Introduction
Taenia hydatigena cysticercosis is a parasitic infection of many wild and domestic animals and is considered an important cause of economic and productive losses in the livestock industry, both in developing and industrialized countries (Getaw et al., Reference Getaw, Beyene, Ayana, Megersa and Abunna2010; Nguyen et al., Reference Nguyen, Gabriël, Abatih and Dorny2016). In Europe, T. hydatigena is a widespread cestode which develops in definitive [i.e. dogs and other carnivores such as foxes (Vulpes vulpes), wolves (Canis lupus), jackals (Canis aureus), European lynx (Lynx lynx), raccoons (Procyon lotor), bears (Ursus arctos) and cats] as well as in many species of intermediate hosts [i.e. pigs, sheep, goats, cattle, buffaloes, wild boars (Sus scrofa), fallow deer (Dama dama), red deer (Cervus elaphus), roe deer (Capreleous capreleous) and moose (Alces alces)] (Chapman and Chapman, Reference Chapman and Chapman1987; Nguyen et al., Reference Nguyen, Gabriël, Abatih and Dorny2016; Filip et al., Reference Filip, Pyziel, Jezewski, Myczka, Wiaczesław Demiaszkiewicz and Laskowski2019). The larval stage (metacestode), formerly known as ‘Cysticercus tenuicollis’, localizes in organs in the abdominal cavity, as well as in the lungs of intermediate hosts. Gravid proglottids containing eggs are excreted in the feces of the definitive host contaminating the environment; when eggs are ingested during grazing by intermediate hosts, the oncospheres migrate via the bloodstream to reach the liver and other organs (Deplazes et al., Reference Deplazes, Eckert, Mathis, von Samson-Himmelstjerna and Zahner2016). In the intermediate hosts, the localization of cysticerci involves the subserous tissue of the abdominal and thoracic cavities; most frequently, they are found on the omentum, mesentery, visceral and parietal peritoneum and, less frequently, on pleura, pericardium and peritoneal ligaments (Scala et al., Reference Scala, Pipia, Dore, Sanna, Tamponi, Marrosu, Bandino, Carmona, Boufana and Varcasia2015). Cysticerci are able to migrate through the liver parenchyma causing traumatic hepatitis cysticercosis (Blažek et al., Reference Blažek, Schramlová and Hulínská1985). In domestic intermediate hosts, cysticercosis may cause production losses due to clinical disease and/or condemnation of organs and offal and high mortality rates (i.e. 19.0% in lambs) due to massive hepatic and pulmonary infections (Scala et al., Reference Scala, Urrai, Varcasia, Nicolussi, Mulas, Goddi, Pipia, Sanna, Genchi and Bandino2016). The risk factors involved in the occurrence of metacestodosis include access of stray dogs to sheep pastures, extensive grazing methods, home slaughtering practices and improper disposal of offal; these factors may also favour the spread of cysticercosis and other important metacestodoses, such as coenurosis and cystic echinococcosis, not only in domestic animals (Varcasia et al., Reference Varcasia, Tosciri, Coccone, Pipia, Garippa, Scala, Damien, Vural, Gauci and Lightowlers2009, Reference Varcasia, Tanda, Giobbe, Solinas, Pipia, Malgor, Carmona, Garippa and Scala2011; Scala et al., Reference Scala, Urrai, Varcasia, Nicolussi, Mulas, Goddi, Pipia, Sanna, Genchi and Bandino2016) but also in wildlife species, such as red foxes and wild boar (Varcasia et al., Reference Varcasia, Tamponi, Tosciri, Pipia, Dore, Schuster, Kandil, Manunta and Scala2015; Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Pacifico, Buono, Neola, Fusco, Santoro, Toscano, Fioretti and Veneziano2019a, Reference Sgroi, Varcasia, Dessì, D'Alessio, Tamponi, Saarma, Laurimäe, Kinkar, Santoro, Caputo, Sarnelli, Fusco, Varuzza, Fioretti, Scala and Venezianob).
In recent years, wild boar populations have increased in many European countries, including Italy (Pittiglio et al., Reference Pittiglio, Khomenko and Beltran-Alcrudo2018) and this ungulate may act as a reservoir of several diseases, both to domestic and wild animals (Meng et al., Reference Meng, Lindsay and Sriranganathan2009). The role of wild boar as an intermediate host of T. hydatigena was previously reported in Italy (Di Nicola et al., Reference Di Nicola, Scacchia and Marruchella2015; Paoletti et al., Reference Paoletti, Della Salda, Di Cesare, Iorio, Vergara, Fava, Olivastri, Dessì, Scala and Varcasia2019), as well as from some European countries, such as Croatia (Rajković-Janje et al., Reference Rajković-Janje, Bosnic, Rimac, Dragičevic and Vinkovic2002), Estonia (Jarvis et al., Reference Jarvis, Kapel, Moks, Talvik and Magi2007) and Spain (de la Muela et al., Reference de la Muela, Hernàndez-de-Lujàn and Ferre2001). However, these studies were performed using a small number of animals (<100 individuals) and European epidemiological data on the distribution of the metacestode of T. hydatigena remain scant. Several studies have reported significant molecular variability in the ND1 and cox1 nucleotide sequences of T. hydatigena from several host species (Rostami et al., Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2013; Boufana et al., Reference Boufana, Scala, Lahmarc, Pointingd, Craig, Dessì, Zidda, Pipia and Varcasia2015), however, data concerning genetic population structure of T. hydatigena from wild boar are limited. This study investigated the prevalence, distribution, genetic variation and population structure of T. hydatigena cysticercosis in free-ranging wild boar from southern Italy.
Materials and methods
Sample size calculation
A sample size of 3321 wild boar was calculated using the open-source software OpenEpi (Dean et al., Reference Dean, Sullivan and Soe2003), inserting the following information: study population (84 000 wild boar; data supplied by Piano Emergenza Cinghiale in Campania – PECC 2016–2019), expected prevalence of T. hydatigena infection of 5–15% (Di Nicola et al., Reference Di Nicola, Scacchia and Marruchella2015), confidence interval (95%) and desired absolute precision (1%).
Study area and sampling
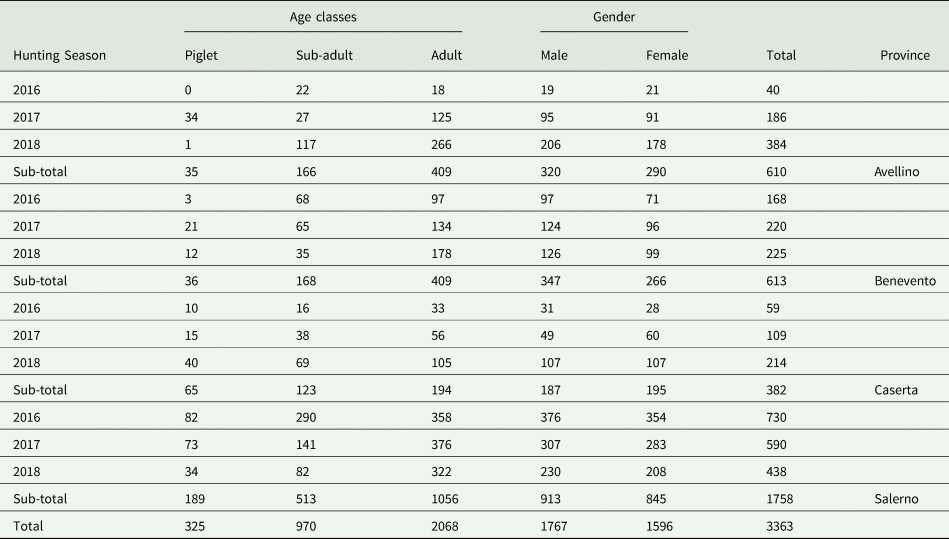
This survey was conducted in four different provinces (Avellino, Benevento, Caserta and Salerno) of the Campania region, southern Italy (total surface 123 417 hectares). Wild boar examined in the present study (n = 3363) originated from three hunting seasons (2016–2018) and were classified according to the season, age class, gender and provinces (Table 1). The examination of the carcasses was performed by 20 veterinarians specialized in meat inspection and involved in the field activities of the regional project ‘Piano Emergenza Cinghiali in Campania – PECC 2016–2019’. The age of the animals was estimated by the examination of the teeth, according to Massei and Toso (Reference Massei and Toso1993). Organs and viscera from abdominal and thoracic cavities were removed from the carcasses and delivered for parasitological examination to the Department of Veterinary Medicine and Animal Productions, University of Naples, Italy.
Table 1. Wild boar (n = 3363) examined in Campania region, southern Italy
Morphological analysis
At post-mortem, the viscera were examined by visual inspection, palpation and serial cuts to investigate the presence of T. hydatigena cysts or necrotic-haemorrhagic tracks of the migrating parasites. Number and localization of the cysts were assessed and cysticerci, in various developmental stages, were removed for microscopical identification, which was performed using keys reported by Rostami et al. (Reference Rostami, Salavati, Beech, Babaei, Sharbatkhori, Baneshi, Hajialilo, Shad and Harandi2013). Massive infections were defined when more than 10 cysts were found in a single animal. In order to confirm the morphological examination, cysticerci were stored at −20°C for subsequent molecular analysis at the Department of Veterinary Medicine, University of Sassari, Italy.
Polymerase chain reaction and sequencing
DNA was extracted from individual cysts using NucleoSpin Tissue (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany) prior to polymerase chain reaction (PCR) and DNA sequencing. Primers JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4.5 (5′-AAAGAAAGAACATAATGAAAATG-3′) were used to amplify a nucleotide fragment (approximately 391 bp in length) within the cytochrome c oxidase subunit 1 (cox1) (Bowles et al., Reference Bowles, Blair and McManus1992). PCR products (amplicons) were purified using a Nucleospin Gel, PCR Cleaned (Macherey-Nagel GmbH & Co. KG, Düren, North Rhine-Westphalia, Germany) and commercially sequenced (Eurofins Genomics, Germany). Generated sequences were edited in MEGA X (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) and compared with those on the NCBI database (https://www.ncbi.nlm.nih.gov/BLAST/).
Data analysis
Data analysis using 386 bp of the cox1 nucleotide sequences amplified in this study was accomplished as previously described (Boufana et al., Reference Boufana, Scala, Lahmarc, Pointingd, Craig, Dessì, Zidda, Pipia and Varcasia2015). In brief, Proseq 3.5 (Filatov, Reference Filatov2002) was used to align and trim sequences and the generated alignment was checked in MEGA X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018) to determine correct reading frames. The flatworm mitochondrial code (Nakao et al., Reference Nakao, Sako, Yokoyama, Fukunaga and Ito2000) was used to infer amino acid sequences. Nucleotide sequences were then aligned in ClustalX2 (Larkin et al., Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007) and transported into DnaSP6 (Rozas et al., Reference Rozas, Ferrer-Mata, Sánchez-DelBarrio, Guirao-Rico, Librado, Ramos-Onsins and Sánchez-Gracia2017) where data on DNA diversity and polymorphism was retrieved. Hapview (Salzburger et al., Reference Salzburger, Ewing and Haeseler2011) was used to generate haplotype networks and determine genealogies. Genetic diversity was accessed using Arelquin (Excoffier and Lischer, Reference Excoffier and Lischer2010) and included the computation of haplotype and nucleotide diversity as well as the evaluation of demographic events such as population expansion and bottleneck using Fu's Fs (Fu, Reference Fu1997) and Tajima's D (Tajima, Reference Tajima1989). The model of evolution was determined using Modeltest 3.7 (Posada and Crandall, Reference Posada and Crandall1998) executed in Paup 4 (Swofford, Reference Swofford2002).
Statistical analysis
A Chi-squared test was used to assess the differences in parasite prevalence and variables analysed (hunting season, age class, gender, provinces) as well as the localization of cysticerci. A value of P < 0.05 was considered significant. Abundance (number of cysts/animal examined) and intensity (number of cysts/positive animals) was also determined. The distribution of positive animals associated with the administrative boundaries of municipalities, provinces and national parks was determined using ArcGIS (version 10.3, ESRI, Redlands, CA, USA). A choropleth map with proportioned circles was designed displaying the following information: province borders, national park, hunting areas, municipalities investigated (positive/negative) and number of positive animals for each municipality.
Results
A total of 301 T. hydatigena metacestodes were found in 229 (6.8%) of the 3363 wild boar examined; the majority of animals (n = 188; 82.1%) were parasitized with a single cyst; the remaining (n = 40) with multiple and a single animal harboured 10 cysts (abundance, 0.09; intensity, 1.3). All cysticerci appeared with their characteristic bottleneck morphology (Fig. 1). Cyst localization among organs and viscera examined was statistically significant (χ 2 = 755.81; P < 0.05); this shows that of the 229 infected pigs, most (187; 81.7%) had cysticerci in the livers. Multiple cyst localization was also observed in 10 animals (4.4% of infected boars). Data on cyst localization are reported in Table 2.
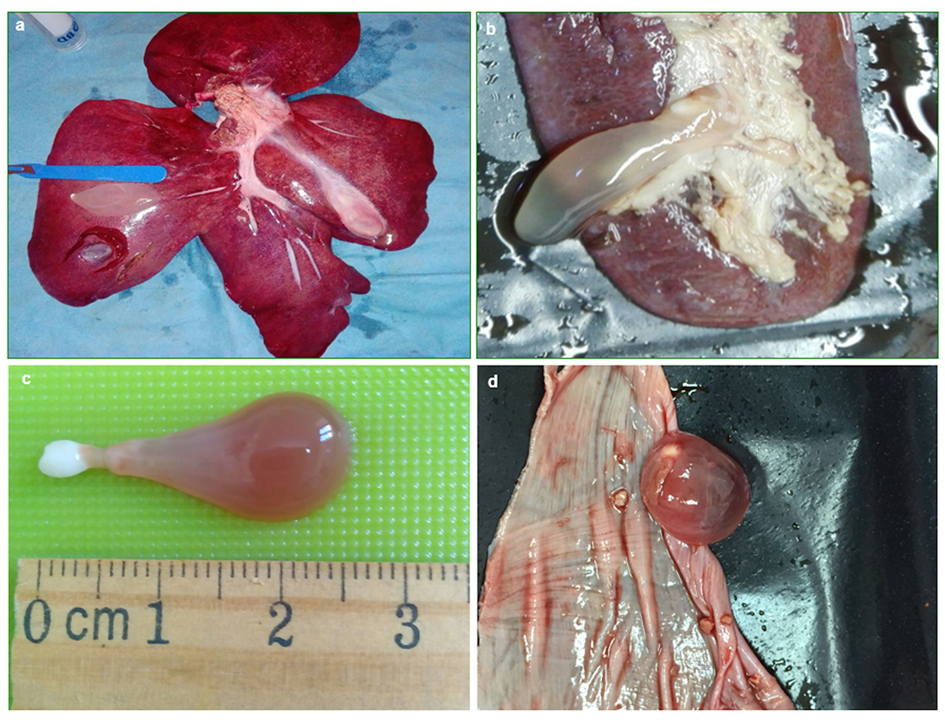
Fig. 1. Cysticerci of Taenia hydatigena found in (a) liver, (b) spleen, (c) isolated cyst, (d) tendinous centre of the diaphragm.
Table 2. Number of wild boar positive to Taenia hydatigena metacestodes according to the organ localization

Details regarding the prevalence of cysticercosis according to hunting season, age class, gender and province are summarized in Table 3. No significant statistical difference (χ 2 = 3.81; P = 0.15) in T. hydatigena infection among the three hunting seasons was found. The age of positive boars ranged from 1 to 9 years (average number 1.3) with the highest prevalence found in adult animals (9.1%), followed by sub-adults (4.2%); no positive piglets were reported (χ 2 = 50.9; P < 0.05). The prevalence of infection detected in males and females was 6.8% (χ 2 = 0.00; P = 0.96). The positivity observed for the study area revealed different scenarios depending on the province considered (χ 2 = 16.17; P < 0.05), with a higher exposure reported for Avellino and Salerno provinces, close to the Cilento National Park. The number and geographical distribution of positive wild boar are shown in Fig. 2.

Fig. 2. Map showing the location of wild boar infected with metacestodes of Taenia hydatigena from different provinces (AV, Avellino; BN, Benevento; CE, Caserta; SA, Salerno; NA, Napoli) and hunting areas in Campania region.
Table 3. Number (percentage) of wild boar positive to Taenia hydatigena metacestodes, according to hunting season, age, gender and province
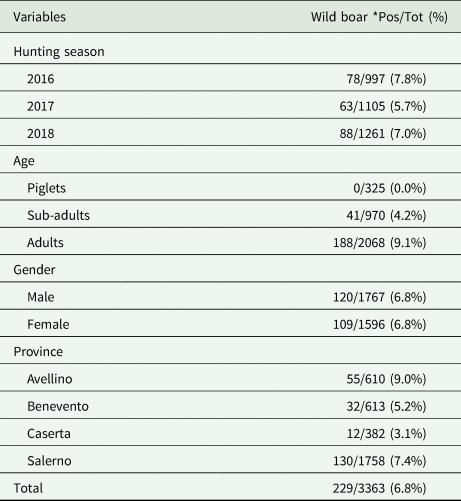
*Pos/Tot = number and percentage of positive samples out of the total examined.
A blast search of the amplified DNA sequences confirmed that wild boar was infected with T. hydatigena. These nucleotide sequences gave a 99% identity with T. hydatigena cox1 sequence from GenBank (e.g. accession number: AB792721). We were able to successfully amplify and sequence DNA from 52 metacestodes spread across the studied area. A total of 20 variable polymorphic sites were detected within the analysed sequences of which 11 were parsimony informative. The generated haplotype network (Fig. 3) consisted of 21 haplotypes separated from each other by 1–4 mutational steps indicating their genetic relatedness, with a main centrally positioned haplotype (Hap 8) that encompassed a quarter (i.e. 13/52–25%) of the isolates. The majority of the haplotypes (57%) had a single T. hydatigena isolate (haplotypes 1, 5–7, 10, 12–13, 17–21). The remaining haplotypes were represented by 2 (haplotypes 14–16), 3 haplotypes (3, 9, 11), 5 (haplotype 4) and 7 (haplotype 2) isolates. Blast search of isolate sequences within Hap 8, showed a 100% identity with haplotype SR07 (accession number: KT372522) that occupied the central haplotype in a study on sheep T. hydatigena from Sardinia (Boufana et al., Reference Boufana, Scala, Lahmarc, Pointingd, Craig, Dessì, Zidda, Pipia and Varcasia2015). The high haplotype diversity for T. hydatigena from wild boar in this study together with the low nucleotide diversity is indicative of rapid demographic expansion (Table 4). Tajima's D was negative indicating an excess of rare polymorphic sites, a feature of recent population expansion. The significantly negative Fu's Fs indicated the presence of rare haplotypes compared to what is expected under neutrality and points to past bottleneck and/or purifying selection events.

Fig. 3. Frequencies of mitochondrial cytochrome c oxidase subunit 1 haplotypes of Taenia hydatigena metacestodes (n = 52) derived from wild boar, southern Italy. Circle size is proportional to haplotype frequency.
Table 4. Diversity and neutrality indices using nucleotide data of the cytochrome c oxidase subunit 1 (cox1) (386 base pairs) mitochondrial gene for Taenia hydatigena metacestdoes removed from wild boar, southern Italy

hn, number of haplotypes; hd, haplotype diversity; πd, nucleotide diversity; s.d., standard deviation.
*Significant at P value ⩽0.001.
Discussion
To the best of our knowledge, this is the first European large-scale survey providing epidemiological and molecular data on T. hydatigena cysticercosis in wild boar populations. The prevalence of infection recorded in this study (6.8%) is consistent with that previously reported from central Italy which ranged from 2.9% (Paoletti et al., Reference Paoletti, Della Salda, Di Cesare, Iorio, Vergara, Fava, Olivastri, Dessì, Scala and Varcasia2019) to 15.0% (Di Nicola et al., Reference Di Nicola, Scacchia and Marruchella2015). Similar prevalence was also detected in Spain (de la Muela et al., Reference de la Muela, Hernàndez-de-Lujàn and Ferre2001) and Croatia (Rajković-Janje et al., Reference Rajković-Janje, Bosnic, Rimac, Dragičevic and Vinkovic2002) with a higher infection rate in wild boar from Estonia (20.0% – Jarvis et al., Reference Jarvis, Kapel, Moks, Talvik and Magi2007). Among the three hunting seasons monitored, the difference in prevalence was not statistically significant (χ 2 = 3.81; P = 0.15), suggesting that infection remained constant in the studied wild boar population, due to the persistence of risk factors (i.e. the improper disposal of boar offal to dogs).
Adult boars showed higher exposure to infection (9.1% – χ 2 = 50.9; P = <0.05) compared to sub-adults (4.7%), and there were no cases in piglets, suggesting that older animals are significantly more likely to ingest parasite eggs (ie: through coprophagy), and their infection intensity may be a reflection of accumulation of parasites over the animals' lifespan. Indeed, this is consistent with observations from the same study area for another metacestodosis (cystic echinococcosis) in wild boar (Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Tamponi, Saarma, Laurimäe, Kinkar, Santoro, Caputo, Sarnelli, Fusco, Varuzza, Fioretti, Scala and Veneziano2019b), as well as in domestic ruminants (Veneziano et al., Reference Veneziano, Rinaldi, Apicella, Garippa and Cringoli2004).
The higher prevalence of infection of T. hydatigena observed in Avellino and Salerno provinces (χ 2 = 16.17; P < 0.05) is probably due to the larger number of dogs enrolled in hunting teams (Varuzza et al., Reference Varuzza, Sgroi, D'Alessio, Neola, Argenio, Caputo, Toscano, Veneziano and Fioretti2019), feeding on raw boar organs and viscera, which may thus increase the parasite transmission. This would suggest a possible relationship between the prevalence of cysticercosis and the number of hunting dogs in a given hunting area. However, some aspects (i.e. number of hunters and their dogs according to the different provinces) which likely affect the circulation of the parasite were not investigated in this study. It should be emphasized that, in rural environments, T. hydatigena infection is historically related to a domestic life cycle, in which farmers fed their dogs with sheep and goat offal (Varcasia et al., Reference Varcasia, Tanda, Giobbe, Solinas, Pipia, Malgor, Carmona, Garippa and Scala2011). Although this habit is decreasing due to the improvement of small ruminant's health management, hunters frequently give raw organs and viscera of hunted animals (mainly wild boar) as a reward to their hunting dogs (Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Tamponi, Saarma, Laurimäe, Kinkar, Santoro, Caputo, Sarnelli, Fusco, Varuzza, Fioretti, Scala and Veneziano2019b). In this scenario, hunters could replace the role previously played by shepherds, allowing the perpetuation of a T. hydatigena semi-domestic life cycle. In addition, the deworming of shepherd/hunting dogs using inadequate drugs, such as extra-label ivermectin is often ineffective against tapeworms (Varcasia et al., Reference Varcasia, Tanda, Giobbe, Solinas, Pipia, Malgor, Carmona, Garippa and Scala2011; Piantedosi et al., Reference Piantedosi, Neola, D'Alessio, Di Prisco, Santoro, Pacifico, Sgroi, Auletta, Buch, Chandrashekar, Breitschwerdt and Veneziano2017) thus favouring the persistence of T. hydatigena infection in dogs. This underlines the importance of informing hunters on hunting hygiene and hunting dog healthcare, including the use of targeted anthelmintic treatments with high efficacy, to avoid the dissemination of parasites, which have an economic impact on farmers and pose a health risk for animals and humans. Therefore, the role of hunters as important players in the management of wildlife species to control and prevent the circulation of infectious and parasitic diseases should be reviewed. Considering over 12 000 wild boars culled per year in the Campania region and 4960 dogs employed in the entire studied area during the hunting season (Veneziano, personal communication), strategic game waste management is a crucial aspect to reduce the spread of meat/offal-borne diseases, including metacestodoses.
In this study, a high prevalence of T. hydatigena metacestodes was recorded in close proximity to the Cilento National Park (i.e. in areas included in the administrative boundaries of Avellino and Salerno provinces). This finding may be due to the widespread distribution of wild carnivores, mainly wolves, in this protected area (Fulgione and Pellegrino, Reference Fulgione and Pellegrino2017). Indeed, in Europe, wolf population growth and its territorial expansion are strictly related to wild boar abundance (Chapron et al., Reference Chapron, Kaczensky, Linnel, Von Arx, Huber, Andrèn, Lòpez-Bao, Adamec, Alvares, Anders, Balčiauskas, Balys, Bedö, Bego, Blanco, Breitenmoser, Brøseth, Bufka, Bunikyte, Ciucci, Dutsov, Engleder, Fuxjäger, Groff, Holmala, Hoxha, Iliopoulos, Ionescu, Jeremić, Jerina, Kluth, Knauer, Kojola, Kos, Krofel, Kubala, Kunovac, Kusak, Kutal, Liberg, Majić, Männil, Mertzanis, Myslayek, Nowak, Odden, Ozolins, Palomero, Paunović, Persson, Potočnik, Quenette, Rauer, Reinhardt, Rigg, Ryser, Salvatori, Skrbinšek, Stojanov, Swenson, Szemethy, Trajçe, Tsingarska- Sedefcheva, Váňa, Veeroja, Wabakken, Wölfl, Wölfl, Zimmermann, Zlatanova and Boitani2014; Galaverni et al., Reference Galaverni, Caniglia, Fabbri, Milanesi and Randi2015). For instance, in protected areas of northern Italy, the presence of boar undigested prey in wolf feces is commonly reported (Meriggi et al., Reference Meriggi, Brangi, Schenone, Signorelli and Milanesi2011), as well as a high copro-molecular prevalence (40.7%) of T. hydatigena (Poglayen et al., Reference Poglayen, Gori, Morandi, Galuppi, Fabbri, Caniglia, Milanesi, Galaverni, Randi, Marchesi and Deplazes2017). Therefore, these findings suggest a crucial role of the wolf in the Taeniidae sylvatic life cycle, as previously shown for Echinococcus granulosus sensu lato (Gori et al., Reference Gori, Armua-Fernandez, Milanesi, Serafini, Magi, Deplazes and Macchioni2015). However, in recent years, rural landscapes were transformed into peri-urban areas, which are extremely attractive as a food source for foxes (Mackenstedt et al., Reference Mackenstedt, Jenkins and Romig2015). Moreover, due to its wide distribution range and huge population size, this canid is considered the most common wild carnivore in Europe (Scott et al., Reference Scott, Berg, Tolhurst, Chauvenet, Smith, Neaves, Lochhead and Baker2014) and constitutes the linkage species between sylvatic and anthropic environments (Plummer et al., Reference Plummer, Davison and Saarma2014). Although foxes are considered reservoirs of several parasites (i.e. Echinococcus multilocularis, Taenia multiceps, Angiostrongylus vasorum and Trichinella britovi) that can infect animals and humans (Otranto et al., Reference Otranto, Cantacessi, Dantas-Torres, Brianti, Pfeffer, Genchi, Guberti, Capelli and Deplazes2015), a very low prevalence (<5%) of T. hydatigena was reported in this species in Europe (Richards et al., Reference Richards, Harris and Lewis1995; Shimalov and Shimalov, Reference Shimalov and Shimalov2003; Saeed et al., Reference Saeed, Maddox-Hyttel, Monrad and Kapel2006) and Italy (Guberti and Poglayen, Reference Guberti and Poglayen1991; Di Cerbo et al., Reference Di Cerbo, Manfredi, Trevisiol, Bregoli, Ferrari, Pirinesi and Bazzoli2008; Fiocchi et al., Reference Fiocchi, Gustinelli, Gelmini, Rugna, Renzi, Fontana and Poglayen2016).
In this study, massive T. hydatigena infections were uncommon in the animals examined, with only a single case reported. This finding is similar to a previous study when up to 265 cysts were found in a single boar from the studied area (Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Pacifico, Buono, Neola, Fusco, Santoro, Toscano, Fioretti and Veneziano2019a). The above case could be due to the fact that the animal was used by hunters as a captive boar to train hunting dogs which could potentially shed in their feces a high amount of T. hydatigena eggs, contaminating the soil in a delimited enclosure, therefore perpetuating the massive infection (Sgroi et al., Reference Sgroi, Varcasia, Dessì, D'Alessio, Pacifico, Buono, Neola, Fusco, Santoro, Toscano, Fioretti and Veneziano2019a). Regarding cyst localization, most of the positive boars showed cysticerci in the liver, as previously reported (Paoletti et al., Reference Paoletti, Della Salda, Di Cesare, Iorio, Vergara, Fava, Olivastri, Dessì, Scala and Varcasia2019). Although the intensity of infection (1.3) observed in this study was low, the epidemiological role of wild boar in the spread of cysticercosis is not negligible, considering the high amount of offal available in the environment for wild and domestic carnivores during the hunting activity.
Finally, the existence of a common lineage for T. hydatigena was further confirmed in this study through the presence of a common central haplotype (Hap 8) shared with sheep, goat, pig, dog (data not shown) and is consistent with that previously described for T. hydatigena (Boufana et al., Reference Boufana, Scala, Lahmarc, Pointingd, Craig, Dessì, Zidda, Pipia and Varcasia2015). Further studies are required using a larger number of isolates, additional mitochondrial genes and longer nucleotide sequences (Boufana et al., Reference Boufana, Scala, Lahmarc, Pointingd, Craig, Dessì, Zidda, Pipia and Varcasia2015).
Concluding remarks
This survey revealed that wild boar may play a role as one of the main intermediate hosts in a semi-domestic life cycle of T. hydatigena cysticercosis in southern Italy. Further studies are required to highlight the role of hunting dogs, wolves and foxes in the circulation of T. hydatigena to better define parasite control strategies. Therefore, based on current results, hunters, as opposed to shepherds may be responsible for the spread of T. hydatigena cysticercosis, particularly in the light of the improvement in small ruminants' practices. Thus, in rural areas, the role of hunters should be reviewed as a prospective sanitary player to avoid the spread of the parasite, being responsible for hunting hygiene, public health and hunting dog healthcare.
Acknowledgements
The authors wish to thank Dr Giorgia Dessì and Dr Claudia Tamponi (University of Sassari, Italy) for technical assistance with molecular procedures and statistical analysis. The authors are grateful to all veterinarians involved in the project for their kind collaboration in carcasses inspection and sample collection: Antonio Apuzzo, Domenico Benedetto, Francesco Celano, Luca De Blasio, Lucio De Maria, Francesco Di Domenico, Vittorio Di Matteo, Simone Iovino, Piero Matonte, Giuseppe Mollo, Antonio Parisi, Simone Palmieri, Boris Pietraggi, Domenico Rufrano, Pasquale Russo, Valerio Toscano, Andrea Santillo, Giampiero Sepe, Pasquale Silvestri and Alessio Vitale.
Financial support
The study was supported by a grant from the Regione Campania UOD Prevenzione e Sanità Pubblica Veterinaria, Wild Boar Emergency Plan in Campania – 2016–2019” (PECC 2016–2019), and by grants from the Ministry of Health of the Italian Republic (IZS ME 04/18 RC and 05/19 RC).
Conflict of interest
None.
Ethical standards
Not applicable.










