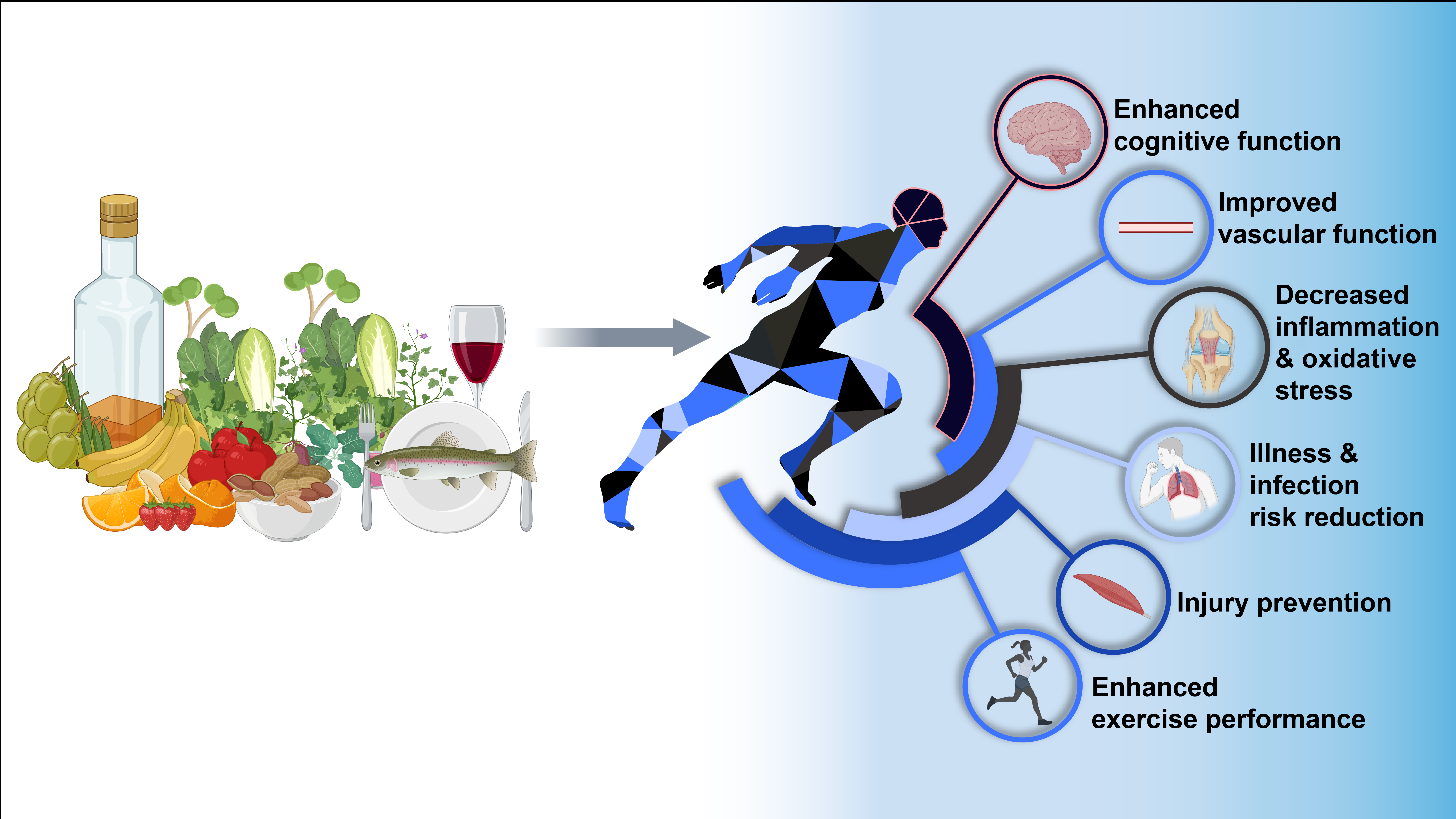
The Mediterranean dietary pattern (MedDiet) reflects the traditional eating habits common among certain rural communities in the Mediterranean Basin prior to the globalisation and westernisation of food systems(Reference Bach-Faig, Berry and Lairon1,Reference Shannon, Ashor and Scialo2) . Salient characteristics of this diet include the high intake of fruits, vegetables, cereals, olive oil, legumes and tree nuts, moderate intake of fish and other seafood, and low intake of sugar-sweetened foods, carbonated beverages, and red and processed meat. In addition, the traditional MedDiet includes a small-to-moderate intake of alcohol, which is typically ingested alongside meals. The potential health-promoting effects of this dietary pattern were first recognised in the 1950s as part of the Seven Countries Study, which was led by the celebrated public health scientist Ancel Keys(Reference Keys, Menotti and Karvonen3). Keys and colleagues identified dietary practices of individuals living in the Mediterranean area, including the use of olive oil as the principal fat, which were associated with a lower risk of CVD(Reference Keys, Menotti and Karvonen3). A number of subsequent epidemiological studies have confirmed the protective effects of a MedDiet against CVD(Reference Martínez-González, Gea and Ruiz-Canela4) and have also identified a potential role of this dietary pattern in decreasing risk of metabolic (e.g. type II diabetes and the metabolic syndrome)(Reference Assaf-Balut, García de la Torre and Duran5–Reference Alvarez León, Henríquez and Serra-Majem7) and neurodegenerative (e.g. Alzheimer’s)(Reference Scarmeas, Stern and Tang8,Reference Scarmeas, Anastasiou and Yannakoulia9) diseases, alongside certain forms of cancer(Reference Schwingshackl, Schwedhelm and Galbete10). The beneficial effects of a MedDiet have also, more recently, been demonstrated in randomised controlled trials, including the large-scale Prevención con Dieta Mediterránea (PREDIMED) trial(Reference Estruch, Ros and Salas-Salvadó11,Reference Estruch, Ros and Salas-Salvadó12) . PREDIMED demonstrated beneficial effects of a MedDiet supplemented with additional nuts or olive oil in the primary prevention of CVD(Reference Estruch, Ros and Salas-Salvadó11,Reference Estruch, Ros and Salas-Salvadó12) , type II diabetes(Reference Salas-Salvadó, Bulló and Babio13) and breast cancer(Reference Toledo, Salas-Salvadó and Donat-Vargas14), amongst others. Scientific interest in the MedDiet continues to grow, with researchers continually seeking new applications for this healthy dietary pattern.
Appropriate nutrition plays a key role in training for, and competing in, competitive sport, and is essential for reducing risk of injury and illness, recovering and adapting between bouts of activity, and enhancing performance(Reference Tipton15,Reference Thomas, Erdman and Burke16) . Nutritional demands for competitive athletes often exceed those of their sedentary counterparts due to the prolonged amounts of time spent at an elevated metabolic rate(Reference Jeukendrup and Gleeson17,Reference Rehrer, Hellemans and Rolleston18) . Nevertheless, guidelines for healthy nutrition are likely to be similar for members of the public and athletes under most circumstances, with some potential exceptions where recommendations may diverge (for further details, see Optimising a MedDiet for Competitive athletes)(Reference Thomas, Erdman and Burke16). Therefore, healthy dietary patterns such as the MedDiet, which are advocated for improving health in the general population(Reference Bach-Faig, Berry and Lairon1), may also play a role in the maintenance of optimal health and performance in athletic cohorts.
The applications of a MedDiet in a sporting context have received very little attention to date. To the authors knowledge, only one study has directly explored the potential performance enhancing effects of this dietary pattern(Reference Baker, DeCesare and Johnson19). Specifically, in a randomised cross-over study by Baker and colleagues(Reference Baker, DeCesare and Johnson19), eleven recreationally active participants consumed either a MedDiet or Western diet for 4 d, after which they completed a battery of exercise performance tests including a 5-km treadmill time trial, a Wingate cycle test, a vertical jump test and an assessment of grip strength via hand-grip dynamometry. Remarkably, 5-km time trial performance was about 6 % faster when participants consumed MedDiet compared with the Western diet, although other performance measures were no different between conditions. These findings require substantiation but tentatively suggest a potential role of the MedDiet for improving (at least certain aspects of) sports performance. In a similar manner, numerous foods or compounds found in a MedDiet have also shown direct ergogenic effects, including nitrate-rich vegetables and vegetable juices(Reference Lansley, Winyard and Bailey20–Reference Shannon, Easton and Shepherd23), n-3 fatty acids readily available in fish amongst other foods(Reference Lewis, Radonic and Wolever24), a variety of different fruits(Reference Cook, Myers and Blacker25,Reference Keane, Bailey and Vanhatalo26) , and certain types of nuts(Reference Yi, Fu and Zhou27).
Building upon these preliminary findings, in the current article, we present the hypothesis that following the key principles of a MedDiet could represent a useful framework for good nutrition in athletes under most circumstances with the potential to elicit physiological changes which positively impact athlete health and exercise performance. We also highlight potential modifications which could be made to this dietary pattern to comply with contemporary sports nutrition practices and identify potential directions for future investigation.
The Meditterranean diet and traditional sports nutrition guidelines
Current nutritional guidelines for athletes are primarily structured around macronutrient consumption(Reference Thomas, Erdman and Burke16). In particular, the quantity and timing of carbohydrate, protein and fat consumption are manipulated to optimise substrate availability and training adaptations(Reference Thomas, Erdman and Burke16).
Sport-specific nutritional recommendations typically state that endurance sports (e.g. prolonged cycling, running, etc) are best supported by diets high in carbohydrate (e.g. 6–12 g·kg·d–1)(Reference Thomas, Erdman and Burke16,Reference Carr, McGawley and Govus28) , especially in the days leading up to an endurance event(Reference Burke, Hawley and Wong29). Carbohydrate recommendations for endurance athletes, therefore, typically exceed those advocated for the general population(Reference Burke, Hawley and Wong29), although direct comparison is complicated by the fact that carbohydrate recommendations for the general population are usually expressed as a percentage of total energy requirement (i.e. 50 % total energy intake(30)) compared with sports-specific recommendations which are usually expressed in grams (per kilogram of body mass per d (g·kg·d–1))(Reference Thomas, Erdman and Burke16). Recommended protein intakes for the general population are 0·75 g·kg·d–1 (UK Reference Nutrient Intake (RNI))(31). By contrast, current recommendations for dietary protein intake in athletes range from 1·2 to 2·0 g·kg·d–1, with values at the upper end of this range particularly relevant for strength-based athletes, individuals attempting to maintain lean mass during periods of energy deficit or those undergoing high-frequency/intensity training(Reference Thomas, Erdman and Burke16,Reference Phillips and Van Loon32) . Finally, dietary fat intake requirements for athletes have received little attention in the scientific literature, with recommendations typically focusing on carbohydrate and protein requirements(Reference Broad and Cox33). However, based around current guidelines, fat intake recommendations for athletes largely align with those of the general population (i.e. 20–35 % of total energy intake)(Reference Thomas, Erdman and Burke16,31) .
The MedDiet has been reported to provide approximately 43 % energy from carbohydrate, 37 % energy from fat and 15 % from protein(Reference Davis, Bryan and Hodgson34). However, regional variations in the macronutrient distribution of this dietary pattern have been reported, and relative carbohydrate intakes as high as about 59 % of energy intake have been documented in Egypt(Reference Karamanos, Thanopoulou and Angelico35). When expressed relative to body mass, for a hypothetical 70-kg endurance athlete with a daily energy intake of 14,644 kJ, 43 % and 59 % energy from carbohydrate would translate into about 5·4 and about 7·4 g·kg·d–1 carbohydrate. This suggests that carbohydrate intake in the traditional MedDiet may be sub-optimal for certain athlete groups (e.g. 6–12 g·kg·d–1 in endurance athletes)(Reference Thomas, Erdman and Burke16,Reference Carr, McGawley and Govus28) , although certain permutations of this dietary pattern may more closely align with recommendations for athletes.
Protein intake in an athletic population adhering to a MedDiet has been demonstrated to be as high as about 1·5 g·kg·d–1(Reference Passariello, Marchionni and Carcuro36), which is comparable to traditional sport-specific recommendations, and exceeds the UK RNI(31). In contrast, fat intakes are slighter higher in the MedDiet compared with healthy eating guidelines (37 % v. 20–35 % total energy intake, respectively), although this is largely from MUFA such as olive oil, which has been associated with numerous health benefits(Reference Shannon, Ashor and Scialo2). Therefore, overall, there are some differences in the macronutrient distribution of the MedDiet compared with both traditional healthy eating guidelines and sports nutrition recommendations. However, as discussed later, it is possible that the macronutrient distribution of the MedDiet could be manipulated on a day-by-day or session-by-session basis by altering the amounts of different foods which are typically consumed as part of this dietary pattern. This could help ensure athletes meet the macronutrient demands of different sporting situations, whilst otherwise retaining the benefits of consuming a MedDiet.
It is acknowledged that ensuring appropriate macronutrient intake is an important consideration for athletes. Nevertheless, focusing exclusively on the macronutrient profile of a diet may be an oversimplification which fails to account for the importance of micronutrients and other bioactive dietary compounds. To this end, current sport-specific and healthy eating guidelines also encourage consumption of a variety of foods (e.g. fruit, vegetables and dairy products) to avoid micronutrient deficiencies and support healthy physiological function(Reference Thomas, Erdman and Burke16). The MedDiet includes many of the components associated with a traditional healthy diet consumed by athletes (e.g. high intake of fruit and vegetables). In addition, the MedDiet also advocates the liberal use of olive oil, high consumption of pulses/legumes, regular intake of nuts and seeds, and small-to-moderate wine intake alongside meals(Reference Shannon, Ashor and Scialo2) Moreover, the fruit and vegetable content of a MedDiet may be higher than typically advocated in health eating guidelines (e.g. 3–6 portions of each per d(Reference Shannon, Stephan and Granic37)). The MedDiet is therefore rich in a constellation of different micronutrients and other bioactive dietary compounds (e.g. polyphenols including resveratrol and quercetin, inorganic nitrate, fibre, n-3 fatty acids) and following the key principles of this dietary pattern could be an effective way to maximise diet quality in athletes with potential health and performance benefits.
Potential health and performance benefits of a Mediterranean diet in competitive athletes
The MedDiet is a healthy, palatable and cost-effective dietary pattern with a rich nutrient profile and widely documented health effects. In this section, we outline the rationale for why a MedDiet could be relevant as a performance-enhancing and health-promoting diet in competitive sporting populations.
Oxidative stress of exercise
High-intensity exercise can increase formation of reactive oxygen species (ROS) and other free radicals, with skeletal muscle representing the primary source of ROS during exercise(Reference Jackson, Vasilaki and McArdle38). High levels of ROS can result in damage to macromolecular structures (e.g. DNA, lipids and proteins) and both high and very low levels of ROS can inhibit muscle force production(Reference Powers, Deminice and Ozdemir39). Although often demonised as a negative by-product of exercise, ROS also play a crucial role in adaptation to exercise via the activation of redox-sensitive signalling pathways, including nuclear factor erythroid 2-related factor 2 (Nrf2), PPAR γ coactivator 1-α (PGC-1α) and mammalian target of rapamycin (m-TOR) pathways(Reference Powers, Duarte and Kavazis40–Reference Done, Gage and Nieto42). Chronic exercise up-regulates the body’s antioxidant defence system, which partly offsets the acute increase in ROS during(Reference Powers, Deminice and Ozdemir39), and in the days following, exercise(Reference Place, Ivarsson and Venckunas43). However, controversy exists over whether competitive athletes should consume high levels of antioxidants, particularly via antioxidant supplements, to further attenuate exercise-induced oxidative stress (defined as a disruption to redox signalling and/or an increased ratio of oxidants to antioxidants)(Reference Powers, Deminice and Ozdemir39,Reference Jones44) .
Current research suggests that a hormetic response may exist, whereby low-to-moderate levels of exercise-induced ROS are beneficial for exercise performance and adaptation, whereas high levels of ROS may compromise muscle force production and attenuate the adaptive response(Reference Radak, Chung and Goto45). Administration of high doses of antioxidant supplements (e.g. vitamin C and E) is typically not recommended in athletes (except in subjects with very low basal levels of antioxidants)(Reference Margaritelis, Paschalis and Theodorou46), given they may significantly alter redox state and impair exercise adaptations(Reference Pastor and Tur41). On the contrary, consumption of healthy dietary patterns which are naturally enriched in foods with antioxidant properties have been proposed as a more suitable strategy to mitigate high levels of oxidative stress without compromising physiological function or the adaptive response to exercise(Reference Barnard, Goldman and Loomis47–Reference Machefer, Groussard and Zouhal49). The differential effects of antioxidant supplements v. an antioxidant-rich diet may be related to the typically higher, and potentially detrimental, total dose of antioxidants provided in supplements(Reference Koivisto, Paulsen and Paur50). Alternatively, the different effects of the two antioxidant vehicles could be related to the fact that antioxidant supplements typically only contain one single antioxidant, whereas plant-based foods contain a constellation of many hundreds/thousands of phytochemicals which provide a network of different antioxidants(Reference Koivisto, Paulsen and Paur50).
In contrast to the findings of studies reporting deleterious effects of vitamin C and E on adaptations during a high-altitude training camp(Reference Gomez-Cabrera, Domenech and Romagnoli51), where production of ROS is typically elevated v. sea level(Reference Wehrlin, Marti and Hallén52), consumption of an antioxidant-rich food diet did not blunt adaptations to a 3-week altitude training camp (2320 m) in national team endurance athletes(Reference Koivisto, Paulsen and Paur50). On the contrary, Hb concentration increased to a greater extent with the antioxidant-rich diet v. control. However, the significance of this finding is unclear and could simply reflect differences in participant hydration status (which was typically lower in control at baseline) given Hb mass was comparable in both groups.
Key position statements from the ACSM and ISSN both advocate a well-chosen diet containing antioxidant-rich foods such as fruits and vegetables(Reference Thomas, Erdman and Burke16,Reference Kerksick, Wilborn and Roberts53) , which could help mitigate against high levels of oxidative stress in athletes. Benefits of consuming such a diet are demonstrated in a study by Watson et al. (Reference Watson, Callister and Taylor54), who contrasted exercise responses in trained athletes consuming a habitual diet with naturally high antioxidant levels compared with a diet with restricted intake of antioxidant-rich foods, which resulted in a threefold reduction in antioxidant intake. Ratings of perceived exertion were lower during exercise and F(2)-isoprostane concentrations, a robust marker of lipid oxidation, were decreased post-exercise when following the high antioxidant diet. The authors concluded that individuals participating in regular, high-intensity exercise may require higher intake of antioxidant-rich foods to protect against exercise-induced oxidative stress than sedentary individuals, which can be met through a healthy habitual diet without the need for dietary supplementation in most circumstances(Reference Watson, Callister and Taylor54).
The MedDiet contains a moderate-to-high intake of fruits and vegetables (e.g. 3–6 portions of each per d(Reference Shannon, Stephan and Granic37)), which is similar to (or slightly greater than) traditional guidelines for athletes. Uniquely, the MedDiet is also rich in olive oil and includes a modest intake of red wine, which may further increase the antioxidant potential of this diet. For example, olive oil phenolics such as hydroxytyrosol and oleuropein have been demonstrated to protect against high levels of oxidative stress and improve mitochondrial function in in vitro., ex vivo and animal model studies(Reference Schaffer, Podstawa and Visioli55,Reference Sun, Wang and Hou56) . In the context of exercise, an interesting study by Musumeci et al. (Reference Musumeci, Maria Trovato and Imbesi57) demonstrated lower markers of oxidative stress (e.g. hydroperoxides and thiobarbituric acid-reactive substances) and increased markers of antioxidant defence (e.g. non-enzymatic antioxidant capacity and Hsp70 expression) following an exhaustive bout of exercise in male Sprague-Dawley rats consuming a habitual diet enriched with extra virgin olive oil v. rats fed standard chow. Meanwhile, in humans, consumption of both wine (240 ml/d or about 1 large glass) and the MedDiet as a whole have been demonstrated to decrease levels of oxidative DNA damage as indicated by lower concentrations of guanine oxidised metabolites, such as 8-oxo-2'-deoxyguanosine (8-OHdG) in peripheral blood leucocytes(Reference Urquiaga, Strobel and Perez58). Similar benefits, including increased total plasma antioxidant status, have also been observed with 300–400 ml/d wine for about 2 weeks(Reference Micallef, Lexis and Lewandowski59–Reference Estruch, Sacanella and Mota61), although such high intakes may not be advocated in athletes (or other populations) and more research into the possible antioxidant effects of lower doses of wine is warranted. Interestingly, consumption of 300 ml/d alcohol-free wine for 7 d has also been shown to increase activity of antioxidant enzymes such as glutathione reductase and superoxide dismutase(Reference Noguer, Cerezo and Donoso Navarro62). Therefore, alcohol-free wine could be considered for consumption by athletes wishing to avoid alcohol intake whilst still benefiting from the antioxidant effects of this key MedDiet component.
Overall, these findings suggest that consumption of a healthy diet such as MedDiet which is naturally rich in foods with antioxidant properties could represent an effective strategy to help ameliorate high level of exercise-induced oxidative stress, without necessitating antioxidant supplements which may have a deleterious effect on exercise-induced adaptations(Reference Pastor and Tur41). Current evidence from the PREDIMED trial(Reference Billingsley and Carbone63) suggests that the MedDiet has superior antioxidant effects to a low-fat diet, which is often practiced by athletes. However, future research is warranted to determine whether the antioxidant effects of a MedDiet are comparable (or superior/inferior) to other healthy diets which may advocated for athletes.
Anti-inflammatory effects
Exercise, particularly when it involves unaccustomed movements, eccentric muscle contractions, or is especially arduous in nature, can result in muscle damage which is associated with an increase in circulating and intramuscular markers of inflammation(Reference Peake, Neubauer and Della Gatta64). High levels of inflammation may slow the recovery process(Reference Pizza, Peterson and Baas65,Reference Yamada, Himori and Tatebayashi66) , and strategies to attenuate exercise-induced inflammation may be valuable when the priority is to ensure rapid recovery between bouts of strenuous exercise (e.g. between heats and finals or during a heavy period of regular competition)(Reference Bell, Walshe and Davison67,Reference Govus, Andersson and Shannon68) . On the contrary, a drastic reduction in inflammation could attenuate exercise-induced training adaptations, including reduced skeletal muscle remodelling(Reference Markworth, Maddipati and Cameron-Smith69). Inflammation is also a key part of the response to injuries(Reference Tipton15), which are often a risk in athletes involved in high-intensity, high-volume training regimens(Reference Bueno, Pilgaard and Hulme70). Excess inflammation can slow the healing process although, considering the importance of inflammation in wound healing(Reference Lin, Kotani and Lowry71), major reductions in inflammation may be counterproductive for the healing response to some injuries(Reference Tipton15).
Adherence to a healthy dietary pattern could help support an appropriate inflammatory response to exercise during regular training and competition, without resorting to specific anti-inflammatory strategies (e.g. non-steroidal anti-inflammatory drugs(Reference Lisowska, Kosson and Domaracka72) or high-dose nutritional supplements(Reference Tipton15)), except in situations where rapid recovery or mitigation of very high levels of inflammation are prioritised. Dietary manipulation rather than medicinal use is likely of benefit given the potential health risk for athletes consuming excessive non-steroidal anti-inflammatory drugs. Associated side effects in exercising athletes include asthma exacerbation, gastrointestinal and renal side effects including acute kidney injury, hypertension and other CVD(Reference Ziltener, Leal and Fournier73). The MedDiet may therefore represent an effective alternative to the use of non-steroidal anti-inflammatory drugs in certain circumstances. Indeed, a number of studies have reported anti-inflammatory effects of the MedDiet as a whole including reduced c-reactive protein, TNF-α and IL-6, which appear, in part, to be related to altered methylation of inflammation-related genes(Reference Arpón, Riezu-Boj and Milagro74) and down-regulation of the NF-κB pathway(Reference Perez-Martinez, Lopez-Miranda and Blanco-Colio75). MedDiet components, particularly fish oils enriched in n-3 fatty acids(Reference Jouris, McDaniel and Weiss76) and certain fruits(Reference McAnulty, Nieman and Dumke77,Reference Howatson, McHugh and Hill78) , have also been demonstrated to reduce high levels of inflammation following damaging exercise. The anti-inflammatory effects of the MedDiet have been demonstrated to help attenuate age-related inflammation (so-called ‘inflammaging’) with attendant reductions in disease risk(Reference Shannon, Ashor and Scialo2,Reference Shannon, Mendes and Köchl79) and could represent an effective strategy for helping support an appropriate inflammatory response during regular exercise training and competition which warrants direct exploration.
Injury prevention
In addition to the potential effects a MedDiet in controlling inflammation, key components of this dietary pattern could also be important for injury prevention or recovery via other mechanisms. For example, the MedDiet has been proposed to play a key role in maintenance of bone health(Reference Malmir, Saneei and Larijani80). Indeed, a recent systematic review and meta-analysis reported greater bone mineral density and reduced risk of fracture with higher v. lower MedDiet adherence(Reference Malmir, Saneei and Larijani80). Importantly, meta-regression analysis revealed a 5 % decrease in risk of hip fractures for each one unit increase in MedDiet score(Reference Malmir, Saneei and Larijani80). As the included studies were conducted in non-athletes, suitable caution needs to be applied when interpreting these findings. However, if present, similar effects could be valuable to competitive athletes in sports that have an increased risk of fractures (e.g. due to traumatic events)(Reference Ryan-Moore, Mavrommatis and Waldron81), stress fractures (e.g. due to cumulative, repetitive stress without adequate recovery)(Reference Rizzone, Ackerman and Roos82) and osteoporosis (e.g. due to prolonged unloading in non-weight bearing sports)(Reference Nagle and Brooks83).
The beneficial effects of a MedDiet on bone health could, in part, be related to the olive oil content of this dietary pattern. Indeed, extra virgin olive oil has been demonstrated to enhance bone mineral density in both rats and humans by increasing osteoblastogenesis and adipogenesis in mesenchymal stem cells(Reference Liu, Huang and Li84). Additionally, intervention with a MedDiet supplemented with additional extra virgin olive oil for 2 years significantly increased serum osteocalcin, suggesting a potentially protective effect on bone(Reference Fernández-Real, Bulló and Moreno-Navarrete85). The MedDiet has also been associated with higher circulating concentrations of 25(OH)D(Reference Zupo, Lampignano and Lattanzio86) and increased Ca absorption and retention, alongside lower Ca excretion, translating into higher bone turnover rates(Reference Seiquer, Mesías and Hoyos87) – factors which could further contribute towards the beneficial effects of this dietary pattern on bone health.
Similarly, n-3 fatty acids, available in fish and other seafood in the MedDiet, have also attracted notable attention in regard to recovery from injuries, particularly those which necessitate a period of immobility or reduced activity(Reference Tipton15). For example, fish oils – a known source of n-3 fatty acids – attenuated atrophy of the soleus muscle in rodents during hind limb immobilisation compared with a corn oil diet control(Reference You, Park and Song88). Mechanistically, n-3 fatty acids were shown to attenuate disturbances in the activation of the Akt pathway through E3 ubiquitin ligases and p70 s6 kinase(Reference You, Park and Song88). Similarly, 8 weeks of n-3 fatty acid supplementation augmented the muscle protein fractional synthesis rate to hyperinsulinaemia-hyperaminoacidaemia, and increased phosphorylation of mTOR and p70 s6 kinase(Reference Smith, Atherton and Reeds89).
Beneficial effects of n-3 fatty acids have also been proposed for traumatic brain injuries in athletes, particularly in sports such as rugby and American Football where these injuries are increasingly common. Murine model studies demonstrate a consistent benefit of n-3 fatty acids (particularly DHA) in the prevention and treatment of traumatic brain injury, which warrants study in humans(Reference Salberg, Yamakawa and Christensen90,Reference Wang, Van and Gavitt91) . By contrast, n-3 fatty acid supplementation has been shown to impair wound healing in rats, which may be related to a supressed inflammatory response(Reference Albina, Gladden and Walsh92).
Overall, the role of a MedDiet in injury prevention and/or recovery in humans warrants direct exploration, with specific consideration over whether effects may differ depending upon injury type.
Illness prevention
Whether or not elite athletes are at a greater risk of infection and illness than the general population remains unclear(Reference Simpson, Campbell and Gleeson93). While regular exercise is undoubtedly beneficial for immune function and reducing the incidence of illness and disease(Reference Campbell and Turner94), several studies suggest that athletes are at greater risk of infection immediately after a strenuous period of training or competitive event(Reference Spence, Brown and Pyne95,Reference Valtonen, Waris and Vuorinen96) . The reasons are multifactorial and remain much disputed(Reference Campbell and Turner94), but suppression of immune cells, travel, fatigue, allergies, increased anxiety, sleep disruption and poor diet could all play a role. Regardless of the precise reasons, the prevalence of illness in elite athletes, most of which are upper respiratory in origin, have been reported to be as high as 7 % during competition(Reference Engebretsen, Steffen and Alonso97,Reference Engebretsen, Soligard and Steffen98) . The resulting increase in the number of training sessions and competitions that are either missed or performed sub-optimally can significantly hinder an athlete’s chances of success throughout their career(Reference Raysmith and Drew99). Therefore, maintaining a healthy immune system capable of mounting effective response to viruses and pathogens is vitally important for competitive athletes. Diet has a profound influence on immune cells(Reference Childs, Calder and Miles100), and consuming a healthy diet may play a key role in maintaining a healthy immune system.
Direct evidence for a beneficial effect of the MedDiet as a whole on illness/infection risk is very limited, although the findings from available studies are promising. One observational study of over 30 000 adults reported 26 % lower risk of sepsis with high v. low MedDiet adherence(Reference Gray, Wang and Martin101). Another study demonstrated that transitioning towards a MedDiet reduced number of catarrhal episodes, degree of intensity of colds, and emergency and hospital admissions in children aged 1–5 years with recurring colds and frequency inflammatory complications(Reference Calatayud, Calatayud and Gallego102). More promising evidence exists to show that key components of the MedDiet may play a role in maintaining a well-functioning immune system, which requires direct substantiation in athletes.
It has been suggested that low intakes of the n-3 fatty acids, available in fish and other sources in the MedDiet but less frequently consumed as part of the classic Western diet, may impair the resolution of inflammation after exposure to a stress or infection(Reference Calder, Carr and Gombart103,Reference Basil and Levy104) . This could be linked to ineffective activation of the specialised pro-resolving mediators that are metabolised from DHA and EPA and play fundamental roles in the cessation of an immune response(Reference Basil and Levy104). By contrast, increasing intake of the n-3 fatty acids EPA and DHA has been shown to enhance some aspects of immune function relevant to infections risk following exercise. For example, 6 weeks consumption of krill oil (2 g/d) increased the cytotoxic activity of natural killer cells (which destroy virally infected cells) 3 h following high-intensity exercise(Reference Da Boit, Mastalurova and Brazaite105). Both this study, and another that provided 3 g/d of fish oil for 6 weeks(Reference Gray, Gabriel and Thies106), found that n-3 fatty acids augmented post-exercise increases in PBMC IL-2 production, which suggests an enhanced T-helper 1 (Th1) cytokine response and possible antiviral benefit. Although further research is needed to determine the optimal dose of n-3 fatty acids to elicit such effects, and their clinical significance, these findings suggest that a diet emphasising adequate intake of n-3 fatty acids could benefit athletes’ immune function. Indeed, recent recommendations suggest that consumption of 250 mg/d EPA and DHA may help optimise immune function(Reference Calder, Carr and Gombart103). This amount is easily achievable with a MedDiet, which typically contains ≥ 3 × 100–150 g servings of fish or 200 g servings of other seafood per week(Reference Estruch, Ros and Salas-Salvadó12). For example, a single 150 g serving of mackerel is estimated to provide over 2000 mg of EPA and DHA, which would meet the entire weekly requirement for these n-3 fatty acids(Reference Rincón-Cervera, González-Barriga and Romero107).
Various (poly)phenol compounds, also abundant in the MedDiet(Reference Medina-Remón, Tresserra-Rimbau and Pons108), may play a role in maintaining a well-functioning immune system and preventing infection. Indeed, several (poly)phenols, including resveratrol from red wine, have been shown to not only modulate aspects of the immune system, but to also exert antibacterial and antiviral effects(Reference Campagna and Rivas109,Reference Paulo, Ferreira and Gallardo110) . Such effects have led some scientists to argue that (poly)phenols could be used as an adjuvant therapy for the highly contagious COVID-19(Reference Paraiso, Revel and Stevens111). Although most studies showing antiviral effects of (poly)phenols are in vitro, there is some evidence of similar effects in vivo (Reference Droebner, Ehrhardt and Poetter112,Reference Ahmed, Henson and Sanderson113) and ex vivo (Reference Ahmed, Henson and Sanderson113,Reference Lackermair, Scherr and Waidhauser114) in athletic populations.
Few studies have examined whether (poly)phenols can reduce infections in athletes, although there is some evidence that the flavonoid, quercetin, may reduce upper respiratory infection (URTI) symptoms (e.g. runny nose, coughing) after strenuous exercise(Reference Nieman, Henson and Gross115). For example, Neiman et al. (Reference Nieman, Henson and Gross115) found that 1 g/d of quercetin reduced URTI symptoms in the 2-week period following 3 d of intense cycling exercise. In addition, 5 weeks intake of a (poly)phenol-rich non-alcoholic beer (predominantly catechins and phenolic acids) decreased URTI incidence by over threefold in the 2 weeks after the Munich marathon in healthy male runners(Reference Scherr, Nieman and Schuster116). Interestingly, quercetin was initially proposed to reduce URTI symptoms by bolstering the immune system and/or attenuating oxidative stress, yet many of the in vivo studies have not observed changes in either following exercise, suggesting that antiviral effects might provide a better explanation for these findings(Reference Nieman, Henson and Gross115,Reference Nieman117) . Although the precise mechanisms to explain how quercetin and other (poly)phenols may reduce URTI symptoms is not well understood, these studies provide tentative support for a role of (poly)phenols in reducing infection rates in athletes.
As recently highlighted in a consensus statement on immuno-nutrition and exercise, future research needs to determine the minimum dose required to reduce URTI symptoms, and whether increasing dietary intake of (poly)phenols can elicit similar effects to high doses of individual (poly)phenols(Reference Bermon, Castell and Calder118). Against this background, it is difficult to say whether the (poly)phenol content (and content of specific (poly)phenols such as quercetin) of a MedDiet, or other healthy diets which may be advocated for athletes, would be sufficient to reduce incidence of illness and infection. For example, achieving the 1 g/d quercetin intake shown to benefit immune function in the study by Neiman et al.,(Reference Nieman, Henson and Gross115) is implausible via a typical healthy diet, even one containing an abundance of quercetin-rich foods such as onions (about 45 mg quercetin/100 g), spinach (about 27 mg quercetin/100 g), lettuce (about 15 mg quercetin/100 g) and red wine (about 3 mg quercetin/100 ml)(Reference Dabeek and Marra119). Nevertheless, some observational evidence suggests that modest intakes of red and white wine (1–7 glasses/week) are associated with a 34 % and 33 % reduced risk of common cold compared with 0 glasses(Reference Ouchi, Niu and Kobayashi120), respectively, suggesting that a small-to-moderate intake of wine, consistent with practices in the MedDiet, could still reduce risk of certain illnesses/infections.
Overall, the evidence presented in this section tentatively suggests that consumption of key MedDiet components (particularly fish and wine), alongside the MedDiet as a whole, could help reduce risk of illness/infection. Further research is needed to substantiate these preliminary findings in athletes (and non-athletes), with a particular focus on whether the amount of bioactive compounds, especially (poly)phenols, consumed as part of the MedDiet are sufficient to enhance immune function without the need for additional dietary supplementation. As noted previously, it is also necessary to determine whether a MedDiet has greater availability of immunomodulating compounds such as (poly)phenols, and subsequently greater effects on illness/infection risk, compared with other healthy diets which may be advocated for athletes.
Vascular function
Exercise is known to protect against CVD, with an efficacy comparable to that of many pharmacological interventions(Reference Naci and Ioannidis121). However, somewhat paradoxically, recent evidence suggests that Masters athletes who have engaged in lifelong competitive exercise may actually have greater risk of atherosclerotic plaque formation compared with sedentary controls(Reference Merghani, Maestrini and Rosmini122,Reference Schwartz, Kraus and Schwartz123) . For example, Merghani et al. (Reference Merghani, Maestrini and Rosmini122) reported presence of atherosclerotic plaques in 44 % of Masters athletes compared with only 22 % of sedentary controls. Similarly, Schwartz et al. (Reference Schwartz, Kraus and Schwartz123) reported increased total, calcified and non-calcified plaque volumes in male marathon runners who had completed ≥ 1 marathon/year for 25 consecutive years v. sedentary controls. The clinical significance of these plaques remains to be elucidated, and it is unclear whether they represent a response to chronic high-intensity exercise or accompanying dietary and other lifestyle factors. Particularly relevant to Masters athletes, Fernandez et al. (Reference Fernández, Rosado-Álvarez and Da Silva Grigoletto124) demonstrated that moderate-to-high-intensity training enhances the positive effects of the MedDiet on the regenerative capacity of the endothelium in older, albeit sedentary individuals with the metabolic syndrome. Thus, the cardio-protective effects of the MedDiet may be potentiated in individuals partaking in regular physical activity (i.e. athletes). Nevertheless, given the paucity of research in this area and the well-documented cardiovascular benefits of a MedDiet(Reference Estruch, Ros and Salas-Salvadó12,Reference Shannon, Mendes and Köchl79,Reference Cowell, Mistry and Deighton125) , the role of this dietary pattern in mitigating formation of atherosclerotic plaques and maintaining cardiovascular health in Masters athletes is worthy of further investigation.
Cognitive function
Cognitive function is a key aspect of sports performance, with athletes across a range of sporting scenarios (e.g. combat sports, endurance sports, invasion games, etc) required to make rapid and appropriate tactical decisions to maximise their chance of success(Reference Shannon, Duckworth and Barlow126). Acute exercise can both positively and negatively impact cognitive performance, depending upon exercise intensity and the nature of the cognitive task(Reference Brisswalter, Collardeau and René127). Nevertheless, strategies to optimise cognitive performance during sport are highly desirable, and nutritional interventions are acknowledged as a potential means of boosting cognitive performance during exercise(Reference Meeusen128).
Numerous observational studies have demonstrated beneficial associations between higher MedDiet adherence and cognitive function across a range of cognitive domains(Reference Shannon, Stephan and Granic37,Reference Petersson and Philippou129,Reference Anastasiou, Yannakoulia and Kontogianni130) . Similarly, intervention with a MedDiet has been shown to enhance cognitive performance in some(Reference Martínez-Lapiscina, Clavero and Toledo131–Reference Valls-Pedret, Sala-Vila and Serra-Mir133), but not all(Reference Knight, Bryan and Wilson134), studies, with the difference in findings between investigations potentially reflecting differences in the cognitive testing battery employed, the participant cohort or the specific construction of the MedDiet administered to participants(Reference Knight, Bryan and Wilson134). Some evidence also exists to show that extracts from key MedDiet components such as vegetables(Reference Thompson, Wylie and Fulford135,Reference Thompson, Vanhatalo and Jell136) and fruits(Reference Philip, Sagaspe and Taillard137) can directly benefit cognitive performance during exercise in young athletic cohorts. Notably, studies by Thompson et al. (Reference Thompson, Wylie and Fulford135,Reference Thompson, Vanhatalo and Jell136) have demonstrated beneficial effects of beetroot juice, which provides inorganic nitrate in an amount achievable through a plant-based diet such as the MedDiet(Reference Shannon, Stephan and Minihane138), on cognitive performance during high-intensity exercise. However, evidence is lacking for an effect of the MedDiet as a whole on cognitive performance during exercise tasks, such that we cannot necessarily infer a beneficial effect of this dietary pattern on cognition in a sporting context. This is especially so given the fact that most competitive athletes are young and healthy, whereas the majority of studies exploring effects of the MedDiet on cognition have included older participants with existing co-morbidities(Reference Petersson and Philippou129). Indeed, to the authors knowledge, only one study has directly explored the effects of a MedDiet intervention on cognitive performance in younger individuals (mean age: 21 years), with inconsistent effects occurring(Reference McMillan, Owen and Kras139). Similarly, it is possible that studies on isolated compounds in the MedDiet may not necessarily reflect effects conferred by this diet as a whole. Therefore, more direct research is needed before the MedDiet can be recommended to athletes as a potential strategy to improve cognitive performance.
Optimising a Mediterranean diet for competitive athletes
In this article, we have presented evidence to suggest that following the key principles of a MedDiet may represent a useful model of healthy eating for competitive athletes under most circumstances. Nevertheless, there are certain situations where strict adherence to this dietary pattern may not be optimal for athlete health and/or performance. In the following section, we provide some examples of where modifications to the MedDiet may be advocated in accordance with contemporary sports nutrition practices.
Macronutrient distribution and fuelling for the work required
As previously discussed, sport-specific nutritional recommendations support high carbohydrate intake (6–12 g·kg·d–1)(Reference Thomas, Erdman and Burke16,Reference Carr, McGawley and Govus28) for endurance athletes in training and competition. This contrasts with the moderate of about 43 % energy provision from carbohydrates in the MedDiet(Reference Davis, Bryan and Hodgson34). For example, a 70-kg endurance athlete with a daily energy intake of 14,644 kJ, including 43 % of energy provided by carbohydrate, would consume 6297 kJ or about 377 g·d–1 via carbohydrate. This equates to about 5·4 g·kg·d–1 carbohydrate and falls short of the lower end of carbohydrate recommendations for this population(Reference Thomas, Erdman and Burke16). It may therefore be necessary for endurance athletes consuming a MedDiet to augment their carbohydrate intake to ensure optimal glycogen storage and availability prior to training and competition(Reference Burke, Hawley and Wong29). This could be achieved by prioritising intake of higher carbohydrate foods such as grains and starchy vegetables, without deviating from the overall healthy eating principles of this dietary pattern. This may necessitate a corresponding decrease in the consumption of fat prior to training and competition, to avoid excess energy intake and ensure athletes are able to meet carbohydrate requirements for exercise. Interestingly, the high carbohydrate intakes observed by some countries within the Mediterranean Basin (e.g. 59 % of energy derived from carbohydrate intake in Egypt)(Reference Karamanos, Thanopoulou and Angelico35) suggest that modest increases in carbohydrate intake may not result in the loss of any associated health benefits, although direct research is required on this topic.
A more nuanced approach may involve following the recently proposed ‘fuelling for the work required’ paradigm(Reference Impey, Hearris and Hammond140). The central tenet of this concept is that athletes could benefit from adjusting carbohydrate intake levels in accordance with the demands of upcoming training sessions, in order to obtain benefits associated with periodically training with low glycogen availability (e.g. increased cell signalling, gene expression and oxidative enzyme activity), without compromising absolute training intensity(Reference Impey, Hearris and Hammond140). Athletes hoping to benefit from this periodised nutritional approach could adjust the content of higher carbohydrate foods (in a diet otherwise based on the principles of a MedDiet) on a day-by-day or session-by-session basis, to ensure exercise is conducted with the optimal muscle glycogen concentrations necessary to meet the training demands. Such an approach would require careful planning by the athlete, in coordination with nutritional support staff and the coaching team.
Dietary protein recommendations for endurance athletes typically range from 1·2 to 1·5 g·kg·d–1, whereas recommendations for strength-based athletes are about 1·6 to 2·0 g·kg·d- (16 141). Protein intakes of about 1·5 g·kg·d–1 have been observed within an athletic population adhering to a MedDiet(Reference Passariello, Marchionni and Carcuro36), which falls within current guidelines for endurance athletes, but may be insufficient to maximise adaptions for strength-based athletes. An individualised approach is likely necessary to determine if incorporation of additional protein to a MedDiet is required to support metabolic adaptation, and skeletal muscle repair and remodelling. This is likely particular pertinent for those individuals whose protein needs may be > 2 g·kg·d–1 (e.g. for those attempting to maintain fat-free mass in an energy-deficit(Reference Jäger, Kerksick and Campbell141)).
Optimisation of protein intake does not simply relate to the amount of protein ingested but also relates the factors such as the timing and type of protein consumed(Reference Aragon and Schoenfeld142). Although debate continues around the optimal timing of protein intake, some evidence indicates that consumption of protein proximal to exercise (either before or after intensive training) may help augment muscle protein synthesis and exercise-induced adaptations compared with a similar intake of this macronutrient at other times of the day(Reference Aragon and Schoenfeld142). Adjusting the timing of protein-rich meals to coincide with exercise may therefore help boost training adaptations(Reference Aragon and Schoenfeld142). Given evidence from both in vivo and in vitro studies suggests that the amino acid leucine may represent an effective ‘trigger’ for muscle anabolism, consumption of foods rich in this amino acid may also be particularly important for maximising muscle protein synthesis(Reference Phillips and Van Loon32,Reference Breen and Churchward-Venne143) . Conveniently, several foods found in the MedDiet appear to be rich in leucine, including certain fish, white meat and legumes/pulses.
Periodic consumption of sugar-sweetened beverages
The MedDiet typically comprises a low intake of sugar-sweetened beverages(Reference Shannon, Stephan and Granic37,Reference Tong, Wareham and Khaw144) . The omission of such beverages could contribute to the salutary effects of this dietary pattern, given negative associations reported between sugar-sweetened drinks and obesity(Reference Malik, Schulze and Hu145), diabetes(Reference Vartanian, Schwartz and Brownell146) and CHD(Reference Fung, Malik and Rexrode147). By contrast, consumption of carbohydrate beverages pre-, during and post-exercise are generally accepted to augment performance(Reference Thomas, Erdman and Burke16,Reference Jeukendrup148,Reference McGawley, Shannon and Betts149) and recovery(Reference Ivy, Katz and Cutler150). This is particularly pertinent for endurance athletes seeking to augment exogenous carbohydrate oxidation rates and/or resynthesise glycogen stores(Reference Griffiths, Deighton and Shannon151,Reference Griffiths, Deighton and Boos152) , thus facilitating high-intensity exercise bouts(Reference Stellingwerff and Cox153). As such, it may be beneficial for athletes otherwise following the principles of a MedDiet to occasionally consume carbohydrate beverages during intensive training periods and competition, given the ergogenic properties of this nutritional strategy. In addition to supporting performance and recovery, a high carbohydrate intake has also been shown to support immune function. Indeed, high carbohydrate availability before prolonged exercise (≥ 60 min), followed by high rates of ingestion during (≥ 30 g/h), attenuates changes in stress hormones (cortisol and adrenaline) and various immune cells, including cytokines, leucocytes and lymphocytes(Reference Nieman and Wentz154). However, there is presently little evidence that this translates into improved clinical outcomes (e.g. reduced infection rates).
Alcohol intake
One of the key factors which distinguishes a MedDiet from other healthy dietary patterns is that it includes a small-to-moderate intake of alcohol (typically red wine), which is usually consumed alongside meals rather than in isolation(Reference Bach-Faig, Berry and Lairon1). Whether athletes following a MedDiet should also consume the alcohol component of this diet, or whether they may be advised to completely or selectively (i.e. immediately before or after exercise) omit this component, will be briefly discussed in this section.
Current evidence indicates that pre-exercise alcohol consumption has a small ergolytic effect on performance during prolonged, endurance exercise, whereas strength is typically unaffected by alcohol intake, even at high doses(Reference Barnes155). By contrast, pre-exercise alcohol intake is prohibited in certain sports (e.g. archery) given perceived performance advantages. When consumed immediately post-exercise, most aspects of recovery are unlikely to be negatively impacted by alcohol consumption with a dose < 0·5 g·kg–1 body mass, which is equivalent to 35 g for a 70-kg individual or roughly half a bottle of wine depending upon strength(Reference Barnes155). This suggests that consuming modest amounts of alcohol, such as a small glass of red wine with an evening meal following the completion of all daily training, in accordance with the dietary practices of the MedDiet, are unlikely to have negative performance implications for athletes under normal circumstances. By contrast, current recommendations are to avoid alcohol consumption during recovery from soft tissue injuries, as small alterations in protein synthesis, tissue blood flow and immune function may hinder recovery(Reference Barnes155).
When considering (a) the lack of evidence to support a positive effect of alcohol on strength/endurance exercise performance, (b) the fact that pre-exercise alcohol intake is prohibited in sports where it is considered to be potentially ergogenic such as archery and (c) potential risks associated with over-consumption, including physical, psychological and social harm(156,Reference Moss157) , it seems unwise to actively encourage alcohol intake in athletes who currently abstain. Moreover, consuming alcohol during recovery from a soft tissue injury should be avoided to minimise recovery impacts(Reference Barnes155). However, it is possible that there could be small benefits of adjusting drinking habits towards those common with a MedDiet in individuals who already consume alcohol. Specifically, consuming small amounts of red wine in preference to spirits or other alcohol could confer benefits linked to the phenolic compounds in wine(Reference Lamuela-Raventós and de la Torre-Boronat158). Moreover, consuming small amounts of wine in the context of a meal may be preferable to alcohol consumption alone, as beneficial synergistic interactions may occur between wine phenolics and other dietary components(Reference Gago, Lundberg and Barbosa159). Nevertheless, this requires direct investigation, and athletes and support staff should carefully consider the costs and perceived benefits of alcohol consumption. As noted previously, one option for athletes wishing to follow the common drinking habits of a MedDiet whilst minimising alcohol intake could be to consume alcohol-free wine, which appears to retain at least some of the benefits associated with wine intake including antioxidant effects(Reference Noguer, Cerezo and Donoso Navarro62).
Sodium intake
The MedDiet is typically low in Na(Reference La Verde, Mulè and Zappalà160), a characteristic which may contribute towards the beneficial effects of this dietary pattern on blood pressure(Reference Cowell, Mistry and Deighton125), endothelial function(Reference Shannon, Mendes and Köchl79) and cardiovascular risk(Reference Estruch, Ros and Salas-Salvadó12). Nevertheless, during certain sporting situations – especially prolonged, intensive exercise in the heat in unacclimatised individuals – increased Na intake may be temporarily advocated in order to mitigate risk of hyponatremia(Reference Anastasiou, Kavouras and Arnaoutis161), preserve muscle contractility and allow more effective restoration of fluid balance post-exercise(Reference Sharp162).
Fibre intake
The high fibre content of a MedDiet may contribute towards reported beneficial effects of this dietary pattern on composition and metabolism of the gut microbiota(Reference Ghosh, Rampelli and Jeffery163,Reference Filippis, Pellegrini and Vannini164) and reduced risk of certain cancers (especially of the digestive tract)(Reference Mentella, Scaldaferri and Ricci165). Fibre is also acknowledged as playing a key role in maintenance of good health in athletes(Reference de Oliveira, Burini and Jeukendrup166). Nevertheless, high intake of fibre before competition has been associated with the development of negative gastrointestinal (GI) symptoms. For example, Rehrer et al. (Reference Rehrer, van Kemenade and Meester167) identified high fibre intake in the pre-race meal to be a risk factor for GI complaints, especially intestinal cramps, in athletes competing in a half Ironman triathlon event. Current guidelines therefore advocate the avoidance of fibre in the day or days prior to competition to minimise risk of GI upset, but with adequate fibre in the habitual diet during training in order to optimise health(Reference de Oliveira, Burini and Jeukendrup166).
Adherence to the Mediterranean diet and traditional sports nutrition recommendations in competitive athletes
Given the potential health and performance benefits of the MedDiet for athletes, it is relevant to explore current athlete adherence to this dietary pattern, which will help identify whether specific MedDiet interventions could be warranted in this cohort. For reference and comparison, we also start by providing a brief outline of athlete adherence to typical sports nutrition recommendations which, as outlined below, is often sub-optimal.
Adherence to traditional sports nutrition recommendations varies substantially between athletes and sports, although current evidence suggests that many athletes fail to achieve traditional sports nutrition recommendations during training and competition, especially in regard to carbohydrate intake levels(Reference Carr, McGawley and Govus28,Reference Bentley, Patterson and Mitchell168) . For example, one study reported that about 81 % of endurance athlete consumed lower than recommended quantities of carbohydrate in their diet(Reference Baranauskas, Stukas and Tubelis169). With regard to protein intake, in one study of over 500 well-trained Dutch athletes, average protein intake levels were 1·5 and 1·4 g·kg·d–1 in men and women, respectively, which compares favourably against current recommended intake values of 1·2–2·0 g·kg·d–1. Nevertheless, the range of protein intake was highly varied (men: 0·5–2·7 g·kg·d–1; women: 0·4–3·6 g·kg·d–1), such that many individuals were outside of the optimal range for intake of this macronutrient(Reference Gillen, Trommelen and Wardenaar170). Finally, fat intake recommendations for athletes of 20–35 % total energy intake appear to be achievable in most circumstances(Reference Broad and Cox33,Reference Burke, Slater and Broad171) , although some athletes may be at risk of under-consuming fat, especially in endurance, weight category and aesthetic sports(Reference Horvath, Eagen and Ryer-Calvin172,Reference Jonnalagadda, Bernadot and Nelson173) . Female athletes also demonstrated insufficient intake of several micronutrients including B vitamins, K, Ca, P, Fe, Mn and Zn(Reference Baranauskas, Stukas and Tubelis169,Reference Hawley, Dennis and Lindsay174) . An inability to meet relevant recommendations may have deleterious effects on performance and increase the risk of injury and illness(Reference Thomas, Erdman and Burke16,Reference Close, Sale and Baar175) . Strategies to understand barriers and enablers to nutritional adherence in high-performance sport is receiving increasing attention in the literature(Reference Bentley, Patterson and Mitchell168). As a palatable, cost-effective dietary strategy, it is possible that the MedDiet may provide one option to help individuals achieve traditional sport-specific recommendations whilst also benefiting from the inclusion of foods and dietary compounds with additional health/performance effects.
To date, few studies have attempted to quantify level of adherence to the MedDiet in athletes, with mixed results emerging(Reference Muros and Zabala176–Reference Sánchez-Benito, Sánchez-Soriano and Ginart Suárez180). Crucially, these studies have been exclusively conducted in Spain – a country in which MedDiet adherence is traditionally high(Reference Bach-Faig, Berry and Lairon1), but may be decreasing due to westernisation of the diet(Reference León-Muñoz, Guallar-Castillón and Graciani181). Therefore, findings are unlikely to be indicative of MedDiet adherence levels in other populations.
A large cross-sectional study by Muros and Zabala(Reference Muros and Zabala176) demonstrated modest adherence to the MedDiet in 4037 Spanish cyclists and triathletes, as determined by scores on the 14-point MedDiet adherence screener (MEDAS score: 7·64)(Reference Muros and Zabala176). The MEDAS scores reported by Muros and Zabala(Reference Muros and Zabala176) are marginally higher than reported recently in non-athletic Spanish cohorts (MEDAS score: 6·34), although both scores are lower than the typical MEDAS threshold used to define high MedDiet adherence (i.e. > 9 points)(Reference León-Muñoz, Guallar-Castillón and Graciani181). A small study of 12 Spanish female Futsal players also reported low and moderate MedDiet adherence in about 58 % and about 42 % of athletes, respectively(Reference Rubio-Arias, Ramos Campo and Ruiloba Nuñez177). In contrast, in a cross-sectional study which evaluated MedDiet adherence in Spanish amateur cyclists (male n 701, female n 84), and inactive individuals (male n 307, female n 411), 48 % of male cyclists reported high adherence to the MedDiet, compared with just 22 % of male inactive individuals(Reference Mayolas-Pi, Munguia-Izquierdo and Peñarrubia-Lozano178). This trend was replicated in females, with 57 % of female cyclists reporting high adherence to the MedDiet compared with 36 % of their inactive counterparts(Reference Mayolas-Pi, Munguia-Izquierdo and Peñarrubia-Lozano178). Finally, in a study of 90 Spanish kayakers(Reference Alacid, Vaquero-Cristóbal and Sánchez-Pato179), only one participant had low MedDiet adherence, whilst 38 and 51 individuals reported medium and high MedDiet adherence, respectively. Nevertheless, low consumption of nuts (a key MedDiet component) and high consumption of sweets and commercially made pastries (typically consumed in low amounts as part of the MedDiet) was reported.
Overall, current evidence tentatively supports the notion that athletic individuals exhibit marginally higher MedDiet adherence levels compared with inactive individuals(Reference Muros and Zabala176,Reference Mayolas-Pi, Munguia-Izquierdo and Peñarrubia-Lozano178) . Furthermore, it appears that athletic women have higher adherence to the MedDiet than men(Reference Mayolas-Pi, Munguia-Izquierdo and Peñarrubia-Lozano178). This may be related to less frequent fast-food consumption during the competitive season(Reference Hull, Jagim and Oliver182), as well as greater nutritional knowledge and education in athletic women compared with men(Reference Spronk, Heaney and Prvan183,Reference Heaney, O’Connor and Michael184) . Additional large-scale studies in other cohorts are needed to better understand current levels of adherence to the MedDiet in both Mediterranean and non-Mediterranean countries. Moreover, larger, more diverse samples of athletes are needed in future research to explore potential differences between individuals depending on their sex, ability level, sport and age. The MedDiet may provide a prudent dietary pattern for the health and performance of athletes worldwide, and a better understanding of current levels of adherence to this dietary pattern will be important in determining the need for potential MedDiet interventions in athletic cohorts.
Conclusions and future directions
In this review, we have provided preliminary evidence to suggest that the MedDiet could represent a useful model of healthy eating in competitive athletes under most circumstances, with the potential to help optimise certain aspects of health and performance. We have also identified certain areas where, in accordance with contemporary sports nutrition practices, subtle modifications could be made to the MedDiet (whilst otherwise adhering to the key principles of this dietary pattern) to maximise potential ergogenic effects.
Going forward, several questions remain unanswered and may represent useful directions for future research. For example, at present, little is known about current levels of adherence to the MedDiet, and potential barriers to adopting this dietary pattern in athletes. However, such information is important as it could help identify individuals who may be suitable for future MedDiet interventions and help optimise the design of studies hoping to increase adherence to this dietary pattern in athletes. The effect of the MedDiet on appetite/satiety also requires consideration, as the satiating effect of the MedDiet could provide challenges to some athletes in ensuring they consume sufficient energetic intake. In addition, numerous studies suggest that consumption of key foods or compounds available in the MedDiet could enhance health and performance in athletes. However, very few studies have focused on the potential effects of a MedDiet as a whole in athletic cohorts. Indeed, as part of this review, we identified only one study by Baker and colleagues(Reference Baker, DeCesare and Johnson19) which directly explored the effects of the MedDiet on exercise performance. This study involved a relatively short-term (4 d) intervention period and included a small set of exercise performance measures(Reference Baker, DeCesare and Johnson19). Further research is therefore needed to understand whether beneficial performance effects of a MedDiet occur immediately upon transitioning to this dietary pattern or whether they require a certain amount of time to fully manifest. Additional studies exploring the performance effects of a MedDiet in different cohorts, and employing a range of exercise tests, are also warranted. Given the MedDiet includes (and excludes) a constellation of different foods, and no uniform definition exists for the specific constituents of this dietary pattern, it is possible that the construction of a MedDiet (i.e. the relative amounts of different foods included and excluded from this dietary pattern) could also influence the health and performance effects observed. Therefore, further research is warranted to identify whether specific permutations of the MedDiet could be especially effective in optimising athlete health and performance.
In summary, preliminary evidence suggests that consumption of a MedDiet, and its individual components, could play a role in optimising certain aspects of health and performance in competitive athletes. Further research in this area could produce fruitful results which are of benefits to athletes, coaches and nutritional practitioners and is strongly encouraged.
Acknowledgements
N/A.
This research was supported by a grant from the UK Nutrition Research Partnership (UK NRP), an initiative supported by the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council (BBSRC) and the National Institute for Health Research (NIHR) (E.S., MR/T001852/1).
This review was conceived and designed by O. M. S. The review was written by A. G., J. M., E. W., P. A-N., T. C., E. S. and O. M. S. P. A-N. produced the graphical abstract. All authors have read and approved the final version of the manuscript.
There are no conflicts of interest.