INTRODUCTION
Infection with Legionella spp. can lead to two distinct clinical entities: Pontiac fever (PF) and Legionnaires' disease (LD). PF is characterized by a self-limiting influenza-like illness (ILI), which usually resolves in 2–5 days. It has a short incubation period of 48 h. LD is a more severe manifestation, associated with pneumonia [Reference Diederen1]. PF as an entity has been infrequently reported in the international literature (about 20 reports in the last six decades). Both PF and LD are notifiable diseases in Ireland. Between 2000 and 2007, 67 cases of LD were notified of which two-thirds were travel-related [2] and no PF case appeared in any official report.
It is considered that under-diagnosis and underreporting of cases of both PF and LD result in significant underestimation of legionellosis in Ireland, as elsewhere in Europe [2, Reference Ricketts and Joseph3]. The non-specific ILI associated with PF makes its recognition difficult. There is no specific test for PF and thus linkage with Legionella spp. is challenging. However, recognition of an outbreak of PF can prompt early intervention and may help prevent LD.
We discuss the investigation of a cluster of Pontiac-like illness which was identified in a work premises following notification of two cases of LD. We highlight issues around the diagnosis of PF, the use of a clinical case definition and risk factors associated with PF.
Background
In July 2008, the Department of Public Health in Dublin, Health Service Executive–East (HSE-E) and the Health Protection Surveillance Centre (HPSC) investigated two cases of LD. The two cases were linked to a workplace, a large Dublin-based company, and had no other common exposure. Symptoms commenced on 17 June 2008 in the first case and on 7 July 2008 in the second case. Both were hospitalized with pneumonia and laboratory confirmed as LD by positive urinary antigen test (UAT). Sputum samples (following antibiotic treatment) did not culture Legionella spp. Sputum of case 2 was positive for L. pneumophila DNA on polymerase chain reaction (PCR) at the UK National Reference Laboratory (Centre for Infections, London), but was insufficient to allow DNA sequence typing of the strain. Investigation focused on two cooling towers, the water systems and the air-conditioning structure as possible sources of infection. Details obtained from the cases of LD and the Human Resource Department on 8 July revealed that ILI resulting in absenteeism had occurred in some employees in June and early July. An outbreak control team from HSE-E initiated epidemiological, laboratory and environmental investigations of the source of illness. A letter from the Department of Public Health was sent by company email to all staff on 3 July after the first case and again on 8 July advising them of cases of LD, and warning them to be alert for symptoms. Local emergency departments (EDs) and general practitioners (GPs) were also informed.
Setting
The workplace was a new seven-storey ‘green building’ occupied for only 3 months. Two separate water systems existed within the office block. System A consisted of two cooling towers located in the underground car park, 5 m from the basement staff smoking area. System B comprised the hot- and cold-water system which serviced sinks and showers and piped chilled water throughout the building. This chilled water was fed into a fan coil unit in various air-conditioned rooms on certain floors to cool room air. Both systems were directly fed from a line taken off the mains water supply and were not physically mixed.
METHODS
Cohort study and case definition
A cohort study of all 400 employees working in the office, including security and delivery staff, was undertaken to establish the extent of illness and to identify risk factors associated with illness. Enquiries about other workers possibly exposed (visitors, independent contractors) added a further two to the cohort. The two LD cases were not part of the cohort.
A self-administered questionnaire was sent by email to the cohort. Focusing on the time period 1 June–7 July 2008, it sought information on demography; clinical symptoms, medical attention; underlying medical conditions; desk location; use of workplace showers; smoking habits; attendance at the smoking area; exposure to air-conditioning; use of the car park, with an estimate of time spent in each place.
A case of Pontiac-like illness was defined clinically as a person who attended the office between 1 June 2008 and 7 July 2008 and reported at least two of the following symptoms: fever, cough, muscle pains and headache, without evidence of pneumonia over the period [Reference Modi4]. Cases could be further categorized as PF cases if they had a >fourfold rise in antibody titre. Two hundred people completed the questionnaire (response rate 50%).
Microbiological investigations
All clinical cases and also those with significant illness requiring attendance at a GP or ED were invited to a serology clinic on 21 July 2008, at least 3 weeks following possible exposure. On advice from the UK Legionella National Reference Laboratory, one serum sample was tested at this time for L. pneumophila antibody estimation against serogroups 1–6 and 8, determined by indirect fluorescent antibody test (IFAT).
Fifteen clinical cases attended for serology. A further 22 employees who did not fit the case definition but had required medical attention also presented for serology.
Of all cases, 24 had a Legionella UAT performed earlier when acutely ill. Ten cases had negative chest X-ray at an ED.
Environmental investigation
An environmental inspection of the workplace was conducted. Samples from both cooling towers and multiple outlets on various floors were sent for PCR analysis and live culture analysis. As a precaution, the cooling towers were deactivated, cleaned and disinfected after the first LD case was reported.
Statistical analysis
Data were entered using Epidata version 2.0 [5] and analysed using Stata version 9.0 [6]. The effect of continuous variables was tested using Student's t test for normally distributed continuous variables (age). Specific attack rates (ARs) and crude risk ratios (RRs) with 95% confidence intervals (95% CIs) were calculated for each specific exposure. Variables were included in a multivariate model if significantly associated with a higher risk of Pontiac-like illness on a univariate analysis at a P value <0·20. The multivariate model was adjusted for age and sex. Collinearity between variables was excluded prior to introduction. Model adequacy was tested using traditional criteria of discrimination: the receiving operating characteristic (ROC) curve and the Hosmer–Lemeshow (H-L) C statistic.
RESULTS
Epidemiological results
Descriptive analysis
Forty-seven (23·5%) of the 200 respondents met the case definition for Pontiac-like illness. The median age of cases was 34 years (range 21–61 years) and the median age for non-cases was 40 years (range 20–72 years) (n.s.). Of the cases, 29 (62%) were female, and in non-cases 63 (47%) were female. Males had a lower risk of being a Pontiac-like case compared to females (18% vs. 29%) (RR 0·6, 95% CI 0·4–1·0).
Dates of onset of illness ranged from 7 June 2008 to 7 July 2008 (Fig. 1). The main symptoms described by cases with Pontiac-like illness (Table 1) were headache (91%), cough (72%) and muscle pain (60%), with median symptom duration 4.5 days (range 1–6 days) Only 30% reported fever. Half of cases had sought medical attention: 14 (30%) had attended their GP and 10 (21%) had attended an ED. The proportion of cases seeking medical attention was the same before and after the first LD case emerged (P=n.s.). Significantly more cases sought medical attention compared to non-cases (24/47 vs. 30/153, χ2=18·1, P<0·01). There was no significant difference in the proportions of smokers represented in cases before and after the date of informing employees of the situation (3 July). Twenty-two cases took sick leave; median duration 3 days (range 1–6 days).
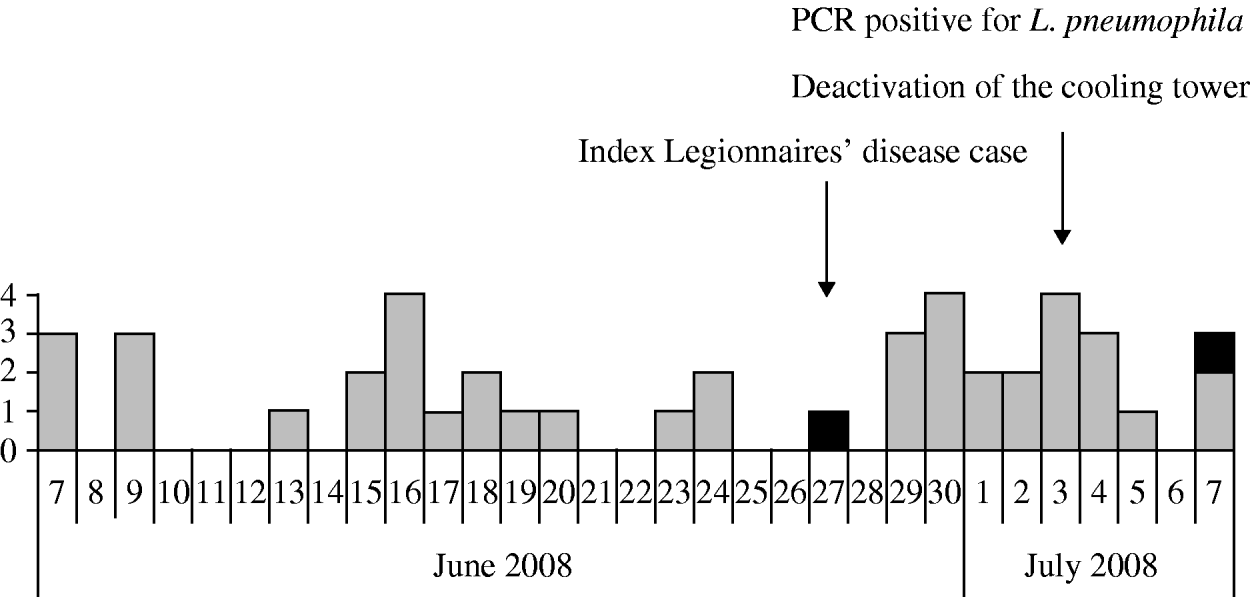
Fig. 1. Date of onset of illness, 2008. ▪, Legionnaires' disease; ![]() , Pontiac-like illness.
, Pontiac-like illness.
Table 1. Symptoms reported by Pontiac-like illness cases (n=47), Dublin, 1 June–7 July 2008
Occupational exposures
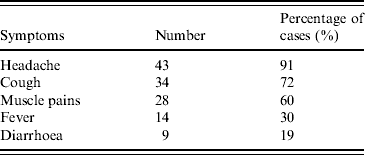
On univariate analysis, attending the basement smoking area was the only exposure associated with being a case (RR 2·6, 95% CI 1·6–4·2). Being a smoker was borderline associated with increased risk of being a case (RR 1·6, 95% CI 1·0–2·7) (Table 2). No significant association was found with being exposed to air-conditioning, having an underlying medical condition, the office floor where employees worked, using the work shower, or visiting the car park.
Table 2. Risk ratio associated with various occupational exposures, Dublin, 1 June–7 July 2008
RR, Risk ratio; CI, confidence interval.
A stratified analysis by smoking status was undertaken as employees were likely to have been exposed to varying risk according to their smoking habits. In smokers, exposure to the basement smoking area was the only occupational location associated with increased risk (12/26 smokers exposed were cases, 1/13 not exposed was a case; RR 6·0, 95% CI 0·9–41·3). There was a significant dose–response effect in smokers who attended the smoking area more than twice per day (9/14 attending more than twice per day were cases; 4/25 who attended less frequently were cases; RR 4·0, 95% CI 1·3–4·2). Based on calculation of the attributable fraction, 83% of cases in smokers could be attributed to using the smoking area.
Controlling for confounding, using a multivariable model the only association of risk that remained significant was exposure to the basement smoking area (OR 4·3, 95% CI 1·8–10·5) (Table 3).
Table 3. Multivariate analysis, adjusted odds ratios, Dublin, 1 June–7 July 2008
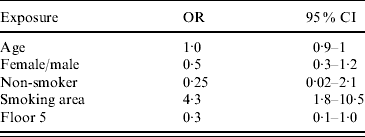
OR, Odds ratio; CI, confidence interval.
Microbiological results
Of the 15 cases (32%) who attended for Legionella serology, none showed serological evidence of Legionella infection on IgG antibody testing. Three workers were detected as having non-negative results; the level of seroconversion was 1:16. Eleven of these cases tested had a negative UAT. A further 15 cases had a negative UAT at the time of illness but did not attend for serology. Ten cases had a normal chest X-ray at the time of initial illness.
A further 23 people who did not meet our case definition but who attended an ED had negative chest X-rays and UATs and negative serology for L. pneumophila.
Environmental results
Samples from both cooling towers were positive on PCR testing for L. pneumophila serogroup (sg)1 at 73 200 000 GU/l in cooling tower 1 and 174 000 GU/l in cooling tower 2. L. pneumophila was cultured at a low level of 100 c.f.u./l in cooling tower 2 and was confirmed as L. pneumophila sg1, monoclonal antibody subgroup ‘Knoxville’. It should be noted that the culture medium showed evidence of competing Pseudomonas spp. that may have inhibited the growth of L. pneumophila.
Although Legionella spp. were cultured in the hot- and cold-water systems further testing was negative for L. pneumophila. The cooling towers were immediately decommissioned, cleaned and disinfected as recommended, at the start of this outbreak [2]. The cooling towers have now been permanently deactivated and replaced by a dry cooling system.
DISCUSSION
We describe a large outbreak of Pontiac-like illness in employees preceding and interspersing a cluster of LD in a workplace. Clinical, epidemiological and environmental evidence suggest that Legionella spp. was the causative agent of the illness seen in these employees. Employees had a non-pneumonic illness with headache, myalgia, cough and fever during the summer time with a short recovery period. Epidemiologically, we demonstrated that the illness was significantly associated with the basement smoking area situated beside the cooling towers where L. pneumophilia sg1 was isolated. Cooling towers have previously been linked to PF outbreaks [Reference Friedman7–Reference Kaufmann11].
The clinical symptoms described in published PF outbreaks have been heterogeneous [Reference Tossa12] making the establishment of a single case definition difficult. Most reports define PF cases clinically [Reference Friedman7, Reference Tossa12–Reference Jones19]. Our clinical case definition required two major symptoms and was more stringent than other published operational definitions [Reference Tossa12]. Headache and cough were reported by the majority of cases with only a third reporting fever.
Laboratory confirmation of the diagnosis of PF is challenging. Seroconversion rates in previous PF outbreaks have been inconsistent [Reference Burnsed14] and sensitivities of serological assays vary considerably both for the diagnoses of LD and PF [Reference Diederen1]. The negative predictive value of IgG testing is estimated at a low rate of 45·5% [Reference Burnsed14] making it difficult to exclude PF when negative. The level of seroconversion suggested to serologically confirm a PF case differs from 1:128 (US case definition) [20] to 1:64 in an outbreak setting (used by HPA) [Reference Gotz17, 21].
Timing of sampling is also an issue. Seroconversion can take from 3 weeks to up to 6–12 weeks after onset of symptoms [Reference Fields, Benson and Besser16, Reference Monforte22] although most seroconversion occurs within 3 weeks [Reference Diederen1]. The reference laboratory advised testing of one sample >3 weeks after potential exposure.
In our study, none of those tested had a fourfold rise in polyclonal titre to ⩾1:128 in the convalescent phase. Similar negative serology results have previously been reported in outbreaks [Reference Benin13, Reference Huhn18]. It is possible, as reported elsewhere [Reference Benin13], that the intermittent short exposures of our cases to the basement area cooling towers may have mitigated against a strong serological response.
PF is attributed to exposure to Legionella spp., but whether the disease is due to dead or live microorganisms, a combination of both, or exposure to their products (endotoxins) remains unknown [Reference Diederen1]. For these reasons, Legionella spp. may not be cultured from the environment despite the emergence of PF cases [Reference Hussong23, Reference Miller24]. Live culture analysis has been the gold standard to detect Legionella spp. in the environment; however, PCR is a rapid quantitative test assisting in timely investigation and control of an outbreak setting. PCR use in Legionella outbreaks is relatively new; therefore guidelines on acceptable levels do not exist. For the same reason there is no database of results from other outbreak investigations from which comparisons may be drawn. Initial PCR results from both cooling towers suggested that high levels of L. pneumophila sg1 were present, particularly in cooling tower 1. The towers were immediately shut down and drained of remaining water. Culture analysis results later unexpectedly detected only low levels of L. pneumophila in cooling tower 2, requiring lesser action according to guidelines with a review of control procedures. The presence of competing organisms may have inhibited growth on the culture plate. However, discrepancies between culture and PCR tests are well documented, especially during summer time [Reference Yaradou25]. Thus, standard methods with additional tests like PCR should be considered in legionellosis investigation.
Differential causes considered for this outbreak of illness were bacteria such as Legionella spp., Mycoplasma pneumoniae, Chlamydia spp., or viruses such as influenza, adenovirus, parainfluenza virus, or picornavirus. Sentinel systems in Ireland reported no influenza/parainfluenza in circulation. Of all differential diagnoses, Legionella spp. and M. pneumoniae are associated with infections in summer [Reference Bartlett26]. However, chest X-rays were normal in ten cases with Pontiac-like illness, ruling out a pneumonic illness. The positive Legionella UAT in both of the LD cases focused attention on legionellosis as the most likely cause of this outbreak.
With a response rate of 50%, we ruled out bias in recruitment by establishing that the characteristics of the cohort did not differ significantly from all employees in the building in terms of sex, age and smoking status. A considerable proportion of workers were on holiday in July, explaining to some extent the response rate and the suboptimal attendance for serology sampling. A possible bias of overrepresentation of smokers in cases due to anxiety was also tested for and excluded.
The dearth of published reports on PF and absence in Irish literature is remarkable given the ubiquity of Legionella spp. in the environment. Lack of specific clinical features, benignity of illness, and absence of reliable microbiological testing may lead to under-diagnosing by the medical community. In this outbreak, advance investigation of ILI in employees in June may have identified the cooling towers as a potential risk leading to earlier intervention. Clinicians should consider the differential diagnosis of PF for those with ILI especially out of the influenza season, taking into account possible exposures to Legionella spp. Early detection is imperative, leading to prevention of potentially severe illness and outbreaks of PF or LD. Challenges in diagnosing PF remain, and further studies are needed to link the occurrence of mild ILI symptoms and exposure to Legionella spp.
ACKNOWLEDGEMENTS
We thank Alicia Barrasa Blanco for her critical feedback. The Epiet fellowship of N. Nicolay was funded by the European Commission DG SANCO. We gratefully acknowledge the advice of Dr Tim Harrison, Respiratory and Systemic Infection Laboratory HPA Centre for Infection, Colindale, UK.
DECLARATION OF INTEREST
None.






