The Centers for Disease Control and Prevention (CDC) estimates that each year there are ∼462,000 episodes of Clostridioides difficile infection (CDI) occur in the United States each year, about half of which occur in patients presenting to or being cared for in hospitals. Reference Guh, Mu and Winston1 Estimates of the hospital-based CDI disease burden are commonly based on claims data or public health surveillance of laboratory-confirmed infections. Regardless of the methodology used, the estimation depends on several steps in the CDI laboratory testing pathway including preanalytic steps and analytic steps (eg, sensitivity of the test). Reference Alcalá, Martin and Marin2 Globally these estimates vary greatly; in general, higher incidences are observed in US-based studies, likely due to relatively higher use of more sensitive tests. Reference Balsells, Filipescu, Kyaw, Wiuff, Campbell and Nair3 Previous studies have focused on variations in sensitivity of specific diagnostic tests to explain differences in some CDI burden estimates, Reference Davies, Longshaw and Davis4–Reference Bauer, Notermans and Benthem7 but few studies have evaluated the impact of test order frequency on differences in estimates of CDI incidence. Reference Kamboj, Brite and Aslam8,Reference Alcalá, Reigadas and Marín9
The CDC Emerging Infections Program (EIP) conducts active population- and laboratory-based surveillance of all incident CDI in 10 geographically diverse parts the United States. Reference Guh, Mu and Winston1 The EIP evaluates all positive tests for C. difficile to identify CDI cases. Events leading to ordering each test (eg, decision to test, testing methods) depend on the individual standard-of-care practice in place at each clinic, hospital, or referral laboratory. Variations in such practices may influence estimated CDI incidences between surveillance sites. Reference Curren, Lutgring and Kabbani10 Some differences in the population-based CDI incidences between sites were noted; however, reasons for these differences have not been fully explored. Reference Lessa, Mu and Bamberg11 We sought to determine whether differences in testing practice depend on patient characteristics and whether any differences in observed incidence of CDI through established surveillance methods reflect differences observed in adjusted testing rates.
Methods
Hospital selection
This observational cross-sectional study was conducted at 5 acute-care hospitals located in 2 EIP surveillance sites. Participants included 3 hospitals in Atlanta, Georgia, from a single healthcare system (hospitals 1–3 at site A), and 2 hospitals in Rochester, New York (hospitals 1 and 2 at site B). Nursing staff at each of the hospitals were oriented to the period-prevalence assessment process by study staff, and they identified efficient methods for reporting all diarrheal episodes (≥3 stools in 24 hours) during the study period. The study protocol was approved by the appropriate institutional review board at each study site. During the study period, each of the participating hospitals utilized NAAT either as a primary C. difficile test or as a secondary reflex test for a negative toxin enzyme immunoassay (EIA) with a positive glutamate dehydrogenase test. CDI testing practices were performed according to local protocols and practice, and each participating hospital had active diagnostic stewardship efforts in place to minimize unnecessary testing of stool specimens for C. difficile. The programs were similar, with an exception: 3 hospitals at site A included an educational effort to nursing staff to identify and suggest CDI testing to providers as an effort to test patients experiencing diarrhea upon of admission (Supplementary Table S1 online).
Period-prevalence assessment
All inpatient adult patient-care units were eligible for participation and were approached for recruitment into the assessment, with an opt-out option if nursing staff shortages interfered with completing the activities. Patient-care units were defined by pre-existing mapping conventions for reporting to the CDC National Healthcare Safety Network (NHSN) to “location types.” We identified 2 distinct 10-day periods in the fall of 2020 and spring of 2021 (ie, 10 consecutive weekdays over a 14-day period) separated by at least 5 months at each facility to perform the prevalence assessment (Supplementary Table S1 online).
Study staff reviewed study processes with nursing staff prior to each assessment. On each assessment day, nursing staff recorded the bed number for each patient observed with diarrhea and stool sample order or collection status. Diarrhea was defined as having at least 3 unformed stools in the prior 24 hours, although no validation or confirmation of frequency and consistency was performed via chart review. Weekend diarrheal episodes were reported to Monday nursing staff to determine whether diarrhea frequency over the previous 24 hours was sufficient for reporting. For each diarrheal episode, limited characteristics of the patient and C. difficile testing results were captured from the electronic medical record. Characteristics included exposures potentially causing diarrhea such as laxatives, chemotherapy, and tube feedings through a nasogastric or percutaneous tube in the 48 hours before first day of diarrhea. Reference Mawer, Byrne and Drake12–Reference Polage, Solnick and Cohen14 Previous C. difficile testing history and positive severe acute respiratory coronavirus virus 2 (SARS-CoV-2) tests were limited to 14 days before the first day of diarrhea. For this analysis, we considered any positive test for C. difficile as a CDI-positive stool, which reflects the established surveillance methods of the EIP population-based estimates.
Definitions and analytic approach
Diarrheal episodes were defined as the cumulative days a patient had diarrhea reported by nursing staff. Diarrheal episodes were classified as “pre-existing” if present prior to the first day of the period assessment or “new” if not present prior to the first day of the assessment. Episodes in patients with any CDI test ordered (regardless of result) in the previous 14 days were also considered pre-existing. New diarrheal episodes were classified as hospital onset if the first day of diarrhea occurred on or after the third day of hospitalization. To evaluate the influence of patient mix on testing practices, we aggregated data by NHSN patient-care type in each hospital. Hospital-based rates (per 1,000 patient days), stratified by site and patient-care location type (wards, oncology, and critical care) were calculated for new diarrheal episodes, hospital-onset episodes, and CDI positivity. Denominators were derived from the number of occupied beds per location per day of the observed study, without excluding any patient days among already CDI-positive patients. The percentage of episodes with CDI test ordered and the percentage of CDI tests positive for CDI, stratified by site and patient care location, were also calculated.
Statistical analysis
Demographic and clinical characteristics of patients with new diarrheal episodes were characterized by study site, and differences in distribution were examined using the χ Reference Alcalá, Martin and Marin2 for categorical variables and the Kruskal-Wallis rank-sum test for continuous variables. Median NHSN unit-specific incidence across study sites, patient-care location types, and participating hospitals were calculated and compared using Kruskal-Wallis tests. Bivariate Poisson regression models with robust error variances were used to examine the unadjusted associations between the clinical and demographic characteristics. Forward selection was used to identify significant correlates of CDI ordering and positivity from among the patient demographic and clinical characteristics.
Among the 860 participants included in the analytic sample, 8.4% were missing race, 2.9% were missing age, and 1.7% were missing jurisdiction of residence. Missing data are a potential source of bias. Reference Cummings15 Therefore, multiple imputations using chained equations were used to create 30 sets of the data set with imputed values for the missing data and to estimate pooled risk ratios. Reference Martin and Austin16
Simulation of population-based estimates
We hypothesized that the magnitude of the difference in testing frequency between the study sites after adjusting for factors influencing likelihood to test (eg, diagnostic stewardship) would be reflected in any observed differences in EIP-derived estimates of CDI incidence. Because the participating hospitals represented only 28% of general acute-care hospitals in the relevant counties (22% of Dekalb–Futon and 50% of Monroe), we did not extrapolate testing practices countywide to avoid biased results. Therefore, we tested the hypothesis simulating EIP case finding using data from the 5 hospitals. We extracted positive C. difficile tests and admission data for a 12-month period that included both of our study periods. The data were limited to only patients residing in the surveillance catchment area of the respective EIP site and were stratified by age, race, and sex, similar to EIP. Reference Guh, Mu and Winston1 We further limited cases to samples collected during the hospital admission. The differences in age, race, and sex-specific CDI incidence were calculated between strata and were summarized using the Cochran–Mantel–Haenszel test. Reference Martin and Austin16
Results
Among the 5 participating hospitals, 107 (96%) of the 112 patient-care units participated in at least one 10-day observation period: 71 (93.4%) of 76 at site A and 36 (94.7%) of 38 at site B. These 107 patient-care units accounted for 2,397 beds and 38,365 patient days and mapped to 26 unique NHSN-defined location types. Patient-care units were most commonly mapped to non–critical-care units: medical (n = 19), surgical (n = 12), oncology specialty (n = 12), medical-surgical combined (n = 11). Among critical care units, cardiothoracic (n = 7), medical (n = 6), coronary (n = 5), medical-surgical (n = 4), and neurosurgical (n = 4) were most common.
In total, 1,588 diarrheal episodes were identified, of which 860 (435 at site A and 425 at site B) were new diarrheal episodes (Supplementary Table S1 online). When comparing patient characteristics, patients in site A, compared to site B, were more likely to be Black (56.3% vs 20.2%), more likely to be aged <50 years (24.6% vs 17.6%), less likely to have received laxatives (33.3% vs 64.5%), more likely to have received chemotherapy (7.4% vs 1.9%), and more likely to have been discharged home (64.5% vs 31.0%). Of the patients with new diarrheal episodes, 62.4% were residents of the EIP surveillance areas (65.0% in site A and 59.8% in site B) (Table 1).
Table 1. Characteristics of Study Participants Patients With New Diarrheal Episodes: Period-Prevalence Survey

Note. IQR, interquartile range; EIP, Emerging Infections Program.
a Pearson χ2 test.
b Kruskal-Wallis rank-sum test.
c Laxatives included docusate sodium, sennosides, polyethylene glycol 3350, biscodyl, fiber, lactulose, and/or psyllium.
d Long-term care included discharge to long-term care facilities, inpatient rehabilitation facility, or skilled nursing facility.
The hospital-based incidence of new diarrheal episodes was 22.4 per 1,000 patient days (28.9 at site A vs 18.4 at site B); 61.5% of episodes (57.9% in site A and 65.2% in site B) were classified as hospital onset (Table 2A). The proportion of new diarrhea episodes tested for CDI was 35.1%, and we detected a large difference in the proportion tested for CDI between the sites: 20.9% at site A versus 49.0% at site B. However, within each site, the proportions of episodes tested were similar between hospitals. The proportions of CDI tests that were positive (16.6%) for CDI were similar at both sites: 17.4% at site A and 14.6% at site B.
Table 2a. Overall Average Rates of Diarrhea Episodes (DE), Clostridiodes difficile Infection (CDI) Test Ordering and Positivity Per 1,000 Patients Days: Fall 2020 and Spring 2021 Period-Prevalence Survey
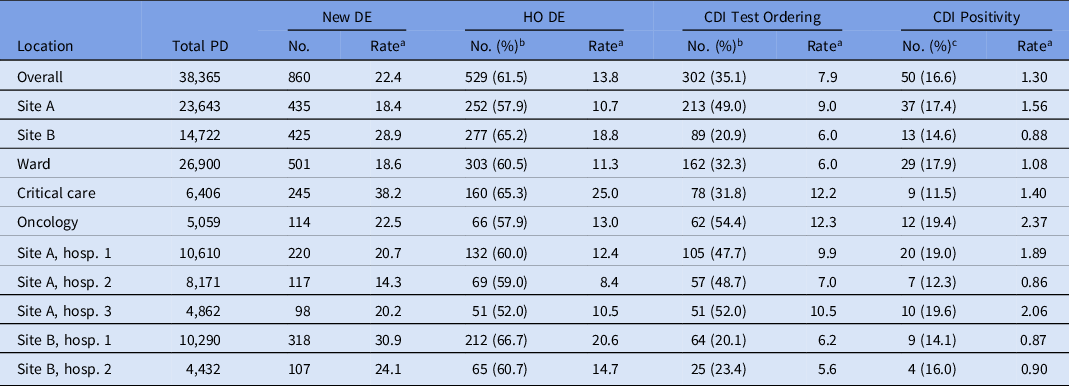
Note. PD, patient days; DE, diarrheal episodes; HO, hospital-onset; CDI, Clostridiodes difficile infection.
a All rates are per 1,000 patient days.
b Percentage of new DE cases.
c Percentage of CDI tests ordered.
Table 2b. Patient Care Locations and Rates (per 1,000 PD) of New Diarrheal Episodes (DE) and Clostridiodes difficile Infection (CDI) Test Ordering Among Hospitalized Patients, By Site, Patient Care Location, and Hospital, Fall 2020 and Spring 2021 Period Prevalence Survey

Note. PD, patient days; NHSN, National Healthcare Safety Network; IQR, interquartile range.
a All rates are per 1,000 patient days.
b Kruskal-Wallis test; Reference Alcalá, Martin and Marin2
c Percentage of new DE cases; Reference Guh, Mu and Winston1
d Fisher exact test.
Hospital-based incidence (per 1,000 patient days) of new diarrheal episodes differed most dramatically between patient location types. It was highest among critical-care locations (33.1 per 1,000 patient days), then oncology (20.1 per 1,000 patient days), followed by other locations (16.0 per 1,000 patient days) (Table 2B and Fig. 1). The proportions of new diarrhea episodes tested for CDI also differed by patient care location, with highest rates among oncology locations (54.4%).
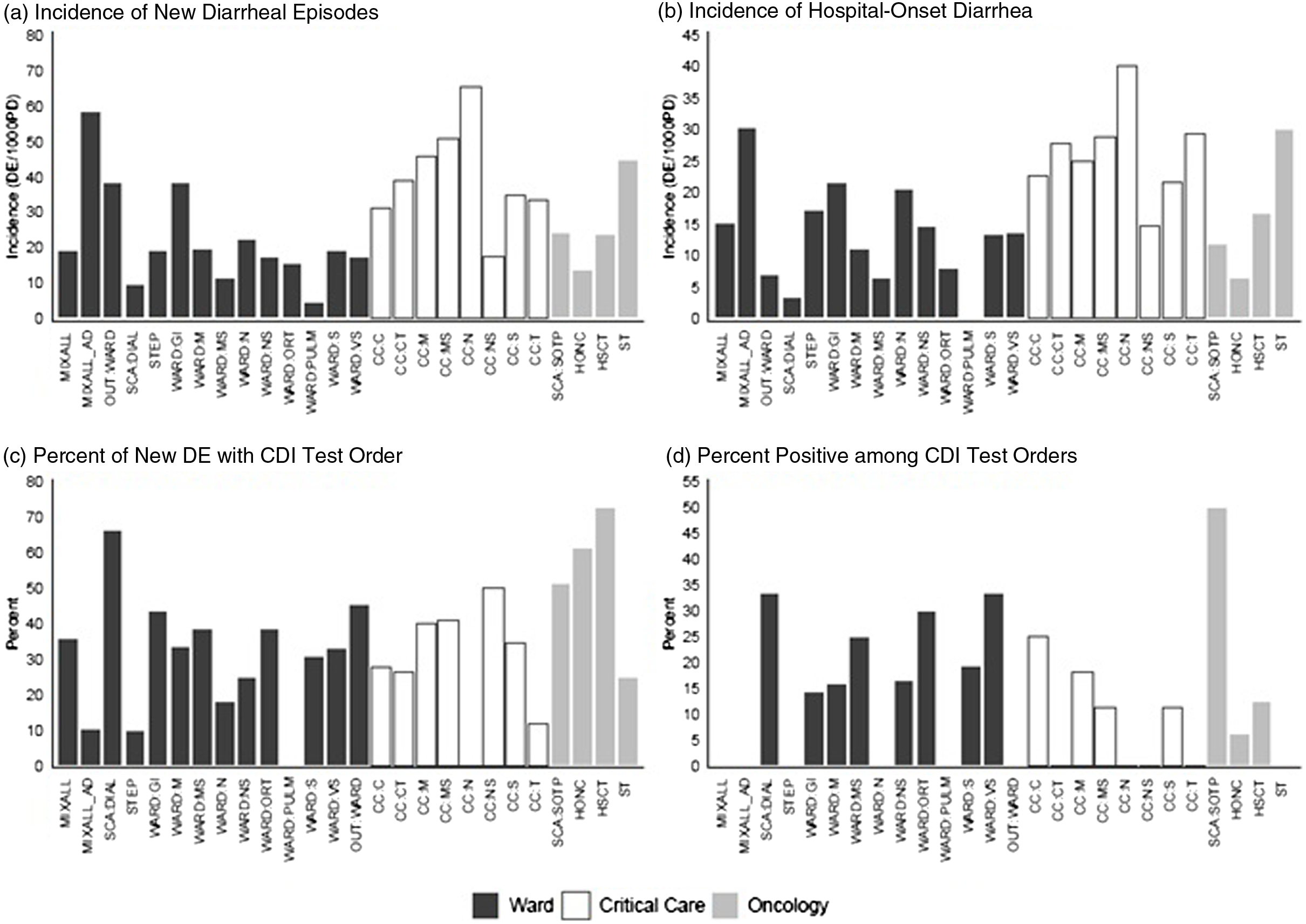
Fig. 1. Incidence (per 1,000 patient days) of new diarrheal episodes (N = 860) and hospital-onset diarrhea, (N = 529), percentage of new diarrheal episodes with CDI test order (N = 302), and percentage of CDI test orders that tested positive (N = 50), among hospitalized patients, Fall 2020 and Spring 2021 period-prevalence survey. Note. CDI, Clostridioides difficile infection.
In univariate analysis, several factors were predictive of testing and were considered in the multivariable analysis (Table 3). In multivariable analysis, independent predictors of testing included care in an oncology location (adjusted RR, 1.57; 95% CI, 1.25–1.98), receiving laxatives (adjusted RR, 0.59; 95% CI, 0.48–0.74), and residing outside the surveillance catchment area (adjusted RR, 0.77; 95% CI, 0.62–0.94). Adjusting for these factors, patients with a diarrhea episode in site B were 49% less likely to receive a test than patients with diarrhea in site A (adjusted RR, 0.51; 95% CI, 0.40–0.63).
Table 3. Predictors of Clostridiodes difficile (CDI) Test Ordering: Fall 2020 and Spring 2021 Period-Prevalence Survey

Note. RR, relative risk; CI, confidence interval.
Using testing results from these 5 hospitals among hospitalized residents of the catchment area to calculate age, race, and sex-specific incidence, both per 1,000 patient days and per 100 admissions, we detected lower hospitalized CDI rates in site B than site A for most strata (Fig. 3). Adjusting for age, race, and sex, the summary rate ratio of the hospitalized CDI rates at site B compared to site A was 0.62 (95% CI, 0.54–0.71), translating to ∼38% lower hospitalized CDI rate among residents of the EIP surveillance area identified from site B compared to site A.

Fig. 2. Period-prevalence survey (2020–2021). (A) Relative rate (and 95% confidence intervals) of selected characteristics for case with new diarrheal episode (N = 860) being CDI tested (N = 302). (B) For CDI test being positive (N = 50) among study participants. Note. CDI, Clostridioides difficile infection.

Fig. 3. Crude and adjusted rate ratios of Monroe County residents compared to Atlanta area residents’ C. difficile incidence estimates calculated using retrospective simulation of Emerging Infection Program methods applied to inpatients at 5 hospitals, October 2020–September 2021. *Race data categorized as other race are not displayed but are included in summary rate ratio. Rate ratios compare rate (CDI positive diarrheal episodes among hospitalized residents per 100 admissions) in each age, race, and sex-specific stratum are crude calculations based on Taylor series. The “All” summary is a Mantel-Hanzel summary rate ratio adjusting for each stratum using OpenEpi version 3 software. Note. CDI, Clostridioides difficile infection.
Discussion
Differences in testing frequency of diarrhea episodes for C. difficile among inpatients at acute-care facilities between 2 geographically distant locations persisted despite adjusting for patient factors that often influence decisions to test. The frequencies of testing inpatients with diarrhea were similar at the hospitals in each site, but they were an estimated 49% less frequent at the Rochester site compared to the Atlanta site, regardless of specific hospital. This observed difference was similar to the 38% lower CDI incidence estimated at the Rochester site compared to the Atlanta site using adjustments as done in the CDC EIP surveillance programs. These differences were essentially identical (within the 95% confidence intervals of each other), suggesting that testing practice may explain the difference in measured incidence observed through population-based surveillance for CDI.
Relying on C. difficile tests with high sensitivity, such as NAAT, might affect population rates CDI rates estimates, Reference Kamboj, Brite and Aslam8,Reference Alcalá, Reigadas and Marín9,Reference Rock, Pana and Leekha17,Reference Gould, Edwards and Cohen18 often leading to higher than true infection incidence because NAAT has lower specificity. Reference Koo, Van and Zhao5,Reference Goodenough, Sefton and Overton6,Reference Gould, Edwards and Cohen18,Reference Buckel, Avdic, Carroll, Gunaseelan, Hadhazy and Cosgrove19 However, our data suggest that differences in the intensity of testing patients, potentially driven by diagnostic stewardship, but definitely driven by differences in patient characteristics (eg, laxative use, oncology status), may also need to be considered when comparing incidence between regions. First, differences in C. difficile testing rates were profound even between the 2 sites, and they likely vary tremendously across diverse geographic areas in the United States and the world. Second, these differences in testing persisted after adjustment for differences in patient mix and potential alternative etiologies for diarrhea among inpatients. These differences may reflect penetration of messaging or programs to promote diagnostic stewardship within a healthcare system or region, or a more general culture of practice within a region. The reason for the differences is purely speculative; we did not systematically study them here.
Our study had several limitations. First, the testing practices between hospitals within each site may only vary minimally because of shared policies between facilities within each site. Also, the number of facilities participating in the study per site was small, reflecting a small proportion of all acute-care hospitals within the counties participating in population-based surveillance efforts for the EIP. This small sample size forced us to use an estimation method of CDI incidence limited to the 5 study hospitals. This study was conducted during the coronavirus disease 2019 (COVID-19) pandemic, which may have influenced testing practices in poorly understood ways. However, periods of study occurred during moderate nadirs in the COVID-19 pandemic, and we considered COVID-19 in the predictive model. Finally, excluding weekends likely introduced some inaccuracies in estimate values, but differences between sites should have been preserved because this method was applied at all sites.
Despite these limitations, our data support findings of other studies that identified positive associations between hospital-wide testing per admission and hospital-onset CDI rates. Reference Balsells, Shi and Leese20–Reference Kamboj, Brite and Aslam23 However, our study approached the study question from the patient perspective, identifying and adjusting for other etiologies of diarrhea. An added strength of this study is estimating diarrheal incidence and testing frequency and rates at the patient-location level. This method allowed for a conservative assessment of the significance of observed differences between patient-location types, using each NHSN-mapped location type as an observation in the analysis. This method also allows for location-specific estimates of testing frequency across many location types, which should be useful for modeling estimates of disease in different surveillance or clinical scenarios.
In summary, we quantified the degree to which frequency of testing differs between patient-location types of acute-care hospitals and which patient factors may drive the likelihood to order such tests. Moreover, differences in testing between surveillance sites persisted even after adjusting for patient factors. This difference was nearly identical to the difference in estimated CDI incidence between the 2 surveillance sites. Comparisons of the estimated incidence of CDI between regions may require some insight into differences in testing practice to best interpret such data.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.205
Acknowledgments
We thank the hospital staff who participated in each phase of this point-prevalence survey effort, as well as the on-site study staff of The Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Emory University, Decatur, GA (Nadine Rouphael, MD, Laurel Bristow, MSc, Kieffer Hellmeister, Jamila Pitts, Mai Kio, Lauren Nolan, PA-C, Ariel Kay, MPH, Vanessa Buster, RN, Cecilia Zhang, Hollie Macenczak, RN, Amer Bechnak, MD, Julie Quach, APRN, FNP-C, Youssef Saklawi, MD, Nina McNair, Eddie Monarrez, MPH, Carly Johnson, MPH, Stephanie Ramer, MPH, Evan Gutter, MPH, Lana Khalil, MD, Joy Winters, Ghina Alaaeddine, MD). We also thank staff from University of Rochester Medical Center (Shayne Hawkins RN, MS, Kate Valcin RN, MS, and Mary Carey PhD, RN, FAHA, FAAN) and staff from Highland Hospital (Joslyn Soule DNP, MS, RN).
Financial support
Study activities were supported through a Service Agreement between Pfizer Inc. and each Emory University and the University of Rochester.
Conflicts of intertest
S.K.F. reports that Emory University has received a services agreement from Pfizer for public health research on C. difficile starting in 2019 for 2 years. G.D. reports that the University of Rochester has received a services agreement from Pfizer for public health research on C. difficile starting in 2019 for 2 years.










