how to assess decisional capacity, and how to accommodate changing preferences.
In general, an early introduction of advance care planning when the person’s cognitive impairment is still mild is facilitative. A community setting would be appropriate for advance care planning, as it would be too late in terms of the person’s capacity when he/she is already institutionalised (Reference Robinson, Dickinson, Rousseau, Beyer, Clark and Hughes9). There are different views as to whether the time of diagnosis is a good time to initiate advance care planning discussion, as it could depend on the person and their family’s readiness, and the primary care team should consider receptiveness or reluctance carefully using a family- and person-centred approach.
Inclusion of all stakeholders, discussions that focus on both medical and social issues with an aim to maintain a normal life, and supporting the integration of emotional and technical issues are required for good practice (Reference Lee, Bamford, Poole, McLellan, Exley and Robinson10). The use of educational strategies that enable shared decision-making for future care can enhance advance care planning discussions (Reference Brazil, Carter, Galway, Watson and van der Steen11).
Practice Point 1: Care Needs for the Next Stage
Family carers need to think ahead about how to prepare for the next stage of the disease. Advance care planning is a communication process (instead of static documentation of decisions made earlier). Primary care providers should maintain communication and support the family to prepare for new needs that are expected to arise in the next stage (see Box 5.1 for a case illustration). For people with Alzheimer’s disease, this could be done considering the expected change in ADL and IADL functioning (see FAST staging in Section 1.3). For example, as the person develops dressing apraxia, suggesting a moderate stage, we could expect the need for assistance in bathing and toileting problems to follow. When the person starts to have speech problems, suggesting an advanced stage of dementia, the family may need to consider a care home arrangement. A care planner could guide the family through these typical disease progression stages and provide information to support better living for the person and their family carers.
Box 5.1 Case Illustration
In our Case 050 (How Did I Spend that Money?), Mr Hui is a typical case of early Alzheimer’s disease. With his current difficulties in handling money and finances as his disease progresses and the expected further cognitive decline, financial management could become a key problem, and conflicts and abuse are potential risks to avoid. On the other hand, the family carer reported that Mr Hui has an unimpaired ability to use transportation, while based on a typical disease trajectory we can expect impairment in these more complex IADL tasks soon. This is a good time to discuss with Mr Hui and the family about future care planning, providing education on areas of functioning where the family already has concerns (i.e., financial management in this case), while explaining other areas with predicted deterioration and risks (e.g., using transport and getting lost in this case), to initiate the advance care planning communication process.
Practice Point 2: Is the Person Mentally Incapacitated?
Whether a person has the mental capacity to make certain decisions should be judged case by case: mental capacity (or mental incompetence) is always relative to the subject matter to be decided on. A person living with dementia, for example, could have the capacity to make a decision about what to have for a meal, but may lack the capacity to manage their property. Livingston et al. (Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames12) provided a succinct summary of the key principles in assessing mental capacity, which include the following:
Assuming capacity until proven otherwise;
All feasible steps have been taken to support the person in making a decision;
Making an unwise decision does not equal a lack of capacity; and
Mental capacity assessment should be based on the person’s ability to understand, retain in their mind, use or weigh the relevant information, and communicate their decision.
In general, people in the early stages of dementia are often still mentally competent to instruct a lawyer to draw up their will and power of attorney. This should therefore be considered in advance care planning, and the primary care team should find the opportunity to discuss with the family member and the person on these matters.
Practice Point 3: Powers of Attorney
Legal planning can be a complicated matter for the person living with dementia and the family, where counselling and advice from the primary care team may be necessary to identify the most suitable help. This advice may include the legal instruments available and what can be done to facilitate decisions and choices with the person’s remaining legal capacity and autonomy (Reference Nikumaa and Mäki-Petäjä-Leinonen13). One of the legal instruments relevant to dementia care is a power of attorney. Depending on the local laws, an enduring power of attorney (EPA) or lasting power of attorney (LPOA) generally allows the person, while s/he still has the mental capacity to do so, to appoint someone to take care of his/her financial matters in the future when s/he becomes mentally incapacitated (see Box 5.2 for a case illustration). Sufficient counselling and early planning are important, due to the complexity of the decision (of handing over one’s power to a trusted other) and risks (e.g., a new risk of financial abuse under an EPA or LPA (Reference Purser, Cockburn, Cross and Jacmon14)) that require sufficient mental capacity.
Box 5.2 Case Illustration
In our Case 089 (Suicidal Ideation and Temper Tantrum), Mrs Yuen’s cognitive impairment has already affected her ability to take medication, cook, or handle finances independently. The reported incidents of her forgetting to collect rent for the properties she owns suggest that it could be beneficial for an attorney to be appointed to take care of her financial matters when necessary. In this case, should Mrs Yuen still have the mental capacity to make such a decision, an EPA or LPA could be an option to protect Mrs Yuen’s rights and benefits. This case also illustrates the potential advantage of early advance care planning, to avoid unnecessary losses (and possible disputes) that have resulted from Mrs Yuen’s current state of reduced ability to handle finances.
Practice Point 4: Advance Directive
Compared with those with other terminal diseases, people living with dementia are more likely to receive aggressive interventions during the end stage of their life, and this appears to be especially true in Eastern cultures compared with the West (Reference Chen, Ho, Huang, Hsu, Chen and Chen15). At the same time, carers might regret their care decisions made on behalf of the person at their end-of-life stage, even when the decision was based on medical advice (Reference Supiano, Luptak, Andersen, Beynon, Iacob and Wong16). It is now known that decisional conflicts and regrets could be minimised, carer satisfaction could be improved, and end-of-life care quality could be enhanced, if there is advance care planning discussion during early dementia (Reference Huang, Lu, Liu and Chang5, Reference Tay, Hum, Ali, Leong, Wu and Chin6) involving the person, the family carers, and health and social care professionals.
As such, an advance directive should be considered a means towards an end, providing an opportunity to engage a person in communicating their preferences in advance care planning, from alpha to omega, including a discussion on end-of-life issues (Reference Tilburgs, Vernooij-Dassen, Koopmans, van Gennip, Engels and Perry17). The person and their family members benefit from the communication to get prepared cognitively, behaviourally, and most importantly, emotionally. Early and ongoing practical and emotional support is essential to prepare the family for potential changes and aid decision-making in the context of the realities of care towards the end of life (Reference Harrison Dening, King, Jones, Vickerstaff and Sampson18). Given the uncertainty surrounding death in dementia, agreement on end-of-life care and interventions tends to be low even among families with good relationships. The emphasis here is therefore the communication opportunity and socioemotional aspect of the process of drawing up an advance directive while the person still has capacity, instead of having a mere legal document, to ensure family consensus and minimise regret.
Practice Point 5: Advance Proxy Care Planning
While an advance directive is applied if the person has decision-making capacity at the time when the directive is completed, advance proxy care planning would be needed for those who have already lost such capacity (Reference Volicer, Cantor, Derse, Edwards, Prudhomme and Gregory19). A proxy decision-maker can be a next of kin, a close family member/friend, a designated power of attorney for care, or a guardian who is trusted to make decisions on the person’s behalf. Some salient points for facilitating the development of a proxy plan include (Reference Volicer, Cantor, Derse, Edwards, Prudhomme and Gregory19) careful consideration of the person’s expressed goals, values, and preferences; regularly reviewing and updating the plan; and conflict resolution mechanisms.
See Box 5.3 for further reading and useful material on advance care planning in dementia.
Box 5.3 Further reading and useful material on advance care planning in dementia
Harrison Dening et al. (2019), Advance care planning in dementia: Recommendations for healthcare professionals (Reference Harrison Dening, Sampson and De Vries2)
discusses the context and importance of a palliative care approach and recommends rationales and strategies for healthcare professionals to support families affected by dementia to better plan for their future care.
Piers et al. (2018) Advance care planning in dementia: Recommendations for healthcare professionals (Reference Piers, Albers, Gilissen, De Lepeleire, Steyaert and Van Mechelen3)
contains 32 recommendations covering eight domains: (1) initiation of advance care planning, (2) mental capacity assessment, (3) holding advance care planning conversations, (4) the role and importance of those close to the person living with dementia, (5) advance care planning when it is difficult or no longer possible to communicate verbally, (6) documentation of wishes and preferences, including information transfer, (7) end-of-life decision-making, and (8) preconditions for optimal implementation.
5.2 Carer Stress and Support
Carer support is core to dementia management, with carer outcomes linked to many dementia outcomes, including disease progression and mortality (Reference Lwi, Ford, Casey, Miller and Levenson20, Reference Tschanz, Piercy, Corcoran, Fauth, Norton and Rabins21). Family carers are key partners in dementia care, while their own health (including mental health) and well-being can greatly influence the outcomes of the person living with dementia – to the extent that some carers could be the ‘invisible second patients’ (Reference Brodaty and Donkin22). While caring for a person living with dementia can be associated with positive gains (Reference Carbonneau, Caron and Desrosiers23), dementia carers have an increased risk of having a high care burden (Reference Etters, Goodall and Harrison24) and other poor psychological and physical health outcomes (e.g., depression, infection, and death) (Reference Schulz, Boerner, Shear, Zhang and Gitlin25–Reference Ory, Hoffman, Yee, Tennstedt and Schulz27). The primary care team should therefore pay attention to the burden and stress associated with dementia care, which are widely documented phenomena (Reference Pearlin, Mullan, Semple and Skaff28, Reference Schulz, O’Brien, Bookwala and Fleissner29). Evidence-based interventions are available to minimise the negative consequences of caregiving and delay institutionalisation, and the primary care team have a role to provide education, psychological support, and mobilise support networks (Reference Cohen, Pringle and LeDuc30).
Key Considerations in Primary Care
The caring experience is diverse. Carer stress is influenced by a range of factors/stressors, including service accessibility, distressed behaviours and neuropsychiatric symptoms, functioning of the person living with dementia, their existing relationship, and the carer’s coping and social support (Reference Pearlin, Mullan, Semple and Skaff28). Many of these factors are modifiable and targets of interventions.
Among these factors, distressed behaviours and neuropsychiatric symptoms have a significant and complex relationship with carer stress and burden. They tend to interact with each other: neuropsychiatric symptoms and personality change can be hard for carers to make sense of or come to terms with; they can increase carer stress and negative emotions (Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez- Pousa and Garre-Olmo31). These could in turn trigger or worsen distressed behaviours, especially when the carers cope with non-adapting strategies and have a low sense of competence in managing distressed behaviours (Reference de Vugt, Stevens, Aalten, Lousberg, Jaspers and Winkens32).
Current guidelines recommend not just one single protocol of carer intervention, but psychoeducation and skills training that are tailored and easily accessible for carers (33).
There can be more than one carer. Family care is sometimes shared among multiple family members (Reference Harvath, Mongoven, Bidwell, Cothran, Sexson and Mason34). Whenever possible, the primary care team should engage the primary carer as well as other family members to facilitate communication and family support.
Caring can happen in the pre-diagnostic and help-seeking periods (Reference van Vliet, de Vugt, Bakker, Koopmans, Pijnenburg and Vernooij-Dassen35). In primary care settings, carers who seek help could have been struggling for a long period of time. Carer burden can be evident during this period (Reference Ng, Leung, Cai and Wong36). In some cases, because of the denial and resistance of the person with suspected dementia and a lack of support from other family members, the carer who first noticed symptoms of dementia may be faced with moral dilemmas and conflicts. Support from the primary care team can help address the burden and stress of help-seekers.
Practice Point 1: Supporting Positive Communication and Understanding
The nature of some common distressed behaviours and neuropsychiatric symptoms, such as delusion of theft and agitation, has a close relationship with psychosocial needs (e.g., social interaction, positive regard, occupation, and identity). Psychoeducation with carers could focus on ways to address these needs, by improving communication skills and creating a positive social environment at home, which should also include fostering self-care in family carers (see Box 5.4 for case illustrations).
Based on the experience from evidence-based multicomponent carer interventions, some common factors in these intervention programmes can be identified (37), including promoting affective expression, enhancing empathy, and increasing tolerance of the person’s neuropsychiatric symptoms. Encouraging carers to validate the person’s feelings behind certain behaviours, instead of focusing on correcting his/her mistakes and confusion, can be helpful (38).
Box 5.4 Case Illustration
In our Case 009 (Where is My Wife?), Mr Tam’s son has noted that he keeps asking about his wife’s whereabouts, forgetting that she is in hospital. While it is a normal reaction for family members to correct or sometimes confront the person with facts, a more helpful way of communication here would be to promote carers’ understanding of the emotional or psychosocial needs or motives behind the behaviour. By allowing Mr Tam to express and by listening to his feelings, opinions, and needs – things that are ‘beyond the realm of fact and correction’ (38) – better communication, relationships, and well-being could result.
In many cases when a family member is targeted, it could be distressing and communication could be challenging. For example, in our Case 006 (Forgotten Home Address), Mrs Cheung suspects that her daughter steals money from her; we can expect her daughter to have negative emotions (e.g., anger and depression) even when the behaviour is recognised as a symptom with treatment indicated. The primary care team could support the carer to understand the behaviour from the person’s perspective through psychoeducation that could help address carer stress: in this case, the behaviour (suspicion of the daughter for stealing) could be understood as a reasonable response, when Mrs Cheung has no recollection of where her money is, and in that situation, it is perhaps logical to suspect someone close (a spouse, child, or in some areas a domestic helper) to be involved. With this understanding, family carers may have greater acceptance of using adaptive coping and communication strategies (e.g., reminders and a safety box) to support the person, rebuilding trust and relationships, which could in turn reduce the behaviour.
Practice Point 2: Meaningful Activities for Behaviours
Family members can also be encouraged to facilitate meaningful activity for the person living with dementia, which, when tailored to their abilities and interests could create a positivity and fulfilment. Participation in meaningful activities could be useful for maintaining social roles and self-identity, encouraging positive expression, and promoting feelings of connectedness. Evidence from individualised, family-centric programmes has shown that family carers can be coached to introduce meaningful activities, communicate effectively, break down tasks, and create a suitable and simplified environment to promote engagement with the person living with dementia, which can reduce distressed behaviours while promoting well-being in both the person living with dementia and family carers (Reference Gitlin, Winter, Vause Earland, Adel Herge, Chernett and Piersol39).
Practice Point 3: Younger-Onset Dementia and Younger Carers
What we know about younger-onset dementia is still relatively little, including its impact on carers. It is likely that carers of both younger-onset dementia and older-onset dementia experience high stress and burden, which would necessarily interact with their life stage (Reference van Vliet, de Vugt, Bakker, Koopmans and Verhey40). For spouses, dependency could be a key concern (Reference Kaiser and Panegyres41). For children, the perception of severe threats in the future is similarly a common theme (Reference Allen, Oyebode and Allen42). This is understandable given that many people with younger-onset dementia are still working and could be the family’s major breadwinner, when the children may still be under schooling.
In this group of carers, there may be increased barriers in accessing services, as very often dementia care services are designed to target older people; unmet needs could be common. Family members may also find it challenging to understand and accept the change in behaviours and personality in younger-onset dementia (Reference Millenaar, Bakker, Koopmans, Verhey, Kurz and de Vugt43). The primary care team can be an important support to provide these families with information and address other unmet care needs. While aids to facilitate family role restructuring are important in dementia carer support in general (37), a reconfiguration of family relationships would probably take even higher priority in younger-onset dementia.
See Box 5.5 for Further reading and useful material on carer stress and support.
NHS Education for Scotland (2020). Promoting Psychological Wellbeing for People with Dementia and their Carers: An Enhanced Practice Resource (38)
an easy-to-read resource designed to enhance the understanding of dementia from a psychological perspective, with practical tips and exercises to enable practitioners to apply the learning to supporting people living with dementia and their families.
American Psychological Association (2011). Principles: Common Factors in Caregiving Interventions (37)
a succinct summary of the key learnings from carer interventions, including characteristics of successful programmes, with reference to the US Resources for Enhancing Alzheimer’s Caregiver Health Studies.
World Health Organization (2019). iSupport for Dementia: Training and Support Manual for Carers of People with Dementia (44)
a skills and training programme for carers of people living with dementia, with five modules and accompanying exercises.
5.3 Formulating and Disclosing Dementia Diagnosis
As this book is about help-seeking cases and the initial encounter(s) with an early detection service and a primary care team, readers will now be accustomed to many of the considerations involved in formulating and disclosing a dementia diagnosis. What cannot be emphasised enough is that delay in help-seeking is common. Late help-seekers, however, have worse functioning and symptoms (Reference Tang, Wong, Ng, Kwok, Lee and Dai45). The benefits and needs of a dementia diagnosis are clear, but timely diagnosis remains a major challenge globally (46, 47). Some primary care professionals may, understandably, have concerns over diagnosing and disclosing dementia to the person and their family carers, as some people may have a strong negative emotional response to the disclosure. Some argue against disclosing for fear of upsetting the person and potential consequences such as suicide. However, existing evidence and reports from primary care physicians suggest that many families are able to handle well the disclosed information (Reference Connell, Boise, Stuckey, Stuckey, Holmes and Holmes48), and the vast majority of people with or without cognitive impairment do want to have the information, for autonomy reasons (Reference van den Dungen, van Kuijk, van Marwijk, van der Wouden, Moll van Charante and van der Horst49), as the knowledge is essential for their future planning and decisions on treatment and care options. How to deliver the message with care is key.
Key Considerations in Primary Care
Primary care professionals are in a good position to facilitate help-seeking and timely diagnosis because:
○ there is an existing rapport with the family, before the presentation of dementia signs and symptoms. Trust in the professional could help the family go through the disclosure process;
○ they have good knowledge of the person’s other conditions, needs, and strengths/resources, which are often important considerations in the diagnosis and subsequent care planning, including advice on legal matters;
○ involvement of primary care may reduce fear and stigma surrounding the diagnosis;
○ family physicians and care services have the advantage of working with the family as a unit, which is particularly important in dementia care;
○ primary care physicians can function in the healthcare system to act as gatekeepers and seek specialist support when indicated.
Practice Point 1: Break Bad News with Care
The formulation and disclosure of a dementia diagnosis is a process rather than an encounter. The primary care team has an important role in ensuring this process is carried out in a way that minimises psychological harm and maximises the health benefits an early diagnosis can bring. Given that individuals have different preferences for accessing health information (their own or that of a person they are caring for), whenever possible, the primary care team should consult the person and the family at the outset to understand their preferences for disclosure, which could range from information-sharing to counselling and instrumental support. In any case, it is important to ensure clarity in the disclosure. Approaches that downplay the significance of the condition and potentially poor prognosis, sometimes motivated by an intention to preserve hope, may nevertheless compromise understanding and informed future planning (Reference Dooley, Bass and McCabe50). Some key behaviours in an appropriate disclosure of a dementia diagnosis include (Reference Lecouturier, Bamford, Hughes, Francis, Foy and Johnston51) preparing for the disclosure, involving the family, responding to the person’s reactions, and communicating effectively.
Disclosing the diagnosis may be particularly challenging if the person lacks insight or when it is something exceedingly difficult for the person and/or some family members to accept (see Box 5.6). As the disease progresses, the person may lose insight into their cognitive impairment and may have fewer complaints than the carer. In some cases, the assessment and diagnostic consultation could be a source of conflict within the family when not all members share the same understanding of the condition. It is thus even more important to not leave the task of breaking bad news to a family carer alone. Having a trusted professional within the primary care team explaining with patience what a diagnosis of dementia means, what the care and treatment options are, and what support will be available (see Section 4.5) can have a significant impact on the person’s and the carers’ outcomes.
Box 5.6 Case Illustration
Diagnosis disclosure may be particularly tricky when the person does not acknowledge any issues, as in our Case 017 (Time and Place Orientation, Mrs Yip) and Case 059 (Denial of Functional Decline and Dementia, Ms Tse). In both cases they denied having dementia: one was defensive and irritable during the assessment, while the other was aware of memory decline but denied other impairments. In these cases, finding out the level of insight may help in deciding the best way to communicate. For example, Mrs Yip’s irritability and defensiveness could indicate either a complete lack of insight (and the assessment viewed as something ungrounded) or good awareness (and denial out of fear about what might happen when she has a confirmed diagnosis). For the latter, reassurance, signposting of post-diagnostic support, and patient education could help. In the former case, if insight is affected by cognitive impairment, confrontation with facts may not be the best strategy, whereas care and intervention that preserve dignity and focus on strengths would be helpful. Carers may also need support in developing communication skills that neither confront the person nor reinforce denial of his/her care needs.
Practice Point 2: Next Steps Are Part of the Process
Some practitioners may hold negative attitudes towards diagnosing dementia due to a perceived suboptimal post-diagnostic management (Reference Giezendanner, Monsch, Kressig, Mueller, Streit and Essig52). The same can be expected of family carers and the person. It is thus an essential component of the assessment and disclosure process that management options are provided. A focus on quality of life and well-being, as well as planning for the future, are therefore also considered part of the best practice in disclosing the diagnosis (Reference Lecouturier, Bamford, Hughes, Francis, Foy and Johnston51).
An individualised approach is important in tailoring the next steps based on assessment findings (e.g., whether the person’s cognitive impairment level may benefit from cognitive stimulation/rehabilitation, whether other more acute conditions and safety should be prioritised, or if no diagnosis can be given at the moment and information/monitoring is advised). At the same time, some general information would be useful for the person and the family to consider the next steps:
communicating the fact that dementia is a chronic condition and the possible prognosis;
emphasising that both drugs and non-drug interventions can help in stabilising symptoms, delaying deterioration, and minimising complications;
highlighting the role of continuing mental and social activities and daily routines.
Remember that a dementia diagnosis is a major life event, and the person and the family will need time to process the information and adjust. While information on treatment options and advance planning is important at this stage to induce hope, it can sometimes be overwhelming with too many other major decisions that need to be made within a short period of time. The primary care team can support by working with the family at the preferred pace and level of support needed in planning for the future, while reassuring them that help is available.
Practice Point 3: Typical Presentation and Assessment Findings
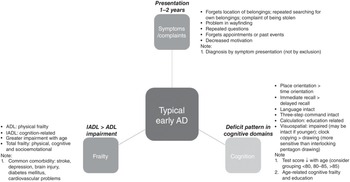
From the cases of Alzheimer’s disease and other cases presented in Chapters 2 and 3, we hope that readers are now familiar enough with the characteristics of the assessment profile to be able to form a clinical impression of Alzheimer’s disease or otherwise, when a person presents with suspected dementia (see Figure 5.1 for an illustration of a typical case presentation in this casebook). In particular:
Age: although Alzheimer’s disease can occur at different ages, the early 80s is the typical age of presentation.
Cognitive assessment findings: while the overall performance (e.g., total score) would depend on age and education, the pattern of impairments should remain similar in people with Alzheimer’s disease. These include better performance in immediate recall than delayed recall; in place orientation compared with time orientation;Footnote 1 and in clock copying versus clock drawing. Since MMSE is sensitive for Alzheimer’s disease, if near-normal MMSE results are obtained alongside other dementia symptoms, frontotemporal dementia or other conditions should be suspected.
Clinical examination findings: in typical Alzheimer’s disease cases, the clinical examination usually shows normal findings, with typical symptomatology and presentation. In moderate Alzheimer’s disease, apraxia in self-care is common. Note that while ADL performance tends to decline with age, in Alzheimer’s disease IADLs should be more impaired than ADLs (unless there are non-cognitive reasons for specific ADL impairments, such as physical frailty). In people who present at a younger age (e.g., in their 50s and 60s), the primary presenting symptom may not be memory impairment, but could be behavioural, speech problems and apraxia; these should lead to the consideration of non-Alzheimer’s dementia and suggest referral for secondary care assessment, including more advanced neuroimaging.
Practice Point 4: Complaints to Watch Out For
The importance of complaints spontaneously made by the person and/or a knowledgeable informant deserves more recognition: they are observations that are significant enough to prompt help-seeking. Evidence from community-based early detection services suggests that the type and pervasiveness of these complaints are associated with illness severity and can provide useful information for triage (Reference Xu, Choy, Tang, Liu, Luo and Lou53).
Common complaints from the carer seen in our cases of uncomplicated early Alzheimer’s disease include repeated searching for belongings, losing items/money frequently, delusions of stealing, difficulty in finding their way, repeated questions, forgetfulness about appointments and recent events, and decreased motivation. Carers of a person with Alzheimer’s disease would typically answer ‘yes’ to most of the questions covered in the GPCOG (on remembering recent events, recalling recent conversations, managing money and finances, managing medication independently, and using transport), except for word finding (see Box 4.2).
Considering that there can be discrepancies between the complaints from the person and the carer, a point to note is to arrange time for separate interviewing. With decreasing levels of insight, the carer’s report is often more important, although the primary care team should always bear in mind how ‘knowledgeable’ the carer is (e.g., whether living together and awareness of the person’s functioning level) in evaluating the validity of the complaint (or the lack of it). In some cases (e.g., Case 003), there may also be insufficient information from a carer, and reports from other informants (e.g., a formal carer or home helper) should also be sought as far as possible.
Practice Point 5: Remember It’s a Clinical Diagnosis
One of the key advantages of the primary care team in the diagnostic process of uncomplicated Alzheimer’s disease is that it is a clinical diagnosis in an older person, who would typically be known to the team for other chronic illnesses and care needs, with clinical symptoms of early Alzheimer’s disease usually presenting for one to two years before help is sought. In considering a dementia diagnosis, comprehensive information about frailty, co-occurring conditions, and family relationships is an important context, which should be taken into account in understanding findings from assessments and examinations, such as the person’s ADL and IADL performance. These other conditions and age-related physical, cognitive, and socioemotional changes could mean that presenting symptoms and assessment results may be modified.
See Box 5.7 for futher reading and useful material on formulating and disclosing dementia diagnosis.
Box 5.7 Further reading and useful material on formulating and disclosing dementia diagnosis
Dementia Australia. Informing the Person with Dementia (54)
a plain-language guide explaining how to prepare for disclosing a dementia diagnosis.
Dementia: Timely diagnosis and early intervention (Reference Robinson, Tang and Taylor55)
a BMJ clinical review with discussion on the role of primary care in dementia diagnosis, investigation, and assessment tools in primary care, assessment of mental capacity, and other tips for non-specialists.
Dementia Diagnosis and Management: A Brief Pragmatic Resource for General Practitioners (56)
www.england.nhs.uk/wp-content/uploads/2015/01/dementia-diag-mng-ab-pt.pdf
a resource pack by the UK NHS aiming to support general practitioners in identifying and appropriately managing people living with dementia in the primary care environment, with case scenarios illustrating when referral is justified and when cases can be safely diagnosed by a general practitioner.
5.4 Issues Surrounding Management
Post-diagnostic management is multidisciplinary and multicomponent, as ‘good dementia care spans medical, social, and supportive care; it should be tailored to unique individual and cultural needs, preferences, and priorities and should incorporate support for family carers’ (Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames12). Important themes surrounding management go beyond treatment (including drug and non-pharmacological interventions); a unique combination of care and support services, which may cover emotional and psychological well-being and practical and integrated support, is needed for the person and/or their family members (Reference Bamford, Wheatley, Brunskill, Booi, Allan and Banerjee57). We have discussed family carer support in Section 5.2; here we will focus on disease management that directly involves the person with dementia.
Key Considerations in Primary Care
The multidisciplinary and multicomponent nature of dementia management means that a team approach is necessary. Depending on the country and local service system context, the primary care team would
○ need to partner with other service providers to facilitate access and adherence to the recommended interventions, care, and support
○ need support from relevant specialist/specialised care services as the disease progresses or when complications arise. Understanding when to refer in line with local collaborative care practice is important
Practice Point 1: Both Pharmacological and Non-Pharmacological Interventions Are Needed
It is increasingly recognised that pharmacological and non-pharmacological interventions, when given early, have equal roles in maintaining cognition, functioning, and quality of life in people with dementia. Cognitive, physical, and social activities have potential benefits (Reference Burke, Hickie, Breakspear and Gotz58): there is dissociation between brain pathology and cognitive symptom presentation (Reference Stern59, Reference Stern, Gurland, Tatemichi, Tang, Wilder and Mayeux60), and cognitive functioning is malleable throughout life even in the context of brain diseases and lesions, which can be altered by everyday experience, including cognitive, physical, and social activities (Reference Fratiglioni, Paillard-Borg and Winblad61).
For drug treatment, in the case of deteriorating cognition in mild to moderate Alzheimer’s disease, physicians may escalate the dose of a cholinesterase inhibitor or add memantine (e.g., Case 025). Memantine may also be prescribed when there is irritability (e.g., Case 017) or in cases of mixed dementia (e.g., Case 020). Response to drug treatment can also be helpful in diagnosis, such as in cases presenting with features of Alzheimer’s disease but when vascular or mixed dementia cannot be ruled out entirely (e.g., Case 010). The presence of neuropsychiatric symptoms does not necessarily indicate antipsychotics and specialist care; for example, the delusional ideas in Case 005 can be handled by non-pharmacological means such as the provision of cues. However, in cases where significant delusion is present, a specialist referral and neuroleptics may be needed. Other situations where referral is indicated include the presence of rigidity (e.g., Case 042) and urgent psychiatric referral in the case of imminent suicide risk (e.g., Case 041).
For non-pharmacological interventions that target cognition and quality of life, group cognitive stimulation that involves social interaction and cognitive processing can be used (Reference Woods, Aguirre, Spector and Orrell62, Reference Huntley, Gould, Liu, Smith and Howard63). Cognitive stimulation has been recommended in England’s National Institute for Health and Care Excellence (NICE) guidelines and by Alzheimer’s Disease International (Reference Prince, Bryce and Ferri64, 65). A manualised version of cognitive stimulation therapy (CST) with evidence of effectiveness and cost-effectiveness when used alone or in combination with medication as maintenance therapy (66, 67) is available in 35 countries (see www.ucl.ac.uk/international-cognitive-stimulation-therapy/international-cognitive-stimulation-therapy-cst-centre). Mental stimulation is therefore one of the most commonly recommended management strategies in the typical Alzheimer’s disease cases presented in this book. Apart from mental stimulation, other common recommendations are regular exercise, a healthy diet, and an active social life. In fact, social interaction is an integral part of CST and may account for some of its benefits. Physical exercise improves activities of daily living and cognitive functioning (especially executive function) (Reference Forbes, Thiessen, Blake, Forbes and Forbes68, Reference Colcombe and Kramer69). Locally developed interventions based on these principles and evidence may be available; for example, in China, an approach integrating cognitive stimulation, physical exercise, and social relationships into traditional Chinese culture has been developed (Reference Adjepong, Amoah-Agyei, Du, Wang, Fenton and Tucker70) for use as an intervention or as a lifestyle approach available from community service providers (see www.eng.hkada.org.hk/about6arts).
Practice Point 2: Managing Distressed Behaviours and Neuropsychiatric Symptoms
Sometimes referred to as ‘challenging behaviours’ and ‘behavioural and psychological symptoms of dementia (BPSDs)’ previously – terms that are now regarded as less helpful (71) – distressed behaviours and neuropsychiatric symptoms can be managed and sometimes prevented. Distressed behaviours that more commonly prompt family carers to seek help include resistance to care, paranoia/suspiciousness, aggression, and restlessness/wandering (71). In the cases presented in this book, for example, suspiciousness of others stealing things from oneself is a common presenting problem (e.g., Cases 025, 035, 047, and 068). Other neuropsychiatric symptoms such as sexual disinhibition, hallucinations, and nocturnal disturbances, on the other hand, may not be easily understood by family carers as related to dementia. These could lead to distress and other undesirable consequences such as premature institutionalisation. The primary care team can help manage and reduce the occurrence of some of these behaviours and symptoms.
Many educational materials and resources are available on managing distressed behaviours and neuropsychiatric symptoms; some non-pharmacological approaches currently being used involve the family carer (see Section 5.2) and include
the Activating event, Behaviour, Consequence (ABC) framework, which focuses on identifying the specific circumstances associated with the behaviour and developing targeted strategies. For example, underlying medical illness, pain, and other potentially modifiable triggers would be explored (Reference Gitlin, Kales and Lyketsos72);
A related Describe, Investigate, Create, Evaluate (DICE) approach that highlights a person–carer–environment triangle, to conceptualise factors related to the person (e.g., unmet needs), carer (e.g., communication issues), and environment (e.g., over-/under-stimulation) (Reference Kales, Gitlin and Lyketsos73);
preventive measures including person-centred care and meaningful activities (see Box 5.8), which may reduce agitation and other behavioural problems, thereby decreasing the use of chemical or physical restraints.
Box 5.8 Case Illustration
In our Case 093 (No Concern for Personal Hygiene), Mr Cheng has the habit of picking up dirty tissues and cigarette butts, which can be disturbing to the family. There can be many ways to understand this behaviour, and one possibility is that the hoarding provides some meaning to Mr Cheng: it could be filling a void, or perhaps he enjoys the feeling of being ‘productive’ and normal in some sense by collecting. Providing an alternative, more adaptive activity in such a case could be a win–win: Mr Cheng can lead a meaningful everyday life while the behaviour is less disruptive to the carer. A point to note is that what is meaningful varies from person to person, depending on factors such as capability, previous interests, identity, and roles. Family carers can be in a good position to identify appropriate activities that are meaningful to the person, with support from the primary care team.
In some situations, when the agitation, aggression, and psychotic symptoms are causing severe distress or posing a threat to the person or others, clinicians may consider offering antipsychotics (65).
Practice Point 3: Optimising Safety, Physical Health, and Mental Health
In Section 1.4, we introduced the biopsychosocial model, which explains the need to intervene on tractable physical, psychological, and social health factors to reduce excess disability in dementia (Reference Spector and Orrell74). We have seen cases in which the recommended management included prevention of health hazards (e.g., fall prevention in Case 002 with a history of hip replacement; home assessment for fire hazards in Case 055) and optimising the person’s physical health (e.g., cardiovascular risks in Cases 001 and 048) and mental health (e.g., depression in Cases 028 and 035).
Figure 5.2 illustrates some important connections among the four ‘Ds’: dementia, depression, delirium, and diabetes mellitus. As their relationships are complex, management strategies should be individualised considering the person’s comorbidity profile. In Case 013, for example, monitoring for mood problems and adjustment disorder is recommended, considering the person’s insight into his cognitive problem. Whereas in Case 035 with comorbid depression at presentation, the temporal sequence between dementia and depression needs to be elucidated to inform the best management. In Case 036, the recommendation is to optimise treatment for the comorbid depression and diabetes on top of the cholinesterase inhibitor prescription, considering the contribution of both depression and diabetes to dementia.
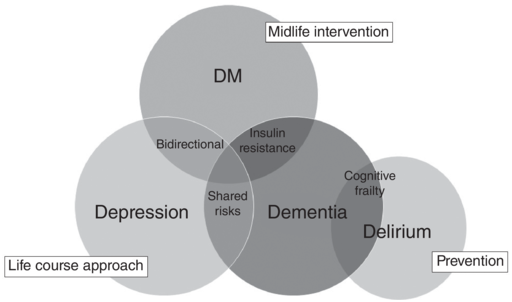
Figure 5.2 The four Ds – dementia, depression, diabetes mellitus, and delirium – as management targets
See Box 5.9 for further reading and useful material on issues surrounding dementia management.
UCL Dementia Training Academy
www.ucldementiatrainingacademy.org/
a web resource signposting to training for professionals on evidence-based psychological and social interventions for people with dementia, their carers, and care staff.
Dementia Centre for Research Collaboration. Behaviour Management: A Guide to Good Practice (BPSD Guide)
https://dementiaresearch.org.au/resources/bpsdguide/
a comprehensive overview of evidence- and practice-based management principles for distressed behaviours and neuropsychiatric symptoms of dementia for clinicians caring for people with dementia.
Alzheimer’s Association. Safety Assessment Checklist
www.alz.org/media/Documents/safety-assess-checklist.pdf
an easy-to-use safety assessment guide covering safety issues such as driving, taking medication, and getting lost.
National Collaborating Centre for Mental Health. The Dementia Care Pathway: Full Implementation Guidance
a resource pack by the UK NHS aiming to support general practitioners in identifying and appropriately managing people living with dementia in the primary care environment, with case scenarios illustrating when referral is justified and when cases can be safely diagnosed by a general practitioner.
5.5 Dementia-Friendly Communities and Prevention
The functions and performance of the primary care team shape and are shaped by the community they are embedded in. A dementia-friendly community usually encompasses activities promoting wider community involvement of people with dementia, care and support services for them and family carers, awareness and education, and environmental design (Reference Hebert and Scales75–Reference Buckner, Darlington, Woodward, Buswell, Mathie and Arthur77). Dementia-friendly communities are developed not only for people with dementia but for everyone (‘what is good for dementia is good for everyone’) (Reference Rahman and Swaffer78, Reference Crampton and Eley79). Such an environment promotes early help-seeking, risk reduction, and primary prevention, with primary care having major roles.
Key Considerations in Primary Care
Emphases of dementia-friendly communities centre around personhood, social inclusion, empowerment, stigma reduction, localised strategies, and community engagement and collaboration (Reference Hebert and Scales75) – concepts and values that are in line with primary care (as compared with secondary or specialised care).
In a dementia-friendly community, a wider collaborative network beyond health and social care providers works with the primary care team to support dementia care.
As much as 40 per cent of dementia risk is potentially modifiable, mostly occurring in midlife and later life (Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames12, Reference Livingston, Huntley, Sommerlad, Ames, Ballard and Banerjee80), and can be addressed through primary care.
Practice Point 1: Dementia Awareness
Stigma and a lack of awareness are barriers to early help-seeking. Late presentation could mean more complications and an increased need for specialised care. Public education on early intervention and prevention is therefore essential for people with dementia to benefit most from primary care. The cases in this book presented to an early detection service in collaboration with primary care practitioners; as the public is generally unaware of the role of primary care in dementia diagnosis and care, there was a need to prepare society in identifying probable dementia as well as recognising primary care as a possible care pathway.
On the other hand, the level of dementia literacy and awareness in the community would affect the perception, experience, and report of the person with dementia and their carer, which should be considered when conducting clinical interviews and assessments. Some examples can be found in the case of Mr Hung (Case 030), where his wife might have missed (and thus under-reported) early signs and symptoms, which can be compared and contrasted with the case of Mrs Cheng (Case 068), where public awareness efforts might have prompted her to present at a preclinical or very early stage. The primary care team have to exercise discretion in weighing the informant’s account.
Dementia awareness in the community is also relevant to social support (or the lack of it). In areas with a high level of awareness and easily accessible diagnostic services, late help-seeking could signal insufficient social support (which was identified as an area for further exploration in Case 019). Where public education targeted gatekeepers other than family carers (e.g., Case 030), people who would otherwise present late (e.g., living alone or with an older spouse only) may be able to access primary care in a timely manner.
Practice Point 2: Collaborative Network
Dementia-friendly communities promote partnerships across public/private sectors and between formal and informal carers for shared care (Reference Twigg81, Reference Kemp, Ball and Perkins82). These may include co-working to increase provision of evidence-based interventions and respite services, and training, coordination, and support for volunteers (Reference Cameron, Johnson, Willis, Lloyd and Smith83) – ‘Dementia Friends Champions’ – who would provide care navigation and lifestyle support (Reference Malby, Boyle, Wildman, Omar and Smith84).
Components of dementia-friendly communities concern people, organisations, communities, and partnerships (Reference Prince, Comas-Herrera, Knapp, Guerchet and Karagiannidou85):
People: involving people with dementia; enhancing public understanding; enhancing caring skills.
Organisations: promoting and providing timely diagnosis and post-diagnostic support by primary care
Communities: for social environment, increasing awareness, reducing stigma, and improving engagement; for physical environment, it includes providing home services and public space.
Partnerships: including cross-sectional support with a collaborative approach and collective commitment.
The point about partnership can be illustrated with Case 002 (repeated buying of groceries), where education and community engagement (e.g., targeting shopkeepers) could potentially improve the disease management, reduce carer stress, and improve the quality of life (better autonomy) of the person with dementia. Case 025 highlighted the role of communities: Ms Wong’s suspicions about her neighbours stealing her safety box key could lead to conflicts; improving understanding and acceptance of dementia in the neighbourhood may help avoid conflicts.
Practice Point 3: The Life Course Approach to Prevention
As mentioned in Section 1.3, Alzheimer’s disease is characterised by a slow, gradual progression of neuropathology over many years. Like most chronic diseases, lifestyle factors contribute significantly to its prevention at different levels. For primary or secondary prevention, modifiable midlife risk factors identified include hearing loss, hypertension, alcohol (>21 units/week), obesity, and traumatic brain injury; in later life, these include smoking, depression, physical inactivity, diabetes, and social isolation (80). The primary care team is in a good position to advise middle-aged and older people on reducing these risks.
For people with dementia, good management of these factors could potentially reduce complications and slow progression as tertiary prevention measures. In some of the cases presented in this book, for example, management of hearing problems is recommended (e.g., Cases 060 and 067). As hearing impairment could reduce sensory and mental stimulation directly, or indirectly through its association with social isolation, it was recommended to consider otoscopic examination, the use of hearing aids, and/or referral to an otolaryngologist in these cases. The same can be said for other known risk factors, which could sometimes be just as important as drug treatment for dementia.
See Box 5.10 for further reading and useful material on dementia-friendly communities and prevention.
Livingston G et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission (80)
an update to the 2017 report, with estimates of early-life, midlife, and later-life risk factors.
Risk reduction of cognitive decline and dementia: WHO guidelines
www.who.int/publications/i/item/9789241550543
an evidence-based recommendation on lifestyle behaviours and interventions to delay or prevent cognitive decline and dementia.
Towards a dementia inclusive society. WHO toolkit for dementia-friendly initiatives (DFIs)
www.who.int/publications/i/item/9789240031531
a toolkit providing practical guidance and tools to support efforts in creating dementia-inclusive societies.