INTRODUCTION
Despite effective treatment options and intensive control programmes, leprosy is still endemic in several of the poorest areas of the world. Since the route of transmission of Mycobacterium leprae, the causative agent of leprosy, is thought to be mainly airborne from person to person, socioeconomic and culturally defined social interaction patterns are considered to be an important determinant in the continuing transmission of this infectious disease.
Bangladesh is one of the countries where the disease remains endemic. Despite reaching the target of eliminating leprosy as a public health problem, defined as less than one registered case/10 000 inhabitants for the whole country in 1998, the prevalence is still above target in some of the poorest areas of Bangladesh [Reference Withington1, 2]. In the poverty-stricken northwest part of the country, the new case detection rate was still 1·25/10 000 inhabitants in 2008.
Studies in this densely populated area showed that physical distance to a patient and severity of the disease (leprosy classification) are risk factors associated with transmission of M. leprae. The host characteristics ‘blood relationship to the patient’ and ‘age’ are risk factors for the development of clinical signs of disease [Reference Moet3]. A qualitative exploration with focus group discussions revealed that the most intensive social contacts in this area occur within the home and take place across different sex and age groups. Outside the home, interaction patterns are assortative for age and sex. Most women and girls have social contacts limited to their home and nearby neighbourhood, while men and boys also report regular contacts outside their neighbourhood. Adult males have the most intensive social contacts both within and outside their neighbourhood S. G. Feenstra (unpublished observations).
In this study we assessed the association between different social contact patterns and the risk of acquiring clinical leprosy disease in the same leprosy-endemic area in northwest Bangladesh. The objective of the case-control study was to identify social contact patterns that contribute to the transmission of M. leprae, with the aim of improving leprosy control activities as a result of this knowledge.
METHODS
Study area and population
A case-control study was conducted in August 2009 in the districts of Nilphamari and Rangpur in northwest Bangladesh. This large (3951 km2), mainly rural area has about 4·5 million inhabitants and is one of the poorest parts of Bangladesh [4, 5].
The first 110 new leprosy patients registered in 2009 in the study area were selected as cases. The Leprosy Mission International Bangladesh (TLMB) or government primary-care facilities diagnosed the patients according to WHO guidelines [6]. Only one patient per household was interviewed to avoid bias due to clustering. From the initially selected group, 10 people could not been reached, while one was excluded because he was living in the same household as another selected patient.
Controls without leprosy were randomly selected from a referent group representative of the general population in the area. This group was selected by a multi-cluster sampling procedure at the start of the COLEP project, a prospective (sero-)epidemiological study on Contact transmission and chemoprophylaxis in leprosy [Reference Moet7]. The study was initiated in 2001 to generate knowledge about risk factors for leprosy and to assess the effect of new interventions. For the current study, which is part of the COLEP project, 15 people were randomly selected from each of the 20 previously assigned clusters by computerized sampling. The 15 selected candidates of each cluster were numbered 1 to 15. Interviewers started to contact the first person and continued following the numbering until 10 people were interviewed or everyone was contacted. Controls were excluded when they were ever diagnosed as a leprosy patient or if they came from the same household as another participant in the study.
Data collection
Research staff of TLMB undertook home visits to conduct interviews with a pre-tested structured questionnaire (see online Supplementary material). Participants were questioned 6–7 months after they were diagnosed as a leprosy patient on personal data, disease status, living circumstances and economic situation (including assets, educational level and periods of food shortage) and social contacts. Any changes in living circumstances or economic situation due to the disease leprosy were specifically enquired about, while changes in economic situation of the household due to other reasons over the last 3 years were also recorded.
The home of the participant was identified as the most important structure from where social contacts take place in Bangladeshi society [Reference Gardner8, Reference Rozario9]. Therefore, social contacts were assessed on three different levels representing the distance of the contact from the home of the participant:
• Level 1: social contacts that take place inside the home.
• Level 2: social contacts that take place outside the home but within a person's neighbourhood, village or urban ward.
• Level 3: social contacts outside the neighbourhood, ranging from the next village or city to contacts outside the country.
Based on a qualitative exploration with focus group discussions conducted before the start of the study, the most common social contact patterns for each level were pre-listed in the questionnaire. Participants were asked to report the frequency of occurrence of the listed social contact patterns, but could also report contacts not pre-listed. For each contact pattern mentioned, they could report how often they usually had this type of contact. They were asked to keep the last year in mind while reporting. Participants were also asked if leprosy had changed their social contact pattern.
Ethical approval
All participants received verbal information about the study in their own language and were asked to sign a consent form. Ethical approval for this study was obtained from the Bangladesh Medical Research Council (reference: BMRC/NREC/2007-2010/2107).
ANALYSIS
Data from the questionnaires was entered into an Access database. After data cleaning, analysis was performed using the statistical package Stata version 10.0 (StataCorp., USA).
A scoring system for the different contact patterns was developed based on the knowledge that both intensity and the duration of contact with a patient is of influence on transmission [Reference Smieszek10, Reference Mossong11]. The following assumptions were made:
• Contacts inside a room or building were assumed to be more intensive than contacts in an open outside area.
• An overnight stay was assumed to be of longer duration and more intensive than a social contact in a room during daytime.
• Regular short contacts were assumed to be as important for the transmission of disease as a contact of long duration.
Each of the contact patterns in the questionnaire was assigned an intensity score between 1 and 3, based on the findings of the qualitative exploration of social contacts performed in the preparation stage of the study (unpublished observations). This intensity score was multiplied by a frequency score, based on the frequency of occurrence of the particular social contact pattern as reported by the participant (Table 1).
Table 1. Scoring system for social contact patterns in Bangladesh

A total score per social contact level was calculated for each participant by adding the results for each contact pattern within the level concerned. Each participant thus received three final scores; one for each of the social contact levels. The higher the score, the more intensive or frequent contacts the participant reported at that particular level. For the first level, inside the home, a measure of crowding was also included. A value for crowding was calculated by dividing the number of household members by the number of sleeping rooms.
Socioeconomic status of the participants was estimated by an asset index. Factor analysis, principal components factor (PCF), was used to construct an asset index to assign a wealth score to all participants [Reference Filmer and Pritchett12]. Data on ownership of different assets in their household was used to calculate a wealth score by weighing the response for each asset of their household by the coefficient of the first factor as determined by application of the factor analysis (PCF), and summing the results (Supplementary Table S1). The first factor accounted for 19·95% of the variance in the data. The control group was assigned to five wealth quintiles according to their final score. Cases were assigned to these quintiles according to the threshold values set by the control group.
To identify possible confounders on the association between social contacts and leprosy, the mean social contact scores for groups of different socioeconomic background, educational level, age and sex were assessed within the control population. Since the social contact scores were normally distributed, the means for variables with two levels were compared with a t test, while an ANOVA test was used for variables with more than two levels.
Univariate and multivariate logistic regression was used to assess the association between clinical leprosy and social contacts. All potential confounding variables with a P value >0·2 in the univariate analysis, were incorporated in a multivariate model. A backwards elimination procedure (P > 0·1) was performed, in which variables without a significant effect on the odds ratio of the main outcome variables were excluded from the final model since they were not confounders. A likelihood ratio test was performed to test whether the variables had a significant effect.
RESULTS
Initially 99 patients (cases) and 199 controls were included in the study population. A deterioration of social contacts, economic situation or living condition due to the disease was mentioned by nine (8·9%) of the cases. Because the objective of this study was to assess social contact patterns as a risk factor for developing clinical signs of leprosy disease, it was important to establish the situation just before symptoms of the disease became apparent. We therefore excluded for further analysis the nine cases, which mentioned that their situation had changed due to the disease, to avoid confusion about cause and effect. Change in economic situation of the household over the last 3 years due to other reasons was similar for case and control groups (16% experienced deterioration and 22% improvement) and therefore no reason for exclusion.
Of the 90 patients included for analysis, the sex ratio (M/F) was 1·2; 21·1% had the multibacillary (MB) form of the disease, while 6·6% was diagnosed with a grade II disability, according to the WHO classification (Table 2). The proportion of children aged <15 years was 15·6%. At the time of the interview, 58·9% of the cases were still on multidrug therapy (MDT), while the other 41·1% had just completed their therapy and were released from treatment.
Table 2. General characteristics for male and female cases of leprosy in the study population, by age group (n = 90)

Both the case and control populations were distributed randomly throughout the study area. The control group was representative of the general population in the area with regard to the household characteristics of religion, household composition, educational level, and neighbourhood (urban/rural), compared to the national statistics, but males of working age (20–39 years) were slightly underrepresented in the control group [4, 5].
The mean social contact score for leprosy cases was higher than the score for the control group at the first and second levels (Table 3). On the first level, inside the home, both cases and controls had the highest scores. To create a better understanding of social contact patterns in the region and to identify possible confounders on the association between social contacts and leprosy, social contact scores for groups of different socioeconomic background, educational level, age and sex were assessed within the control population (Table 4). By comparing the means with a t test, it was observed that on the first level (in the household) there was a significant difference in mean score by household size, age (adult/child) and educational level (P < 0·05). The mean score was higher for people from large households, aged <20 years and with a higher educational level. Within the neighbourhood (level 2), males and people aged <20 years had a significantly higher mean score than females and older people (P < 0·05). Social contacts outside the neighbourhood were limited and the scores were relatively low. However, the mean score was significantly higher in males compared to females (P < 0·05).
Table 3. Summary of the social contact scores for each distance level for cases and controls
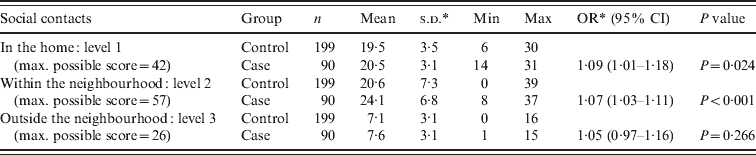
OR, Odds ratio; CI, confidence interval; s.d., standard deviation.
* Univariate logistic regression.
Table 4. Mean social contact scores per level for subgroups of the control population

† Educational level: low, highest educated person in the household had <6 years of schooling; high, highest educated person in the household had ⩾6 years schooling.
‡ There was a recent period of food shortage reported (in the year before the interview).
* t test for the difference between means: P < 0·05.
Leprosy was associated with a higher score for social contacts in the home (OR 1·09, 95% CI 1·00–1·19, P = 0·043) and in the nearby neighbourhood (OR 1·07, 95% CI 1·03–1·11, P = 0·001), even after correction for age in the multivariate analysis (Table 5). The variables sex and socioeconomic status as measured by the wealth index did not change the odds ratio of the main outcome variables in the multivariate analysis, therefore these variables were not confounders and were dropped in the final model. A significant association between leprosy and a period of food shortage in the last year (OR 2·03, 95% CI 1·17–3·52, P = 0·012), the 20–29 years age group (OR 4·07 95% CI 1·33–12·47, P = 0·014) and the >50 years age group (OR 5·17 95% CI 1·56–17·11, P = 0·007) was observed in the final model. We have reported the issue of food shortage in relation to leprosy disease in detail elsewhere [Reference Feenstra13].
Table 5. Results of univariate and multivariate logistic regression analysis with a backwards elimination procedure

OR, Odds ratio; CI, confidence interval; s.d., standard deviation.
* Educational level: low, highest educated person in the household had <6 years of schooling; high: highest educated person in the household had ⩾6 years schooling.
† There was a recent period of food shortage reported (in the year before the interview).
DISCUSSION
Clinical leprosy in the endemic area of northwest Bangladesh is associated with a more intensive social contact pattern within the home and nearby neighbourhood.
The strength of this case-control study is that it takes into account recently diagnosed leprosy cases while patients who reported deterioration in social contacts, living situation or economic status due to their disease were excluded. Since 70% of the participating patients mentioned that their symptoms appeared recently (less than 6 months before the diagnosis) we could assume that the situation around the time of diagnosis represented the situation before any symptoms of disease appeared, allowing assessment of social contact patterns as risk factor for acquiring leprosy disease. Only one patient mentioned improved social contacts due to the disease, therefore this was not used as exclusion criterion for analysis. Positive changes, however, might be underreported and more patients may have improved social lives due to the disease.
We emphasize that we could only study the association between social contact patterns and clinical leprosy disease. Individuals infected with M. leprae without clinical signs of disease are difficult to identify. They do not present themselves at a health facility and there is no reliable test for infection with M. leprae. The average incubation time of leprosy is estimated to be 2–5 years, but it can take 20 years or longer before clinical disease becomes apparent after a person is infected. Changes in social contact patterns are possible during such a long period. However, common alterations due to, e.g. ageing or changing environment are expected to be similar for cases and controls and therefore accounted for by the study design. Such alterations are not expected to be caused or influenced by subclinical infection with M. leprae.
A limitation of the study is the use of self-reported data on social contacts as measured by a questionnaire, which is by definition subjective. Although we tried to compose simple questions with categories of social contacts that are familiar to the people in the study area, there may be differences in interpretation and valuing of social contacts due to the knowledge level of people with different educational background or age. People were asked to report on their regular pattern of social contacts at the time of interview, but recall bias will be of influence on social contacts patterns that do not occur regularly (e.g. only a few times a year). By asking cases and controls exactly the same questions, we attempted to reduce the effect of the above forms of bias. Another possible source of bias was the slight underrepresentation of males of working age (20–39 years) within the control group, because they were not always available during household visits. In the multivariate analysis, age group and sex were taken into account to correct for this underrepresentation.
We developed a scoring system specifically for this study based on a one-time measurement of social contact patterns, because no method was available that could be adapted to our situation. A diary method was used in Europe and Vietnam to study contact patterns relevant for the spread of infectious diseases [Reference Mossong11, Reference Horby14]. However, a diary method requires either registration over a long period or a very large study population. Because leprosy has a relatively low prevalence and keeping a diary for a long time is difficult in a developing country with high levels of illiteracy, using such method was not feasible. An advantage of a newly developed method is that it could be designed for the study area and that intensity as well as duration and frequency of social contacts could be included. A disadvantage is that the results are not completely applicable to other areas and that it is difficult to compare the results of this study with other studies. The validity of the method was assessed by comparing the score results of the control population with the expected pattern of social contacts for the area [Reference Gardner8, Reference Rozario9] (unpublished observations) and by a detailed analysis of the variables within each level (Supplementary Table S2). As expected, social contacts on the first level, inside the home, were the most intensive for both cases and controls in our study, while males had higher scores for social contacts outside the home than females.
Because we used general categories and a simple scoring system, the overall pattern found in this study could be compared with other studies on airborne infectious diseases and social contact patterns. Most of the studies identified were conducted in developed countries with different cultural practices. However, contact profiles and implications for infectious disease transmission of these studies have similarities with our results. In a European study on airborne infectious diseases, households were also identified as an important connective place for people of different age and sex groups [Reference Kretzschmar and Mikolajczyk15]. These authors conclude that households play a bridging role in the transmission of airborne infectious diseases between subgroups. In two other studies social contacts outside the home were found to be highly associated with age and sex [Reference Mossong11, Reference Glass and Glass16]. The conclusion of these studies was that contact patterns were highly assortative for age and sex, which has major implications for disease transmission patterns. In our study we found significant differences in social contact scores for age and sex groups, indicating differences in behaviour between these groups.
Social contact scores outside the home (levels 2 and 3) were significantly higher for males. Since a higher social contact score in the neighbourhood (level 2) was strongly associated with clinical leprosy, we concluded that males have potentially a higher risk of becoming infected with M. leprae due to their social contact patterns.
A higher risk for males in Bangladesh is reflected in the male/female distribution of leprosy in this region, which has always been in favour of males [Reference Richardus17]. The male/female ratio of newly detected cases for the study area was 1·35 in 2008. Similar sex ratios are observed in other Asian countries, but the new case detection rate of leprosy is the same for both sexes in Africa and South America. Although suggested in literature, there is no hard evidence for a biological reason to explain the difference in case detection rate between males and females [Reference Varkevisser18]. Therefore differences in social contact patterns between the sexes in Bangladesh could be an important factor that contributes to the higher risk of males to acquire leprosy in this area.
To measure economic status of households, we used an asset index as proxy measurement of wealth. Although this index measurement is objective, a limitation is that the score of the index depends greatly on the set of assets used [Reference Houweling, Kunst and Mackenbach19, Reference Filmer and Scott20]. We measured socioeconomic status with an asset index similar to the index used in the USAID-sponsored Demographic and Health Survey, carried out in 84 developing countries, because this is a method with proven value for public health purposes [Reference Rutstein and Johnson21]. We used a set of assets based on the local version of the Demographic and Health Survey for Bangladesh. Besides wealth index we also took a recent period of food shortage, educational level and household size into account. Although none of these socioeconomic parameters had a confounding effect on the association between social contacts and leprosy in our analysis, we should point out that measuring the socioeconomic status of households is an issue of debate and controversy and using a different method might yield different results [Reference Falkingham and Namazie22].
Existing control measures are mostly targeted at household contacts of leprosy cases. These interventions are very effective, because household contacts of leprosy patients have the highest risk of being infected and are an easy-to-reach target for disease control measures. However, control measures in an endemic area should not be limited to the households of patients. Social contacts between leprosy patients and susceptible individuals inside their neighbourhood are very important for continuing disease transmission, since these contacts cause infections to spread from household to household over a larger area. We therefore advise extending disease control measures in endemic areas to high-risk groups within the neighbourhood (villages or urban wards) of leprosy patients. Social contact profiles can be used to identify people at risk, while meeting places in the neighbourhood can be used to get in touch with people at high risk.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268812000969.
ACKNOWLEDGEMENTS
We thank the staff of the Rural Health Program of TLMB in Nilphamari for their dedication and hard work in organizing and conducting the interviews. We gratefully acknowledge The Netherlands Leprosy Relief for their financial support of the study.
DECLARATION OF INTEREST
None.







