Introduction
Social cognition encompasses a range of abilities that support interpersonal interactions (Fiske, Reference Fiske1993; Forbes & Grafman, Reference Forbes and Grafman2010), such as emotion recognition, evaluation of relevant social and emotional signals, social and semantic knowledge, moral reasoning, theory of mind and empathy (e.g., Adolphs, Reference Adolphs2009; Beer & Ochsner, Reference Beer and Ochsner2006; Decety & Jackson, Reference Decety and Jackson2004, Reference Decety and Jackson2006; Decety & Lamm, Reference Decety and Lamm2006; Forbes & Grafman, Reference Forbes and Grafman2010). Empathy refers to the ability to understand and respond to the emotional experience of another person (Decety & Jackson, Reference Decety and Jackson2006). Existing literature suggests that empathy can be parsed into two separate components subserved by partially dissociable brain regions: A cognitive component, which involves taking another's perspective; and an affective component, which involves the capacity to experience an affective response towards another person and regulate one's own emotions (e.g., Decety & Jackson, Reference Decety and Jackson2006).
Abnormalities in aspects of social cognition, particularly emotion recognition, have been increasingly recognised in individuals with dementia (Elamin, Pender, Hardiman, & Abrahams, Reference Elamin, Pender, Hardiman and Abrahams2012; Kumfor & Piguet, Reference Kumfor and Piguet2012). Recently, research has also revealed disturbances of empathy in some dementia syndromes, including behavioural-variant frontotemporal dementia and semantic dementia (Dermody et al., Reference Dermody, Wong, Ahmed, Piguet, Hodges and Irish2016; Irish, Hodges, & Piguet, Reference Irish, Hodges and Piguet2014; Oliver et al., Reference Oliver, Mitchell, Dziobek, MacKinley, Coleman, Rankin and Finger2015; Rankin, Kramer, & Miller, Reference Rankin, Kramer and Miller2005). The degree to which empathy is affected in dementia syndromes that present with predominant language impairment, however, has been relatively unexplored.
Primary Progressive Aphasia (PPA) refers to a group of progressive neurodegenerative disorders in which the earliest and primary clinical feature is language impairment (Mesulam, Reference Mesulam2003). Clinically, PPA syndromes are heterogeneous and can be further classified into three subtypes based on clinical presentation, neuroimaging findings and neuropathology: the fluent variant, known as semantic dementia and two non-fluent variants: progressive non-fluent aphasia (PNFA) and logopenic progressive aphasia (LPA) (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011). This study focusses on the non-fluent presentations of PPA.
PNFA is characterised by agrammatism in language production and/or apraxia of speech (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011). Individuals with PNFA show slow and effortful speech. Cognitively, PNFA patients show impairments on tasks of verbal executive functioning (Gorno-Tempini et al., Reference Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamy, Rosen and Miller2004), single word repetition (Leyton et al., Reference Leyton, Savage, Irish, Schubert, Piguet, Ballard and Hodges2014; Piguet, Leyton, Gleeson, Hoon, & Hodges, Reference Piguet, Leyton, Gleeson, Hoon and Hodges2015), digit repetition and letter fluency (Libon et al., Reference Libon, Xie, Wang, Massimo, Moore, Vesely and Grossman2009). Atrophy in PNFA is typically observed in the left posterior frontoinsular regions, including the left inferior frontal gyrus, anterior insula and premotor cortex (Gorno-Tempini et al., Reference Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamy, Rosen and Miller2004; Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011). With disease progression, atrophy extends into left frontal regions and bilateral subcortical areas (Brambati et al., Reference Brambati, Amici, Racine, Neuhaus, Miller, Ogar and Gorno-Tempini2015), with changes in white matter more pronounced in the right hemisphere over time (Lam, Halliday, Irish, Hodges, & Piguet, Reference Lam, Halliday, Irish, Hodges and Piguet2014). Pathologically, PNFA is generally associated with abnormal accumulation of the tau protein (Chare et al., Reference Chare, Hodges, Leyton, McGinley, Tan, Kril and Halliday2014).
Individuals diagnosed with LPA also present with non-fluent speech output. In this syndrome, however, the language profile is characterised by slowed speech output marked with word-finding pauses (Gorno-Tempini et al., Reference Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamy, Rosen and Miller2004). On assessment, LPA patients show impairment in areas such as verbal executive functioning, working memory and visuospatial abilities, together with deficits in word retrieval, sentence repetition and syntactic comprehension (Foxe, Irish, Hodges, & Piguet, Reference Foxe, Irish, Hodges and Piguet2013; Gorno-Tempini et al., Reference Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamy, Rosen and Miller2004; Piguet et al., Reference Piguet, Leyton, Gleeson, Hoon and Hodges2015; Rohrer & Warren, Reference Rohrer and Warren2010). Atrophy in LPA typically involves the left temporoparietal junction (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011) and with disease progression may also include lateral/posterior temporal and medial parietal regions (Brambati et al., Reference Brambati, Amici, Racine, Neuhaus, Miller, Ogar and Gorno-Tempini2015). In contrast to PNFA, LPA is overwhelmingly associated with Alzheimer's disease pathology (Chare et al., Reference Chare, Hodges, Leyton, McGinley, Tan, Kril and Halliday2014; Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011).
Whilst the majority of research in PNFA and LPA has focussed on language impairment, brain regions undergoing atrophy in PNFA and LPA have also been identified as key structures supporting affective and cognitive empathy. The anterior insula cortex, which is affected in PNFA, plays a crucial role in affective components of empathy and emotional experiences via its role in representing and integrating internal body states (e.g., Bernhardt & Singer, Reference Bernhardt and Singer2012; Craig, Reference Craig2009; Ibanez & Manes, Reference Ibanez and Manes2012; Kurth, Zilles, Fox, Laird, & Eickhoff, Reference Kurth, Zilles, Fox, Laird and Eickhoff2010), as well as in representing pain experienced by others (Lamm, Decety, & Singer, Reference Lamm, Decety and Singer2011). In contrast, the temporoparietal junction plays a central role in aspects of cognitive empathy, such as mentalising and perspective taking (Ruby & Decety, Reference Ruby and Decety2004; Samson, Apperly, Chiavarino, & Humphreys, Reference Samson, Apperly, Chiavarino and Humphreys2004; Saxe, Reference Saxe2006). Indeed, impaired cognitive empathy has been associated with atrophy of the temporoparietal junction in patients with Alzheimer's disease (Dermody et al., Reference Dermody, Wong, Ahmed, Piguet, Hodges and Irish2016).
Mounting evidence indicates that aspects of social cognition, such as emotion recognition and interpreting emotional prosody, are compromised in PNFA (Couto et al., Reference Couto, Manes, Montanes, Matallana, Reyes, Velasquez and Ibanez2013; Kumfor, Irish, Hodges, & Piguet, Reference Kumfor, Irish, Hodges and Piguet2013; Kumfor et al., Reference Kumfor, Miller, Lah, Hsieh, Savage, Hodges and Piguet2011; Rohrer, Sauter, Scott, Rossor, & Warren, Reference Rohrer, Sauter, Scott, Rossor and Warren2012), consistent with the frontoinsular involvement in this syndrome. The limited research on empathy in PNFA has yielded mixed findings (Eslinger, Moore, Anderson, & Grossman, Reference Eslinger, Moore, Anderson and Grossman2011; Rankin et al., Reference Rankin, Gorno-Tempini, Allison, Stanley, Glenn, Weiner and Miller2006), although existing studies in this syndrome have been limited by small sample sizes (n < 8) and may have been underpowered to detect empathy changes. In contrast, social cognition deficits appear to be rare in LPA, although few studies have formally investigated the presence of these deficits. For instance, Piguet et al. (Reference Piguet, Leyton, Gleeson, Hoon and Hodges2015) investigated social cognition and episodic memory in PNFA and LPA, and identified emotion recognition deficits in PNFA, whereas LPA showed impaired episodic memory but intact emotion recognition. In contrast, Rohrer et al. (Reference Rohrer, Sauter, Scott, Rossor and Warren2012) observed deficits in emotional prosody in both PNFA and LPA. Here, we aimed to explore empathy profiles in the two non-fluent PPA syndromes: PNFA and LPA. Based on the existing literature and the pattern of brain atrophy observed in these syndromes, we hypothesised that PNFA would show changes predominantly in affective empathy, whereas in LPA, cognitive empathy may be more affected.
Whether the co-occurrence of deficits in aspects of cognition or social cognition contributes to empathic impairments remains unclear. For example, in behavioural-variant frontotemporal dementia, conflicting evidence exists regarding the relationship between executive dysfunction and loss of empathy (Eslinger et al., Reference Eslinger, Moore, Anderson and Grossman2011; but see Lough, Gregory, & Hodges, Reference Lough, Gregory and Hodges2001; Lough et al., Reference Lough, Kipps, Treise, Watson, Blair and Hodges2006). Interestingly, reduced cognitive and affective empathy have been associated with lower fluency as well as worse abstract reasoning abilities, in behavioural-variant frontotemporal dementia and semantic dementia (Rankin et al., Reference Rankin, Kramer and Miller2005). In PNFA and LPA, impairment in areas of language and communication are the primary presenting features. It is not known, however, how language disruption may influence empathy in non-fluent PPA. It is also increasingly recognised that in LPA, other cognitive skills beyond the language domain rapidly decline (Leyton, Hsieh, Mioshi, & Hodges, Reference Leyton, Hsieh, Mioshi and Hodges2013), yet how this decline influences social interactions has not been explored. Thus, a secondary aim of this study was to consider the relationship between loss of empathy, cognition and social cognition in these syndromes. Finally, loss of empathy in other dementia syndromes, such as frontotemporal dementia, has been associated with increased carer burden and loss of care within relationships (Hsieh, Irish, Daveson, Hodges, & Piguet, Reference Hsieh, Irish, Daveson, Hodges and Piguet2013a). Given the role of empathy in interpersonal relationships, we also explored potential associations between empathy and carer burden, with the hypothesis that loss of empathy would result in increased feelings of burden and poorer psychological wellbeing of carers.
Methods
Participants
Twenty-three PNFA and 16 LPA participants were recruited from FRONTIER, the younger onset dementia clinic located in Sydney, Australia. All participants underwent neuropsychological assessment, were assessed by an experienced behavioural neurologist and had an MRI scan. Diagnosis of either PNFA or LPA was reached by the multi-disciplinary team based on the current diagnostic criteria (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa and Grossman2011).
Twenty-four control participants were recruited from the NeuRA volunteer healthy control database for comparison. All controls scored above 88/100 on the Addenbrooke's Cognitive Examination-Revised (ACE-R) (Mioshi, Dawson, Mitchell, Arnold, & Hodges, Reference Mioshi, Dawson, Mitchell, Arnold and Hodges2006) or the Addenbrooke's Cognitive Examination-III (Hsieh, Schubert, Hoon, Mioshi, & Hodges, Reference Hsieh, Schubert, Hoon, Mioshi and Hodges2013b). Patients and controls were excluded based on the following: current or prior history of psychiatric illness; significant head injury; alcohol or substance abuse; presence of another neurological disorder or limited proficiency in English.
Approval for this study was granted by The South Eastern Sydney Local Health District and the University of New South Wales ethics committees. Participants or their Person Responsible provided informed written consent in accordance with the Declaration of Helsinki. Participation was voluntary and participants were reimbursed for travel costs.
Informants
An informant was available for all PNFA and LPA participants. The majority of informants were spouses (30, 76.9%). Others were the patient's child (4, 10.3%), child's spouse (2, 5.1%), patient's friend (2, 5.1%) or the patient's sibling (1, 2.6%). The distribution of informants (spouse vs. others) did not differ between patient groups (χ2 = 1.02, p = .31). The majority of informants were female (24, 61.5%) and informant sex did not differ according to patient diagnosis (χ2 = .60, p = .44).
Materials
Neuropsychological Assessment
The ACE-R (Mioshi et al., Reference Mioshi, Dawson, Mitchell, Arnold and Hodges2006) or ACE-III (Hsieh et al., Reference Hsieh, Schubert, Hoon, Mioshi and Hodges2013b) were administered to assess general cognition. Digit Span (Wechsler, Reference Wechsler1997) and the Trail Making Test (Tombaugh, Reference Tombaugh2004) were used to assess attention and working memory. The Rey Complex Figure (RCF) was used to assess visuoconstructional skills and non-verbal episodic memory (Rey, Reference Rey1941). The Sydney Language Battery (SYDBAT) was used to test naming, word comprehension, semantic association and word repetition (Savage et al., Reference Savage, Hsieh, Leslie, Foxe, Piguet and Hodges2013). Letter fluency was used to assess word generativity (Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen1991). The Emotion Selection Task was employed to assess emotion recognition (Kumfor et al., Reference Kumfor, Sapey-Triomphe, Leyton, Burrell, Hodges and Piguet2014b; Miller et al., Reference Miller, Hsieh, Lah, Savage, Hodges and Piguet2012). In this task, participants view arrays of seven faces of the same person displaying the six basic emotions and a neutral expression. Participants are required to point to the face corresponding to the label spoken by the examiner (e.g., ‘Point to the happy face’), with verbal responses also accepted. Responding for this task is untimed and no feedback is provided. Non-morphed images from the NimStim database were used (www.mac-brain.org), which were cropped to remove non-facial information (i.e., hair) and converted to greyscale (see Kumfor et al., Reference Kumfor, Sapey-Triomphe, Leyton, Burrell, Hodges and Piguet2014b for an example of the stimuli used).
The Frontotemporal dementia Rating Scale (FRS) was used as a dementia staging tool to assess changes in everyday functioning abilities and behaviours (Mioshi, Hsieh, Savage, Hornberger, & Hodges, Reference Mioshi, Hsieh, Savage, Hornberger and Hodges2010). The FRS provides an index of disease severity (very mild, mild, moderate, severe, very severe and profound) and an associated Rasch score. Higher FRS Rasch scores reflect higher functional capabilities.
Measure of Empathy
The Interpersonal Reactivity Index (IRI) was used to investigate aspects of cognitive and affective empathy (Davis, Reference Davis1983). The IRI is a 28-item questionnaire consisting of four seven-item subscales. Perspective Taking explores the capacity to imagine another person's perspective in a situation (e.g., ‘When he/she is upset at someone, he/she usually tries to “put him/herself in their shoes” for a while’). The Fantasy subscale assesses the capacity to identify with characters represented in fictional situations such as films/books (e.g., ‘When he/she watches a good movie, he/she can very easily put him/herself in the place of a leading character’). Empathic Concern measures the capacity to feel warmth, concern or compassion for others (e.g., ‘He/she is often quite touched by things that he/she sees happening’). Personal Distress measures an individual's anxiety and emotional reactivity as a result of observing another's negative experience (e.g., ‘Being in a tense emotional situation scares him/her’). The Perspective Taking and Fantasy subscales measure components of cognitive empathy, whereas Empathic Concern and Personal Distress measure aspects of affective empathy (Davis, Reference Davis1983). For patients, informants completed the modified version (worded in the third-person), and rated how well the statement describes the patient on a five-point scale ranging from 0 (does not describe the patient well) to 4 (describes the patient well). For patients, ratings from informants were based on two time periods: (i) before the illness and (ii) the present time. Both time periods were rated by informants on the same day and asked informants to first consider the patient ‘before illness’ and secondly at ‘present’ for each item. Controls completed a self-rated version of the IRI for the ‘present’ time only.
Cronbach's alpha was calculated for each subscale of the IRI across all groups and showed strong internal consistency, ranging from .71 to .85. Raw scores for each subscale were converted into percentage scores, taking into account missing items on each subscale. Then, difference scores (‘present’ minus ‘before illness’) were calculated for each subscale, to measure relative change following disease onset. To ensure reliability of subscale scores and to avoid potential bias, subscale scores were only included where at least four of the total seven questions of the corresponding subscale were completed (Hsieh et al., Reference Hsieh, Irish, Daveson, Hodges and Piguet2013a). One LPA participant was excluded from the study for this reason. Additionally, one PNFA participant had data available for the Perspective Taking subscale only. Finally, difference scores were not available for three participants (two PNFA, one LPA) due to missing data for the IRI ‘before illness’.
Carer Burden and Wellbeing
The Zarit Burden Interview (ZBI) was used to measure levels of carer burden (Bédard et al., Reference Bédard, Molloy, Squire, Dubois, Lever and O'Donnell2001). This 12-item informant-rated measure is rated on a five-point scale ranging from 0 (never) to 4 (nearly always). Areas assessed include physical health, psychological wellbeing and finances of the carer. The maximum score for the ZBI is 48. Scores ≥ 12 have been shown to indicate high levels of burden (Higginson, Gao, Jackson, Murray, & Harding, Reference Higginson, Gao, Jackson, Murray and Harding2010).
The Intimate Bond Measure (IBM) was used to assess the informant's perceived quality of their relationship with the patient (Wilhelm & Parker, Reference Wilhelm and Parker1988). This 24-item questionnaire generates two separate scores used to assess the patient's level of ‘Care’ and ‘Control’ within the relationship. Items on the IBM are scored on a four-point scale from 0 (not true) to 3 (very true), with a maximum score of 36 calculated for the Care and Control subscales. Higher scores on the Care subscale indicate higher perceived care provided by the patient (i.e., a positive perception). In contrast, higher scores on the Control subscale indicate higher perceived controlling behaviour of the patient (i.e., a negative perception).
The 21-item Depression Anxiety Stress Scale (DASS) was used to assess the informant's current psychological wellbeing (Lovibond & Lovibond, Reference Lovibond and Lovibond1995) and was converted to 42-item DASS scores following completion. In addition, the total of each subscale was combined to give a Total score, with higher scores denoting poorer psychological wellbeing.
Statistical Analyses
Data were analysed using IBM SPSS (Version 23). Categorical variables (e.g., sex) were analysed using chi-square. Demographic variables, neuropsychological tests and emotion recognition assessments were analysed using univariate analyses of variance (ANOVA). Data were normally distributed for all subscales of the IRI, except for Empathic Concern in PNFA patients. Both non-parametric and parametric analyses were conducted for this subscale, which yielded similar results. Thus, parametric analyses are reported throughout. Comparison of present functioning between groups on the IRI subscale percentage scores (Fantasy, Perspective Taking, Empathic Concern, & Personal Distress) were analysed using univariate ANOVA. Sidak post-hoc tests were conducted to investigate differences between groups whilst correcting for multiple comparisons. To compare before illness and present IRI subscale percentage scores within PNFA and LPA, planned paired sampled t tests were employed. Statistical significance was set at p < .05.
Finally, to investigate the relationship between IRI subscale percentage difference scores and measures of cognition, emotion processing and carer burden correlational analyses were conducted. Correlational analyses were restricted to those IRI subscales that significantly changed following disease onset. Due to our a priori hypotheses about the direction of relationships between variables of interest, one-tailed Spearman's rank correlations were employed. In addition, we conducted partial correlations between empathy and carer burden whilst taking into account disease severity. Statistical significance was set at p < .01 for all correlation analyses to account for multiple comparisons.
Results
No significant differences were found between patients and controls for age, sex or education (all p values > .10). Patient groups did not differ significantly in disease duration or functional ability (Table 1).
TABLE 1 Demographics Characteristics of PNFA, LPA and Healthy Controls
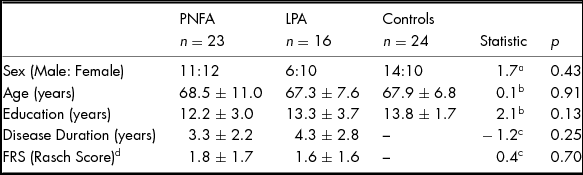
Note: Values are mean ± standard deviation. FRS, Functional Rating Scale. Higher FRS Rasch scores denote higher functioning. aChi-square value. bANOVA F Statistic. c Independent t-test value. d FRS score missing for one PNFA patient.
Neuropsychological Assessment
Performance on standard neuropsychological tests was consistent with the cognitive profiles typically seen in PNFA and LPA (Table 2). In brief, PNFA patients showed worse general cognition compared to controls (ACE: p < .001). Additionally, compared to controls, PNFA showed significant deficits in attention and verbal working memory (Digits-F: p < .001; Digits-B: p < .001). PNFA also showed widespread language dysfunction compared with controls (Letter fluency: p < .001; Naming: p < .001; Semantic: p < .001; Comprehension: p = .001 and Repetition: p < .001). However, PNFA patients demonstrated relatively intact visuospatial abilities (RCF Copy: p = .13) and non-verbal episodic memory (RCF 3-min Recall: p = .12). In terms of emotion recognition ability, PNFA were impaired compared with controls (p = .01).
TABLE 2 Cognitive Performance in PNFA, LPA and Healthy Controls

Note: Values are mean ± standard deviation. Maximum scores are provided in parentheses where applicable. ACE, Addenbrooke's Cognitive Examination; Digits-F, Digit Span Forwards (maximum span); Digits-B, Digit Span Backwards (maximum span); RCF, Rey Complex Figure; SYDBAT, Sydney Language Battery. Missing scores: Digit Span: two PNFA; Trials A: two PNFA; SYDBAT naming: two PNFA; SYDBAT semantic: one PNFA, two control; SYDBAT comprehension: one PNFA, one LPA; SYDBAT repetition: four PNFA; RCF: two PNFA; Letter Fluency: five PNFA, one LPA; Emotion Selection: seven PNFA, three LPA, one Control. Discontinued: Digit Span: one PNFA; Trials B: eight PNFA, four LPA; SYDBAT naming: one PNFA; SYDBAT Semantic: one LPA; RCF Recall: two LPA; Letter Fluency: one LPA.
LPA patients also showed general cognitive impairment compared to controls (ACE: p < .001). On tasks assessing language, LPA were significantly impaired (Letter Fluency: p < .001, Naming: p = .01; Semantic: p = .003; Comprehension: p < .001); however, single-word repetition was similar to controls (p = .12). Performance on tasks assessing verbal attention and working memory was reduced compared to controls (Digits-F: p < .001; Digits-B: p < .001). Visuomotor processing speed and mental flexibility was also significantly impaired in LPA compared to controls (Trails A: p = .006; Trails B: p < .001). Additionally, LPA showed reduced emotion recognition performance compared to controls (p < .001).
Direct comparisons between the patient groups revealed that PNFA patients performed significantly worse on single-word repetition than LPA (p = .01). In contrast, LPA showed worse non-verbal episodic memory (RCF Recall: p = .01) and single-word naming (p = .01) than PNFA. No other significant differences were found between the patient groups across the cognitive or emotion recognition tasks (all p values > .05).
Burden, Relationship Quality and Carer DASS
Levels of carer burden and psychological wellbeing according to diagnosis are provided in Table 3. Whilst no significant differences between PNFA and LPA were observed for perceived carer burden, 36% of carers (nine PNFA; five LPA) experienced high burden (ZBI score >12/48). No significant differences between PNFA and LPA carers were observed for Depression, Anxiety or Stress subscales. Importantly, however, six (five PNFA; one LPA) carers reported moderate to severe depression, three (two PNFA; one LPA) carers reported moderate to severe anxiety and five (three PNFA; two LPA) carers reported moderate to severe stress. Perceived quality of relationship on the IBM Care and Control subscales were not significantly different between PNFA and LPA.
TABLE 3 Carer burden and Psychological Wellbeing in PNFA and LPA Informants
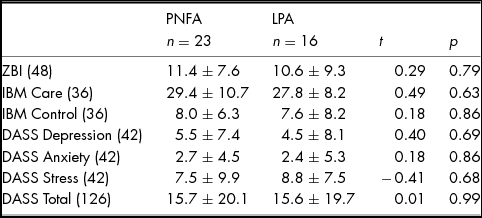
Note: Values are means ± standard deviation. Maximum scores are provided in parentheses. ZBI, Zarit Burden Interview; IBM, Intimate Bond Measure; DASS, Depression, Anxiety and Stress Scale. ZBI: Scores ≥ 12 indicative of high burden. Missing Scores: IBM Care & Control: three PNFA; DASS: two PNFA.
Interpersonal Reactivity Index (IRI)
Present levels of empathy in PNFA and LPA compared with controls are shown in Figure 1. On the Perspective Taking subscale, a significant effect of diagnosis was observed, (F(2, 60) = 3.88; p < .05), with PNFA patients rated lower than controls (p = .05), whereas LPA were rated similarly to controls (p = .14). In addition, a significant effect of diagnosis was observed on the Fantasy subscale (F(2, 59) = 10.20; p < .001), with both PNFA (p < .001) and LPA (p = .02) having lower Fantasy scores than controls. No significant effect of diagnosis was observed for Empathic Concern (F(2, 59) = .10; p = .38) or Personal Distress, (F(2, 59) = 1.83; p = .17).

FIGURE 1 Comparison of IRI subscales relating to present functioning for PNFA, LPA and Control participants. Note: Scores are percentage scores for each subscale. Error bars represent ± standard error of the mean. *: Significantly different from the other groups. Missing scores: Fantasy: one PNFA; Empathic Concern: one PNFA; Personal Distress: one PNFA.
The difference between pre-morbid and present functioning on each of the IRI subscales within PNFA and LPA groups is presented in Figure 2, and pre- and post-illness percentage scores are reported in Table S1. In PNFA, significantly reduced Perspective Taking (t(20) = 2.90, p = .009) and lowered Empathic Concern (t(19) = 2.14, p = .046) at present compared to pre-morbid functioning was identified. Moreover, following disease onset, PNFA patients displayed higher levels of Personal Distress (t(19) = −4.18, p = .001). No significant differences were observed on the Fantasy scale, (t(19) = 1.52, p = .14).

FIGURE 2 Difference scores for the four subscales of the Interpersonal Reactivity Index questionnaire in PNFA (circles) and LPA (squares). Note: Difference scores represent present percentage score minus pre-morbid functioning percentage score. Error bars represent ± standard error of the mean. Dashed line represents no change between present and pre-morbid ratings. * Significantly different from pre-morbid to present ratings at p < .05. Missing Scores: Fantasy: three PNFA, one LPA; Perspective Taking: two PNFA, one LPA Empathic Concern: three PNFA, one LPA; Personal Distress: three PNFA, one LPA.
In LPA, current Perspective Taking capacity was significantly lower than pre-morbid scores (t(14) = 2.36, p = .03). In addition, Personal Distress was higher following disease onset (t(14) = −2.40, p = .03). A trend for decreased Empathic Concern was also observed (t(14) = 1.82, p = .089), with no significant difference on the Fantasy subscale (t(14) = .28, p = .79).
Relationship between Empathy and Cognition, Emotion Recognition and Carer Wellbeing
Correlational analyses were conducted between IRI percentage difference scores and measures of cognition, emotion recognition and carer wellbeing, for the subscales which changed following disease onset (Perspective Taking, Empathic Concern, Personal Distress) (See Table 4). In PNFA, reduced Perspective Taking was associated with worse emotion recognition (Emotion Selection: r = .574, p = .01). In LPA, reduced Perspective Taking was associated with lower visuospatial abilities (RCF copy: r = .605, p = .01) and reduced Empathic Concern was associated with increased carer burden (ZBI: r = −.681, p = .01). No other correlations reached significance following correction for multiple comparisons.
TABLE 4 Correlations Between the Interpersonal Reactivity Index (IRI) Percentage Difference Scores and Cognitive and Carer Wellbeing Measures According to Diagnosis

Note: Values shown in bold are * p < .05; ** p < .01. FRS, Frontotemporal dementia Rating Scale; ACE, Addenbrooke's Cognitive Examination; RCF, Rey Complex Figure; ZBI, Zarit Burden Interview; IBM, Intimate Bond Measure; DASS, Depression, Anxiety and Stress Scale. Missing Scores: PNFA: FRS: 3; ACE: 2; SYDBAT Naming: 5; SYDBAT Repetition: 6; RCF 4; Emotion Selection: 8; ZBI: 2; IBM Care: 5; IBM Control: 5; Carer DASS: 3. Note: Scores on Empathic Concern and Personal Distress: unavailable for one PNFA. LPA: FRS: 1; ACE: 1; SYDBAT 1; RCF: 1; Emotion Selection: 4; ZBI: 1; IBM: 1; Carer DASS: 1.
To ensure that the observed associations with carer burden did not solely reflect increased disease severity, we conducted partial correlations controlling for FRS scores. In PNFA, partial correlations showed a trend between Empathic Concern and carer burden after controlling for disease severity, r = −.43, p = .04. In LPA, the association between Empathic Concern and carer burden remained statistically significant when accounting for disease severity, r = −.71, p = .005.
Discussion
This study investigated the capacity for empathy in the two non-fluent PPA syndromes: PNFA and LPA. Our results revealed subtle alterations in the capacity for empathy across dementia subtypes. Although preliminary, our findings suggested that these changes might reflect distinct cognitive mechanisms. Moreover, our findings shed light on the potential contribution of changes in empathy on carer burden in these syndromes. Here, we discuss how these findings inform our understanding of social cognition profiles in PNFA and LPA.
Empathy Characteristics of PNFA and LPA Patients
In PNFA, we demonstrated significant alterations in the capacity for empathy, confirming that symptoms in PNFA extend beyond the domain of language. As hypothesised, we found reduced affective empathy in PNFA following disease onset, a finding that converges with the emotion recognition deficits and reduced emotional enhancement of memory reported in this syndrome (Kumfor, Hodges, & Piguet, Reference Kumfor, Hodges and Piguet2014a; Kumfor et al., Reference Kumfor, Miller, Lah, Hsieh, Savage, Hodges and Piguet2011; Rohrer et al., Reference Rohrer, Sauter, Scott, Rossor and Warren2012). Contrary to our expectations, we also observed reduced cognitive empathy following disease onset, suggesting a pervasive change in empathic capacity within this syndrome, which has been previously underappreciated.
In LPA, we demonstrated a decline in cognitive empathy following disease onset. Contrary to our predictions, a trend for a decline in affective empathy was also observed, despite other studies reporting relative preservation of social cognition (Piguet et al., Reference Piguet, Leyton, Gleeson, Hoon and Hodges2015; but see Rohrer et al., Reference Rohrer, Sauter, Scott, Rossor and Warren2012). Emerging evidence suggests that LPA patients show a rapid decline in cognition, together with widespread neurodegeneration accompanying disease progression (Leyton et al., Reference Leyton, Hsieh, Mioshi and Hodges2013; Rogalski et al., Reference Rogalski, Cobia, Martersteck, Rademaker, Wieneke, Weintraub and Mesulam2014). Moreover, recent studies suggest that some LPA patients progress more rapidly than others (Leyton et al., Reference Leyton, Hodges, McLean, Kril, Piguet and Ballard2015). It is possible that as brain atrophy becomes more widespread, affective empathy also becomes compromised, a hypothesis that future longitudinal studies should address.
Consistent with our within group analyses, both PNFA and LPA showed reduced cognitive empathy capacity compared to controls. Unlike our within group contrasts, however, PNFA and LPA did not significantly differ from controls in empathic concern or personal distress. Importantly, visual inspection of these subscales suggested this was likely due to insufficient power. Recent studies have demonstrated that controls underestimate themselves on self-rated questionnaires of social functioning (Hutchings, Hodges, Piguet, & Kumfor, Reference Hutchings, Hodges, Piguet and Kumfor2015), which likely influences the ability to capture the magnitude of change in patients versus controls. Future studies should consider the use of informant-rated measures in controls, or objective measures of empathy in both patients and controls (e.g., Baez et al., Reference Baez, Morales, Slachevsky, Torralva, Matus, Manes and Ibanez2016) to address this issue.
Relationship Between Empathy, Cognition and Social Cognition
Our correlational analyses suggested that empathy disruption is associated with decline in some aspects of cognition, which are specific to each syndrome. In LPA, changes in cognitive empathy were associated with visuospatial skills. This association may reflect a common neural substrate of visuospatial skills and cognitive empathy, such as the temporoparietal junction. The temporoparietal junction is commonly implicated in theory of mind and the ability to direct attention to socially relevant information in healthy individuals (Saxe & Kanwisher, Reference Saxe and Kanwisher2003). Of relevance here, the temporoparietal junction is a key region of atrophy in LPA (Leyton et al., Reference Leyton, Hodges, McLean, Kril, Piguet and Ballard2015). Whilst neuroimaging analyses were beyond the scope of this study, this hypothesis will be important for future studies to consider.
Additionally, whilst facial emotion recognition was impaired in both groups, this was associated with aspects of cognitive empathy in PNFA only, with a trend for a similar association for affective empathy. Interestingly, Couto et al. (Reference Couto, Manes, Montanes, Matallana, Reyes, Velasquez and Ibanez2013) reported impaired facial recognition and theory of mind in PNFA, with both abilities associated with the integrity of the insula. The insula plays a key role in both emotion recognition and empathy, representing the interface between the body's internal physiological state and the external conscious affective state (Bernhardt & Singer, Reference Bernhardt and Singer2012; Craig, Reference Craig2009; Ibanez & Manes, Reference Ibanez and Manes2012; Kurth et al., Reference Kurth, Zilles, Fox, Laird and Eickhoff2010). Our results suggest that the association between empathy and emotion recognition in PNFA observed here, reflects the early and ongoing degradation of the insula in these patients. The observed impaired facial emotion recognition in LPA was somewhat unexpected; however, this may reflect the cognitive demands of our task. Unlike other facial emotion recognition tests (e.g., Ekman 60), the Emotion Selection Test employed here, comprises arrays of seven faces and the participant is required to point to the face that matches an aurally presented emotional label. Whilst the current task minimises language demands, it arguably has greater visuospatial scanning and working memory demands than other facial emotion recognition tasks (Miller et al., Reference Miller, Hsieh, Lah, Savage, Hodges and Piguet2012). Future studies will be necessary to determine the conditions under which LPA patients are able to interpret social information, and the extent this capacity declines with disease progression.
In addition to changes in affective and cognitive empathy, we also identified an increase in personal distress in both groups following disease onset. Importantly in both groups, we found no clear association between personal distress and any of the cognitive variables of interest, suggesting that the observed increase in personal distress does not simply reflect changes in language function, as previously suggested (Eslinger et al., Reference Eslinger, Moore, Anderson and Grossman2011). Increased personal distress may reflect the relatively preserved insight in these patients (Banks & Weintraub, Reference Banks and Weintraub2008; Fatemi et al., Reference Fatemi, Boeve, Duffy, Petersen, Knopman, Cejka and Geda2011; Medina & Weintraub, Reference Medina and Weintraub2007), although we did not formally measure insight or awareness of deficits. Future studies investigating the relationship between personal distress, insight and neuropsychiatric symptoms are needed.
Impact of Empathy Decline on Carers
Exploration of the potential impact of empathy decline revealed important associations with carer burden. Reduced affective empathy was associated with increased carer burden in LPA, with a similar trend observed in PNFA. This association remained significant after controlling for disease severity in LPA and approached significance in PNFA. We have previously demonstrated that in PPA, carer burden is closely related to non-language changes, including empathy, autobiographical memory and emotional memory (Hsieh et al., Reference Hsieh, Irish, Daveson, Hodges and Piguet2013a; Kumfor et al., Reference Kumfor, Hodges and Piguet2014a; Kumfor et al., Reference Kumfor, Teo, Miller, Lah, Mioshi, Hodges and Irish2016). This apparent elevation of carer burden in response to the non-language features of PPA may reflect inadequate psychoeducation for carers and family members regarding the evolution of the disorder. Our results lend further support to this hypothesis and suggest that improved carer education regarding non-language features in PPA represents an important management strategy.
The current study represents a novel investigation of empathy in unique patient populations. However, some caveats should be noted. The current study did not directly measure empathy deficits in the patient groups (e.g., using psychophysiological techniques) (e.g., Baez et al., Reference Baez, Morales, Slachevsky, Torralva, Matus, Manes and Ibanez2016; Lamm, Batson, & Decety, Reference Lamm, Batson and Decety2007). Future research should consider concurrent objective and subjective measures of empathy in PNFA and LPA in order to further corroborate our findings. In addition, future neuroimaging studies in PNFA and LPA will help to clarify the mechanisms that give rise to the observed changes in empathy in these syndromes and in turn identify potential intervention strategies to improve socioemotional functioning in these patients.
In summary, this study is the first to explore empathy profiles in both PNFA and LPA, revealing a new dimension to the social cognitive deficits experienced by these patients, beyond the domain of language. Investigation of non-language symptoms in PPA is essential to extend our knowledge of these disease phenotypes and to develop effective interventions to improve quality of life of both patients and carers.
Acknowledgements
The authors are grateful to the participants and their families for supporting our research.
Financial Support
This work was supported in part by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neurone disease, from the National Health and Medical Research Council (NHMRC) of Australia programme grant (APP1037746) and the Australian Research Council (ARC) Centre of Excellence in Cognition and its Disorders Memory Node (CE110001021). MI is supported by an Australian Research Council Discovery Early Career Researcher Award (DE130100463). OP is supported by an NHMRC Senior Research Fellowship (APP1103258). FK is supported by an NHMRC-ARC Dementia Research Development Fellowship (APP1097026).
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








