Dietary factors have been shown to contribute to the development of glucose intolerance. Foods with high dietary glycaemic index and load, low dietary fibre, carbohydrate-rich foods(Reference Villegas, Liu and Gao1, Reference Sluijs, van der Schouw and van der A2), excessive energy intake(Reference Wang, Luben and Khaw3) and high fat intake (particularly saturated fats)(Reference Thanopoulou, Karamanos and Angelico4) may predispose to glucose intolerance. On the other hand, high intakes of fish(Reference Patel, Sharp and Luben5), potato, vegetables, legumes and vitamin C(Reference Carter, Gray and Troughton6) are inversely associated with the development of type 2 diabetes. However, these individual nutrients or foods alone probably explain only a small part of the dietary effect on glucose metabolism.
Recently, dietary pattern analysis has emerged as an alternative and complementary approach to examining the relationship between diet and the risk of chronic diseases(Reference Hu7). Several studies have examined dietary patterns and diabetes incidence(Reference van Dam, Rimm and Willett8–Reference Erber, Hopping and Grandinetti10). However, these studies were conducted in Caucasians, who may differ significantly from Chinese in terms of lifestyle, diet and body physiology. The traditional Chinese diet, with a low fat content and plenty of vegetables(Reference Woo, Woo and Leung11), would be expected to reduce the likelihood of development of diabetes but only rarely has this been investigated prospectively in the Chinese population(Reference Villegas, Yang and Gao12).
Studies have also highlighted the role of food variety in body fat accumulation(Reference McCrory, Fuss and McCallum13, Reference Woo, Cheung and Ho14). A wide variety of sweets, snacks and carbohydrates, coupled with a low variety of vegetables, appears to promote long-term increases in energy intake and body fat(Reference McCrory, Fuss and McCallum13). In the Hong Kong Chinese population, increased variety of snack consumption was associated with increased risk of developing overweight(Reference Woo, Cheung and Ho14). Therefore, it would be of particular interest to examine snack intake and variety of snacks as predisposing factors to diabetes in this population.
In a 9–14-year follow-up of participants recruited into a territory-wide dietary and cardiovascular risk factor prevalence survey carried out in 1995–1996, in which detailed dietary information was also obtained from a subsample, we aimed to identify dietary patterns in a Chinese population and examine the relationship of dietary pattern and dietary intake, including dietary glycaemic load, rice intake, snack intake and variety of snacks, with the development of diabetes in this population.
Methods
Study population
The Hong Kong Dietary Survey was conducted at baseline from October 1995 to May 1996 as part of the territory-wide Cardiovascular Risk Factor Prevalence Survey in ethnic Chinese. A detailed description of the sample for the Cardiovascular Risk Factor Prevalence Survey has been published elsewhere(Reference Janus, Watt and Lam15). In brief, participants were contacted by a random telephone survey and invited to a hospital-based centre at the Queen Mary Hospital, Hong Kong SAR, China, for physical examination and blood tests. Information on demographics, current smoking status, alcohol intake, participation in exercise/sports and family history of diabetes was also obtained using an interviewer-administered questionnaire. Current smokers were defined as those who reported having smoked at least one cigarette per day for at least 6 months before the interview. Drinkers were defined as those who drink at least once a month. Participants were also categorized as ‘participated in exercise/sports’ if they reported that they had been, or were currently, participating in exercise/sports 1 month before the interview. Family history of diabetes was defined as having at least one first-degree relative with diabetes.
Participants were divided by gender and age (five age groups: 25–34, 35–44, 45–54, 55–64 and 65–74 years). Thus, a total of ten groups were established. Dietary assessment was carried out consecutively on those who attended, until 100 or more participants were recruited into each of the ten sex- and age-specified groups. The response rate from the telephone survey was approximately 80 %; of those who responded, 40 % participated in blood tests and in recording anthropometric measurements. The sample closely matched the Hong Kong general population, since there was no difference in age distribution or socio-economic characteristics between subjects attending for blood tests and measurements, those who participated in the telephone survey, and the population as a whole as described in the 1996 Hong Kong by-census. There were also no significant differences in physical or laboratory parameters between subjects from the three geographical regions (Hong Kong Island, Kowloon and New Territories)(Reference Janus16).
The overall Cardiovascular Risk Factor Prevalence Survey included 2900 attendees aged 25–74 years, of whom 1010 (510 female and 500 male) underwent dietary assessment. The mean age of the dietary study participants was 45·6 (sd 11·7) years. From January 2005 to December 2008, the original cohort was invited to re-attend repeat blood tests and recording of anthropometric measurements, including weight, height and circumference measurements of waist and hip. A total of 690 of the 1010 participants returned (68·3 %). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committees of the Chinese University of Hong Kong and the University of Hong Kong. Written informed consent was obtained from all participants.
Dietary assessment
Dietary assessment was carried out at baseline during 1995–1996 using an FFQ, the validity of which has been examined elsewhere(Reference Woo, Leung and Ho17). This consisted of 266 items in the following seven categories: bread/pasta/rice (sixteen items); vegetables (sixty-three items); fruit (twenty-six items); meat (thirty-nine items)/fish (thirty-one items)/egg (five items); beverages (thirty-seven items); dimsum/snacks (thirty-nine items); soups (ten items); and oil/salt/sauces. Wherever possible, participants were instructed to maintain a brief dietary record of the preceding 7 d before the visit, during which time a survey on a week's diet would be carried out. On the day of the interview, each participant was asked to complete the questionnaire with information pertaining to the food item, the size of each portion and the frequency of consumption on a daily and weekly basis. Portion size was explained to the participants using a catalogue of pictures of individual food portions.
Data were cross-checked by examining the dietary pattern (e.g. if meals were skipped) to determine whether it corresponded to the number of times staple foods such as rice or noodles were consumed over a 1-week period. In case of discrepancies, the questionnaire was re-checked with the participant. The amount of cooking oil was estimated according to the method of preparing different foods: 0·2 tablespoon for steaming fish or for stir-frying half a portion of vegetables and one tablespoon for stir-frying one portion of vegetables or one portion of meat. The type of oil used was also documented to allow an estimation of the quantity of fat used in cooking. Quantification of nutrients was carried out using food tables for Hong Kong compiled from McCance & Widdowson's The Composition of Foods (Reference Holland, Welch and Unwin18) and two food tables used in China published by Zhongshan University(Reference Tsang and Yung19) and the Institute of Health of the Chinese Medical Science Institute(20).
To identify dietary patterns, individual food items from the FFQ were first aggregated into groups. We formed thirty-one separate food groups on the basis of similarity of type of food and nutrient composition (Appendix). Some individual food items were preserved either because it was inappropriate to incorporate them into a certain food group (e.g. coffee, mayonnaise and tomatoes) or because they were suspected to represent distinct dietary patterns (e.g. preserved radish). Principal component analysis (PCA) was conducted with Varimax rotation to the thirty-one food groups. Factors with eigenvalues ≥1 were retained. Four factors with eigenvalues ≥1·14 and explaining 50·8 % of the variance were identified (Table 1). We labelled the first factor as more snacks and drinks, the second factor as more vegetables, fruit and fish, the third factor as more meat and milk products and the fourth factor as more refined grains. A factor score was then calculated for each participant for each of the four patterns, in which the standardized intakes of each of the thirty-one food groups were weighted by their factor loadings and summed.
Table 1 Food group factor loading for four dietary patterns in the Hong Kong Adult Dietary Survey

Positive loadings <0·15 and negative loadings >−0·10 were omitted for simplicity.
The food groups are presented in descending order of loading values on the snacks and drinks dietary pattern.
The glycaemic index values for each food item in the FFQ were obtained from the international table of glycaemic index and glycaemic load values of foods(Reference Foster-Powell, Holt and Brand-Miller21), as well as from several publications on the glycaemic index of commercially available foods in the UK(Reference Henry, Lightowler and Strik22, Reference Henry, Lightowler and Dodwell23), from the China Food Composition Table(Reference Yang, Wang and Pan24) and also from a recent article about the glycaemic index in cereals and tubers produced in China(Reference Yang, Wang and Cui25). The dietary glycaemic index for each participant was calculated by summing the products of the percentage contribution of each individual food to daily available carbohydrate intake multiplied by the food's glycaemic index value. Available carbohydrate was calculated as total carbohydrate minus dietary fibre(Reference Foster-Powell, Holt and Brand-Miller21). The dietary glycaemic load was also calculated by multiplying the dietary glycaemic index by the total amount of daily available carbohydrate intake (divided by 100).
Variety of snacks was calculated on the basis of the percentage of different food items consumed within the snack food group, regardless of the frequency with which they were consumed, as well as their portion. In addition, the quality of diet was examined by applying the Dietary Quality index-International (DQI-I)(Reference Kim, Haines and Siega-Riz26), which has been used to evaluate the quality of the Mediterranean diet(Reference Tur, Romaguera and Pons27). Essentially, four major aspects of the diet are assessed: variety, adequacy, moderation and overall balance, each with subcomponents. The range is 0–100, with a high score representing high quality. In the present study, we did not have sufficient information to calculate the category of empty-energy foods under the aspect ‘moderation’. Therefore, the range of scores for moderation was 0–24 instead of 0–30, and the DQI-I total score was 0–94 instead of 0–100.
Outcome ascertainment
The WHO Study Group (1998) criteria for glucose intolerance and diabetes were used to classify participants into glucose tolerance groups. Diabetes was diagnosed if fasting glucose was ≥7·0 mmol/l and/or the 2 h post-glucose load was ≥11·1 mmol/l. Impaired glucose tolerance (IGT) was diagnosed if fasting glucose was <7·0 mmol/l and the 2 h post-glucose load was ≥7·8 mmol/l but <11·1 mmol/l. Impaired fasting glucose (IFG) was diagnosed if fasting glucose was ≥6·1 mmol/l but <7·0 mmol/l and the 2 h post-glucose load was <7·8 mmol/l.
Statistical analysis
The Student t test and the χ 2 test were used to test for differences in mean age, obesity indices and selected dietary factors, as well as for differences in distribution of the characteristics of participants who were alive and had completed interviews and those who were lost to follow-up. Multivariable Cox proportional hazards regression was used to calculate the OR and 95 % CI of incident diabetes by 1 sd increase in continuous dietary pattern scores of each PCA-derived dietary pattern. Age (in years, continuous), sex, BMI (in kg/m2, continuous), waist-to-hip ratio (WHR; continuous), current smoking status (categorical), alcohol intake (categorical), participation in exercise/sports (categorical) and family history of diabetes (categorical) were considered as potential confounders. The above analyses were also performed for dietary glycaemic load (continuous), rice intake (in g/week, continuous), snack intake (in g/week, continuous), variety of snacks (in %, continuous) and DQI-I (continuous). Moreover, the Cox models above were repeated using the prevalence cases of IGT/IFG and diabetes at follow-up as the outcome. Prevalence cases of IGT/IFG and diabetes included those with IGT/IFG or diabetes at baseline and again at follow-up, in addition to the incident cases of IGT/IFG or diabetes (i.e. cases in the N-I, N-D, I-I, I-D and D-D groups). A value of P < 0·05 was used to denote significant difference. All analyses were performed using the Statistical Package for the Social Sciences statistical software package version 17·0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 1010 participants included at baseline, 690 (68·3 %) completed interviews at a mean of 11·8 years of follow-up and 320 (31·7 %) were lost to follow-up. Those who were lost to follow-up were slightly older (P < 0·01), had higher WHR (P < 0·01) and had slightly higher dietary glycaemic load (P = 0·096) and rice intake (P < 0·05). Nevertheless, there were no differences in BMI, snack intake, variety of snacks and DQI-I between those who were lost to follow-up and those who returned (Table 2).
Table 2 Comparison between those alive at 10-year follow-up and those lost to follow-up, Hong Kong Dietary Survey

DM, type 2 diabetes; WHR, waist-to-hip-ratio; DQI-I, Dietary Quality index-International.
Of the 654 participants without diabetes at baseline, seventy-four (11·3 %) developed diabetes over the follow-up period (Table 3). Other categories of glucose tolerance changes are also shown (e.g normal to normal, IGT/IFG/diabetes remaining IGT/IFG/diabetes, IGT/IFG/diabetes becoming normal).
Table 3 Ten-year changes in glucose tolerance categories, Hong Kong Dietary Survey

N-N, normal at baseline and follow-up; N-I, normal at baseline and IGT/IFG at follow-up; N-D, normal at baseline and DM at follow-up; IGT, impaired glucose tolerance; IFG, impaired fasting glucose; I-N, IGT/IFG at baseline and normal at follow-up; I-I, IGT/IFG at baseline and follow-up; I-D, IGT/IFG at baseline and DM at follow-up; DM, type 2 diabetes; D-D, DM at baseline and follow-up.
*P ≤ 0·05 by t test comparing between sexes.
†Incidence of DM was calculated as N-D and I-D divided by the total number of participants without DM at baseline.
As shown in Table 4, a 1 sd increase in more vegetables, fruits and fish pattern score was associated with a 13 % lower diabetes risk (OR = 0·77; 95 % CI 0·59, 0·99) after adjustment for age, BMI, WHR, current smoking status, alcohol intake and participation in exercise/sports. Additional adjustment for family history of diabetes did not change the associations (OR = 0·76; 95 % CI 0·58, 0·99). In contrast, a 1 sd increase in more meat and milk products pattern score was associated with a 39 % increased risk of diabetes (OR = 1·39; 95 % CI 1·04, 1·84).
Table 4 OR and 95 % CI of developing DM by an sd increase in continuous dietary pattern scores of each PCA-derived dietary pattern, Hong Kong Dietary Survey
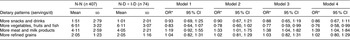
DM, type 2 diabetes; PCA, principal component analysis; N-N, normal at baseline and follow-up; N-D, normal at baseline and DM at follow-up, I-D, IGT/IFG at baseline and DM at follow-up; IGT, impaired glucose tolerance; IFG, impaired fasting glucose.
*OR is per sd increase (using sd of the normal group as reference).
Model 1: adjusted for sex and age.
Model 2: adjusted for sex, age, BMI and waist-to-hip-ratio (WHR).
Model 3: adjusted for sex, age, BMI, WHR, current smoking status, alcohol intake and participation in exercise/sports.
Model 4: adjusted for sex, age, BMI, WHR, current smoking status, alcohol intake, participation in exercise/sports and family history of diabetes.
Table 5 shows the risk of developing diabetes for other dietary factors. There were suggestions of inverse associations of DQI-I and rice intake with the development of diabetes after adjustment for BMI, WHR, current smoking status, alcohol intake, participation in exercise/sports and family history of diabetes, but the relationships were not statistically significant (OR for DQI-I = 0·89, 95 % CI 0·69, 1·14; OR for rice intake = 0·87, 95 % CI 0·78, 1·34; Table 5). Further analyses on the relationships of diet with glucose intolerance and diabetes using prevalence cases of IGT/IFG and diabetes at follow-up as the outcome showed similar results (data not shown).
Table 5 OR and 95 % CI of developing DM by an sd increase in continuous dietary factor scores and dietary intake, Hong Kong Dietary Survey

DM, type 2 diabetes; N-N, normal at baseline and follow-up; N-D, normal at baseline and DM at follow-up; I-D, IGT/IFG at baseline and DM at follow-up; DQI-I, Dietary Quality index-International; IGT, impaired glucose tolerance; IFG, impaired fasting glucose.
*OR is per sd increase (using sd of the normal group as reference).
Model 1: adjusted for sex and age.
Model 2: adjusted for sex, age, BMI and waist-to-hip-ratio (WHR).
Model 3: adjusted for sex, age, BMI, WHR, current smoking status, alcohol intake and participation in exercise/sports.
Model 4: adjusted for sex, age, BMI, WHR, current smoking status, alcohol intake, participation in exercise/sports and family history of diabetes.
Discussion
In the present 9–14-year follow-up study of Hong Kong Chinese adults, we identified four dietary patterns using PCA, namely, more snacks and drinks, more vegetables, fruit and fish, more meat and milk products and more refined grains. The more vegetables, fruits and fish pattern, which was rich in vegetables, fruit, legumes and fish, was associated with a lower risk of diabetes. In contrast, the more meat and milk products pattern, which was rich in red meat, milk products and refined grains, was associated with a substantially higher risk of diabetes. These associations were independent of age, BMI, WHR, current smoking status, alcohol intake, participation in exercise/sports and family history of diabetes. However, no significant associations were observed between dietary glycaemic load, rice intake, snack intake, variety of snacks and the development of diabetes.
Several prospective studies have examined dietary patterns and diabetes incidence, but most of these studies were confined to Caucasians(Reference van Dam, Rimm and Willett8–Reference Erber, Hopping and Grandinetti10) and have rarely been carried out in the Chinese(Reference Villegas, Yang and Gao12). In general, two major patterns have been reported. A prudent or healthy diet, characterized by high consumption of vegetables, fruit, fish, poultry and whole grains, was associated with reduced risk of diabetes, whereas a Western diet, characterized by high consumption of red and processed meat, fried foods, high-fat dairy products, refined grains, sweets and desserts, was associated with increased risk. Our results are in agreement with these studies reporting that the more vegetables, fruits and fish pattern was associated with reduced risk of diabetes. The protective effects of fruit and vegetables on the development of diabetes could be attributed to their antioxidant properties, as well as to their dietary fibre and Mg content(Reference Schulze, Schulz and Heidemann28, Reference Larsson and Wolk29). However, evidence regarding the role of fish intake in relation to diabetes risk has remained inconclusive(Reference Patel, Sharp and Luben5, Reference van Woudenbergh, van Ballegooijen and Kuijsten30). It is possible that a high intake of fish may generally be an indicator of a more health-conscious attitude; therefore, a diet rich in fish may also be accompanied by a high consumption of vegetables and fruit, and thus reduced risk of diabetes.
The association between the more meat and milk products pattern and diabetes risk observed in the present study is also consistent with studies that showed an increased risk of diabetes for participants adhering to a Western diet(Reference van Dam, Rimm and Willett8–Reference Erber, Hopping and Grandinetti10). The adverse effects of meat, milk products and refined grains have been attributed to their higher saturated fat and carbohydrate content, which may lead to hyperglycaemia and hyperinsulinaemia, and therefore higher risk of diabetes(Reference Villegas, Liu and Gao1, Reference Fung, Rimm and Spiegelman31, Reference Sun, Spiegelman and van Dam32).
Although the dietary patterns observed in the present study were similar to the diets in Western populations, levels of whole grains consumption were rather low in our participants. Only about one-quarter of the study population consumed food containing whole grains, with the average intake being 0·11 (sd 0·59) servings/d. A diet high in whole grains has previously been associated with reduced risk of diabetes(Reference van Dam, Rimm and Willett8, Reference Fung, Hu and Pereira33). A recent study also showed that substitution of whole grains for white rice would produce a 36 % reduced risk of diabetes in both men and women(Reference Sun, Spiegelman and van Dam32). Given the potential benefits of whole grains on glucose metabolism, further work would be needed to determine their role on diabetes prevention.
Studies have also highlighted the role of glycaemic load in the development of glucose intolerance and diabetes, but results have been inconsistent. Several prospective studies suggested positive associations of glycaemic index and glycaemic load with diabetes risk(Reference Villegas, Liu and Gao1, Reference Salmeron, Manson and Stampfer34, Reference Salmeron, Ascherio and Rimm35), whereas others did not show a positive association(Reference Meyer, Kushi and Jacobs36–Reference Mosdol, Witte and Frost38). A lack of association between glycaemic load and measures of insulin sensitivity, insulin secretion and adiposity was also observed(Reference Liese, Schulz and Fang39). In our study, no associations were found between dietary glycaemic load and diabetes risk. Villegas et al.(Reference Villegas, Liu and Gao1) suggested that divergent findings between studies could be due to differences in study methods. An FFQ that does not address carbohydrate quality in detail may provide inaccurate results. The FFQ used in the present study was validated and fairly detailed; however, higher dietary glycaemic load and rice intake were observed in those who were lost to follow-up, which may have introduced a bias into the estimation of the incidence of diabetes.
It has been pointed out that diets high in refined carbohydrates may lead to hypertension, dyslipidaemia and metabolic intermediaries of insulin resistance(Reference Liu and Manson40), and thus to diabetes risk. A recent study also found that a higher intake of white rice was associated with a 78 % increased risk of diabetes(Reference Sun, Spiegelman and van Dam32). Previously, we reported a higher consumption of rice in participants with diabetes who had normal BMI(Reference Woo, Ho and Sham41). However, in the present follow-up study, rice intake was not related to the risk of diabetes. It is possible that the diabetogenic potential of rice as a staple diet observed in some other studies is ameliorated by a higher consumption of vegetables, fruit and fish, components of the prudent diet. It is also possible that associations might be apparent with only a wide variation in the level of rice consumption in the study population in which rice is the major staple food.
Consumption of snacks and fast food has been associated with weight gain and obesity(Reference McCrory, Fuss and McCallum13, Reference Woo, Cheung and Ho14), the most important predisposing factor for diabetes. However, we have previously shown no difference in snack consumption among the glucose tolerance groups(Reference Woo, Ho and Sham41). The findings from the present longitudinal study further support the suggestion that snack consumption was not a risk factor.
The limitations of our analysis should be noted. The number of new cases of diabetes was small; therefore, some predisposing factors may not have achieved statistical significance. Our measurement of diet was based on a single FFQ administered at baseline that may not have been representative of consumption over the long term. Furthermore, although the complete follow-up rate is 68 %, the possibility of selection bias from differential survival and other loss to follow-up is considerable.
In conclusion, our findings suggest that dietary patterns in the Hong Kong Chinese can predict risk of diabetes. However, no relationships of dietary glycaemic load, rice intake, snack intake and variety of snacks with the development of diabetes were observed. These findings add to the existing evidence that dietary patterns are important predictors for diabetes; however, further work is needed to determine their role in diabetes prevention, and thereby reduce the risk of diabetes in the Chinese population.
Acknowledgements
The present study was supported by grants from the Research Grants Council, Hong Kong Government (Grant number: HKU 7626/07M), the Centre for Nutritional Studies, Faculty of Medicine, the Chinese University of Hong Kong, and the Health and Health Services Research Fund (Grant no. 06070951). The authors have no conflict of interest to declare. R.Y. contributed to the writing of the manuscript and to data analyses; J.W. developed the concept, planned the data analyses, designed the study and contributed to data analyses and writing of the manuscript; R.C. contributed to the development of glycaemic index coding; A.S. contributed to data handling and analyses; S.H. contributed to dietary data collection and analyses; A.T., B.M.Y.C., T.H.L. and K.S.L.L. contributed to determining the follow-up cohort and clinical diagnoses, as well as to data collection and writing of the manuscript. The authors thank all individuals for their participation.
Appendix
Food groupings used in the dietary pattern analysis of the Hong Kong Dietary Survey








