Breast milk has undergone hundreds of thousands of years of product development during the evolution of our species. It can, with confidence, be claimed to be the ideal form of nutrition for the human infant during the first months of life and the ideal supplement to solid nutrition during the infant's first years. In addition to providing developmentally appropriate nutrition, breast milk contains a multitude of immunologically active components. In the same manner that breast milk has evolved to fit the needs of our infants, it can be assumed that our infants have evolved to develop in the presence of breast milk. Despite being well-equipped for extra-uterine life at the time of birth, the newborn infant also has several shortcomings, and the most profound are those faced by the immature immune system. The infant undergoes a transition from the sterile environment experienced in utero to one that is filled with microbes, and he must also begin to feed. It is, thus, essential that his immune system learns to differentiate between hostile and innocuous antigens.
Breast milk may help infants in these essential adaptations. It provides infants with direct anti-pathogenic effects via maternal microbe-specific Ig and various other antimicrobial substances(Reference Newburg and Walker1–Reference Jeliffe and Jeliffe3), and has an extensively documented ability to ward off infective disease, such as infantile diarrhoea, in developing countries(Reference Ruiz-Palacios, Calva and Pickering4–Reference Arifeen, Black and Antelman6), but also common causes of pathogen-related infant morbidity in western settings(Reference Wilson, Forsyth and Greene7–Reference Kramer, Chalmers and Hodnett10). There is also an impressive amount of data to suggest that its recipients are protected from common disruptions of the immune system such as type 1 diabetes(Reference Rosenbauer, Herzig and Giani11), inflammatory bowel disease(Reference Barclay, Russell and Wilson12), coeliac disease(Reference Akobeng, Ramanan and Buchan13) and atopic diseases, including allergies and asthma(Reference Kramer, Chalmers and Hodnett10, Reference Lucas, Brooke and Morley14–Reference Vandenplas, Deneyr and Sacre21). Breast-feeding promotes gut colonisation with beneficial bifidobacteria in infants(Reference Newburg and Walker1, Reference Rautava and Walker22–Reference Harmsen, Wildeboer-Veloo and Raangs24), and this finding may be linked with many of the advantages associated with breast-feeding.
Nevertheless, results on the potency of breast milk in reducing the risk of atopic disease remain unclear and contradictory, with evidence for both decreased(Reference Lucas, Brooke and Morley14–Reference Vandenplas, Deneyr and Sacre21, Reference Friedman and Zeiger25) and increased(Reference Sears, Greene and Willan26–Reference Rusconi, Galassi and Corbo31) risk of atopic disease in breast-fed children, and no proven dose–response pattern. The effects of breast-feeding, or the lack of it, on infant immune physiology have thus remained elusive.
In the present study, we determined blood plasma concentrations of key cytokines and circulatory antibody-secreting cells in exclusively breast-fed infants, and compared them with those of infants who had experienced early termination of breast-feeding and had been primarily fed with formula. The present study aimed to shed light on whether differing immune physiology is evident in breast-fed and formula-fed infants.
Materials and methods
Design
The infants in the study were selected from a prospective study (n 98) that had followed them up from birth to the age of 12 months. The majority of the participants were genetically at high risk for atopy, with the mother or other first-degree relatives with atopic disease. This family history was self-reported. A case–control design was chosen, as it is not feasible to conduct a randomised study on breast-feeding. The inclusion criteria for the formula-fed group were the complete cessation of breast-feeding before 3 months of age and consequent exclusive formula feeding at that age. Infants who were exclusively breast-fed at 3 months of age were chosen as controls. Infants with chronic disease were excluded from the study. Altogether, eighteen formula-fed and twenty-nine exclusively breast-fed infants fulfilled the criteria of the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human patients were approved by the Turku University Central Hospital Ethics Committee. Written informed consent was obtained from all patients.
Determining plasma cytokine concentrations using cytometric bead assays and ELISA
Venous blood samples were drawn, heparinised and stored at − 80°C for later analysis at the ages of 1, 3, 6 and 12 months. The samples of formula-fed infants were drawn between September 2000 and February 2002 and the samples of breast-fed infants between May 2000 and August 2002 in almost parallel follow-up periods. The concentrations of the cytokines interferon γ, TNF-α, IL-10, IL-5, IL-4 and IL-2 were measured simultaneously from the same blood plasma sample through use of a multiplexed bead-based flow cytometric assay between August and September 2003, and all samples had been stored at the same facility in similar conditions. A commercial Human Th1/Th2 Cytokine Kit, BD Cytometric Bead Array, a FACSCalibur flow cytometer and BD CBA Software (BD Immunocytometry Systems and BD Biosciences Pharmingen) were used for the analysis according to the manufacturer's specifications. The detection limits for these cytokines according to the manufacturer were 7·1 pg/ml for interferon γ, 2·6 pg/ml for IL-2, 2·6 pg/ml for IL-4, 2·4 pg/ml for IL-5, 2·8 pg/ml for IL-10 and 2·8 pg/ml for TNF-α.
Cytometric bead assay analyses were carried out simultaneously during August and September 2003. The plasma concentrations of transforming growth factor β2 (TGF-β2) were measured in duplicate using commercial sandwich ELISA specific for the molecule (R&D Systems). Activation of latent TGF-β2 and all determinations were carried out according to the manufacturer's instructions.
Determining the number of Ig-secreting cells
The total number of Ig-secreting cells and the number of cells producing antibodies directed specifically against the cows' milk allergen, casein, were measured using the enzyme-linked immunospot assay, as detailed in previous work (Isolauri et al), from blood samples obtained at the ages of 3, 6 and 12 months. On the day of sample collection, peripheral blood mononuclear cells were isolated using Ficoll-Paque gradient centrifugation. Isolated cells were washed three times in Hanks balanced salt solution and suspended in Roswell Park Memorial Institute 1640 medium containing 10 % fetal calf serum and adjusted to a concentration of 1–2 × 106 cells/ml.
To determine the total number of Ig-secreting cells, the wells were coated for 2 h at 37°C with rabbit anti-human IgA, IgG and IgM (Dako A/S) diluted in the ratio 1:100 in PBS (pH 7·4), and to detect the number of cells secreting casein-specific antibodies, the coating was performed with β-casein (20 μg/ml; Sigma Chemical Company). Uncoated binding sites were blocked with 1 % bovine serum albumin (Boehringer Mannheim) in PBS (pH 7·4) for 30 min at 37°C. After washing, the lymphocyte suspension was incubated on antigen-coated flat-bottomed ninety-six-well microtitre plates (Immunoplate RI, A/S Nunc) at 37°C for 2 h. The detection of antibodies secreted during that time was performed with alkaline phosphatase-conjugated goat antiserum to human IgA, IgG and IgM (Sigma Chemical Company), diluted in 1 % bovine serum albumin–PBS (pH 7·4) and incubated overnight at room temperature, followed by a substrate agarose overlay and observation of the coloured spots.
Statistical evaluation
The clinical data are described as means with ranges. The differences in clinical characteristics between the study groups were assessed using Student's t test and the χ2 test. The results are expressed as geometric means with 95 % CI. The differences between serum cytokine concentrations in the breast-fed and formula-fed infants during the course of the follow-up were assessed using ANOVA for repeated measures after logarithmic transformation. If the ANOVA suggested a difference between groups, further analyses at each time point were conducted using Student's t test after logarithmic transformation. A P value of less than 0·05 was considered statistically significant.
Results
Clinical data
The clinical characteristics of the participants are shown in Table 1. The study groups were comparable with regard to gestational age, sex, birth weight and the mode of delivery, while differing on the mode of feeding according to the study design. Due to the design of the original prospective cohort, the family history of atopy differed between the formula-fed and breast-fed groups. A total of fourteen of the eighteen (77 %) formula-fed infants had a first-degree relative with atopic disease compared to all of the twenty-nine (100 %) breast-fed infants (P =0·04). A total of twelve of the eighteen (67 %) formula-fed infants had a mother with clinical atopic disease compared to all of the twenty-nine (100 %) in the breast-fed group (P =0·009). The groups are, thus, significantly different regarding family history of atopy. However, the cytokine results observed persist and remain significant even when only the participants with atopic mothers are evaluated (i.e. when the six formula-fed participants without an atopic mother are excluded from the evaluation), suggesting that the differences witnessed in cytokine concentrations are not due to the differences in family history of atopy (Table 2).
Table 1 Clinical characteristics of participants (Mean values and ranges; number of participants and percentages)

AD, atopic disease.
Table 2 Serum cytokine concentrations in formula-fed and breast-fed infants (Geometric means and 95 % confidence intervals)

TGF-β2, transforming growth factor β2; IFN-γ, interferon γ.
Differing plasma cytokine concentrations in formula-fed and breast-fed infants
ANOVA for repeated measures showed a significant difference in TNF-α concentrations (P =0·0019), with elevated serum TNF-α concentrations in formula-fed infants compared to their breast-fed counterparts (Fig. 1). Serum IL-2 concentrations (P =0·001) were also significantly higher in formula-fed than in breast-fed infants, with significant differences seen throughout the first year of life (Fig. 2). A contrary situation was evident with TGF-β2. The concentrations of TGF-β2 (P≤ 0·0001) were significantly lower in formula-fed than in breast-fed infants throughout the first year of life, with up to ten-fold differences seen (Fig. 3).
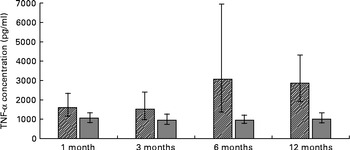
Fig. 1 Serum TNF-α concentrations in formula-fed (![]() ) and breast-fed infants (
) and breast-fed infants (![]() ). ANOVA for repeated measures showed a significant difference in TNF-α concentrations (P =0·0019). Statistically significant differences were evident for 1 month (P= 0·039), 3 months (P= 0·010), 6 months (P< 0·0011) and 12 months (P= 0·039).
). ANOVA for repeated measures showed a significant difference in TNF-α concentrations (P =0·0019). Statistically significant differences were evident for 1 month (P= 0·039), 3 months (P= 0·010), 6 months (P< 0·0011) and 12 months (P= 0·039).

Fig. 2 Serum IL-2 concentrations in formula-fed (![]() ) and breast-fed infants (
) and breast-fed infants (![]() ). ANOVA for repeated measures showed a significant difference in IL-2 concentrations (P≤ 0·001). Statistically significant differences were evident for 1 month (P= 0·0003), 3 months (P= 0·001), 6 months (P= 0·0001) and 12 months (P≤ 0·014).
). ANOVA for repeated measures showed a significant difference in IL-2 concentrations (P≤ 0·001). Statistically significant differences were evident for 1 month (P= 0·0003), 3 months (P= 0·001), 6 months (P= 0·0001) and 12 months (P≤ 0·014).

Fig. 3 Serum transforming growth factor β2 (TGF-β2) concentrations in formula-fed (![]() ) and breast-fed infants (
) and breast-fed infants (![]() ). ANOVA for repeated measures showed a significant difference in TGF-β2 concentrations (P≤ 0·0001). Statistically significant differences were evident for 1 month (P≤ 0·0001), 3 months (P≤ 0·0001), 6 months (P= 0·039) and 12 months (P≤ 0·0001).
). ANOVA for repeated measures showed a significant difference in TGF-β2 concentrations (P≤ 0·0001). Statistically significant differences were evident for 1 month (P≤ 0·0001), 3 months (P≤ 0·0001), 6 months (P= 0·039) and 12 months (P≤ 0·0001).
No statistically significant differences were evident between the serum concentrations of interferon γ (P =0·10), IL-10 (P =0·05), IL-5 (P =0·84) or IL-4 (P =0·28) (Table 2)
Differing numbers of Ig-secreting cells in formula-fed and breast-fed infants
The different levels of cows' milk antigen exposure between the two study groups were well-reflected in the enzyme-linked immunospot assay results for casein-specific Ig. The numbers of casein-specific IgA- and IgG-secreting cells were significantly greater in formula-fed than in breast-fed children at 3 and 6 months of age, but not at a later age (Table 3). In the infants receiving formula, the highest numbers of casein-specific Ig-secreting cells were seen at 3 months, and the total number of secreting cells decreased with age. The opposite was true in the breast-fed group, where low amounts of casein-specific Ig-secreting cells were seen at 3 months, and the number of total secreting cells increased with age so that by age 12 months, no significant difference remained between the groups.
Table 3 Numbers of Ig-secreting (Ig-scr.) cells in formula-fed and breast-fed infants (enzyme-linked immunospot assay) (Geometric means and 95 % confidence intervals)

Discussion
The gold standard and optimum goal of early infant nutrition should be the healthy, breast-fed infant. Defining correct nutrition by detailing the precise composition of what the infant feeds on is an approach that is prone to erroneous conclusions. The composition of breast milk varies from one individual to another, and can also change in each lactating woman due to diet, parity, season and stage of lactation. The biologically active components of breast milk are diverse and show even more variation than the nutritive components. The dynamics of how cytokines and other biologically active breast milk components affect each individual infant are impossible to predict. Thus, we must study and compare the physiology, in the case of the present study, the immune physiology of infants under different modes of feeding in order to assess the nutritive and physiological appropriateness of each diet.
One of the most striking differences between breast-fed and formula-fed infants was evident in the serum concentrations of the TGF-β isoform, TGF-β2, with breast-fed infants exhibiting significantly higher levels of this anti-inflammatory cytokine. TGF-β2 has a wide variety of effects extending from regulation of cell proliferation and differentiation to modulation of immune responses(Reference Li, Wan and Sanjabi32–Reference Nakamura, Kitani and Fuss35). It also appears to contribute to mucosal barrier function by inducing IgA production(Reference Ogawa, Sasahara and Yoshida36). Several studies have indicated the importance of TGF-β2 in the process of tolerisation(Reference Verhasselt, Milcent and Cazareth37, Reference Penttila38). Higher serum concentrations of the potentially pro-inflammatory cytokines TNF-α and IL-2 were seen in formula-fed than in breast-fed infants. TNF-α is a pleiotropic cytokine with wide-ranging pro-inflammatory effects, and its ability to disrupt mucosal barrier function is well-acknowledged(Reference Sanders39, Reference Desjeux and Heyman40). Elevated concentrations of TNF-α prevailed in formula-fed infants throughout the first year of life. The present results thus suggest that early consumption of formula is associated with pro-inflammatory immune responsiveness during the first year of life. In contrast, exclusive breast-feeding promotes an anti-inflammatory cytokine milieu, which is maintained throughout infancy.
There are several possible explanatory models for the observed results. We propose it is likely that the differences seen between breast-fed and formula-fed infants could be due to direct immunoregulatory effects of breast milk TGF-β2, the effects of breast-feeding on infant gut microbiota, functional immune cells and other immunoactive substances contained in breast milk.
It is likely that breast-fed infants have ingested more exogenic TGF-β2 than the infants fed with formula. In a mouse model, enterally given TGF-β emerged as systemic, immunologically active TGF-β in nursing mouse pups(Reference Letterio, Geiser and Kulkarni41). In another animal experiment, enterally given TGF-β was shown to induce endogenous TGF-β production(Reference Ando, Hatsushika and Wako42). We, and others, have shown in previous studies that the concentrations of breast milk cytokines not only mirror the clinical immunological status of the mother, but are also reflected in the cytokine concentrations and clinical manifestations of their child(Reference Jones, Holloway and Popplewell43–Reference Hoppu, Kalliomaki and Laiho45). For example, high breast milk TGF-β2 concentrations are associates with reduced risk of atopic disease in the infant(Reference Kalliomaki, Ouwehand and Arvilommi46). Serum cytokine levels not only reflect the present state of immune activity, but also have the potential to guide the development and polarisation process of various immune cells, yielding long-lasting, perhaps even permanent, effects on the individual's immune function.
Indeed, the elevated serum TGF-β2 in breast-fed infants was seen to persist throughout the first year of life, despite reversion to a similar diet as the formula-fed group due to the consumption of solid foods and cows' milk, as well as the cessation of breast-feeding. In light of the present finding, it seems that the consumption of breast milk may not only have direct immunoregulatory effects, but also the potential to guide the infant's own immune system towards the production of regulatory T cells and healthy immune function.
The persistent anti-inflammatory effects of breast-feeding may also be due to the effects of breast milk on infant gut microbiota. In addition to active microbes(Reference Gueimonde, Laitinen and Salminen47–Reference Abrahamsson, Sinkiewicz and Jakobsson49) and molecules operative in microbial recognition(Reference LeBouder, Rey-Nores and Raby50), breast milk contains an abundance of complex oligosaccharides that the microbial populations in the developing intestine utilise(Reference Chichlowski, German and Lebrilla51–Reference Asakuma, Hatakeyama and Urashima53). These oligosaccharides are believed to facilitate development of a healthy, infant intestinal microbiota rich in bifidobacteria. Recent genomic analyses suggest an intricate co-evolution among the human host, milk glycans and the microbes they enrich, as human milk oligosaccharides appear to very specifically favour colonisation with certain microbial species that have previously been shown to be beneficial to health(Reference Sela, Chapman and Adeuya54), further underlining the unique role of breast milk in the immunological education of the neonate.
The present results showed a similar phenomenon regarding casein-specific Ig-secreting cells, as others focusing on antibody levels have done previously(Reference Kaila, Arvilommi and Soppi55–Reference Jenmalm and Björksten58). The higher levels witnessed in formula-fed infants may simply be a reflection of the higher antigen exposure that the formula-fed infants are subjected to, and, consequently, antibody levels in themselves should not be regarded as an indication of permanently altered immune development. The witnessed enzyme-linked immunospot assay results validate the present study group division and show that formula-fed infants are exposed to foreign antigens at an earlier age and to a greater extent than breast-fed infants. This phenomenon in itself could also contribute to the immunological differences witnessed between the groups, but conclusions on causality cannot be drawn from the present study. It is interesting to note that differences in serum cytokine concentrations outlast the differences witnessed in the numbers of cows' milk-specific Ig-secreting cells, again suggesting a type of immune programming by breast milk.
The breast-fed infants in the present study manifested high levels of TGF-β2 and low levels of pro-inflammatory cytokines throughout the first year of life and exceeding the duration of breast-feeding. Such an immunological environment limits IgE production and hyper-responsiveness and promotes tolerisation, possibly prohibiting the onset of allergic disease. In the pursuit for improved forms of alternative infant nutrition, manufacturers should not necessarily solely endeavour to mimic the composition of breast milk, but rather aim to achieve similar physiological effects in the infant.
Acknowledgements
We would like to thank Mika Korkeamäki and Heikki Arvilommi for their contributions towards the present study. Funding for the present study was received from the Academy of Finland and the Finnish Cultural Foundation Varsinais-Suomi regional fund. The authors are not aware of any conflict of interest. All authors contributed intellectually to the manuscript to the extent that they can defend the contents.








