The epidemic of tobacco use among young people is defined as a major public health problem in developed and developing countries. The World Health Organization (WHO) European Region Report (World Health Organization, 2010) indicates that in 2009, the number of cigarette smokers among US adults was estimated to be 24.3% (males 26.4%, females 22.3%). Smoking rates are even higher in Turkey. WHO's 2011 Global Adult Tobacco Survey estimated regular smoking rates in Turkey as 31.1% among all individuals, with 47.8% of males reporting smoking and 15.1% of females (Küresel Yetişkin Tütün Araştırması, 2008; WHO, 2011a). The relatively few studies of smoking rates in Turkey have been conducted mostly in Turkish school or university populations, probably because of ease of sampling. There are no family or twin studies of smoking in Turkey. Erdogan and Erdogan (Reference Erdogan and Erdogan2008) studied 3,659 students from six universities in Ankara. They found that 33.4% of interviewed students were regular smokers, and females had a lower tendency to smoke. Of 1,753 male students, 39.5% were regular smokers, 14.9% occasional smokers, and 45.5% were non-smokers. Of 1,901 females, 27.8% smoked regularly, 14.7% smoked occasionally, and 57.5% did not smoke (Erdogan & Erdogan, Reference Erdogan and Erdogan2008). Another study of Turkish students found very similar rates of smoking. Öncel et al. (Reference Öncel, Gebizlioğlu and Alioğlu2011) assessed 1,734 students (869 males and 866 females, both smokers and non-smokers) at Kırıkkale University, in Kırıkkale, Turkey. They found that 548 study participants (31.6%) were identified as smokers, smoking every day for a month or longer. Of the smokers, 66.1% were males and only 33.9% were females. The risk of smoking was three times higher in males than in females. Other identified risk factors included having a smoking twin, mother, or father, and having a high family income (Öncel et al., Reference Öncel, Gebizlioğlu and Alioğlu2011). When comparing Öncel et al.'s (Reference Öncel, Gebizlioğlu and Alioğlu2011) results with US data, we found a higher frequency of smoking in males (66.1% vs. 22.8%) and smoking levels in females (33.9% vs. 17.4%) in Turkish youth as compared with US youth aged 18–24 years (Centers for Disease Control and Prevention, 2010). Rates of smoking in Turkey contribute to considerable health burden. Cardiovascular diseases and cancer are the top two causes of mortality in Turkey. Eighty-seven percent of deaths from lung cancer and about 30% of other cancer-related deaths are caused by smoking in developed countries (Bozkurt et al., Reference Bozkurt, Şahinöz, Özçırpıcı, Özgür, Şahinöz, Acemoğlu and Akkafa2006).
It is also notable that rates of smoking among women vary significantly across countries. The WHO European Region Report (World Health Organization, 2010) found that patterns of smoking between the sexes largely fall into three distinct groups. In the Nordic and some Western European countries, smoking rates for women and men are similar, and are declining. For example, the proportions of male and female smokers are 31% and 28% in Norway, 31% and 26% in Ireland, and 28% and 22% in the Netherlands and Finland, respectively. In many countries of Central and Southern Europe, more men than women smoke, although rates among women are also high (63% of men vs. 41% of women in Greece, 47% vs. 45% in Austria, and 48% vs. 27% in Bulgaria). Finally, in the newly independent states (NIS) of the former USSR, smoking rates are high among men and relatively low among women (61% vs. 3% in Armenia; 53% vs. 24% in Latvia, and 43% vs. 9% in Kazakhstan; World Health Organization, 2010). Nevertheless, smoking rates among women are rising rapidly in these countries. The gender difference in smoking rates is also narrower among young people. According to the WHO Global Youth Tobacco Survey (GYTS) conducted from 1999 to 2009 across the European Region, 21% of boys and 17% of girls had smoked cigarettes in the previous 30 days (World Health Organization, 2011b).
Case-control-, twin-, and sib-pair-based investigations suggest that genetic factors play an important role in smoking behavior and nicotine dependence (ND; Kendler et al., Reference Kendler, Neale, Sullivan, Corey, Gardner and Prescott1999; Li, Reference Li2003a; Munafo & Johnstone, Reference Munafò and Johnstone2008; Neale et al., Reference Neale, Sullivan and Kendler2005; Pergadia, Reference Pergadia, Agrawal, Heath, Martin, Bucholz and Madden2010). To the best of our knowledge, there are no studies about the smoking status of Turkish twins in the literature. The purpose of this study was to determine the risk factors for smoking behavior in Turkish twins in the context of cultural differences, and to investigate genetic and environmental factors affecting individuals’ smoking status and related phenotypes. Smoking is a widespread habit in Turkey and a major public health problem worldwide. As the first step of the twin study, we interviewed twins living in the Kırıkkale and Ankara regions of Turkey. Our study provides valuable information regarding factors related to smoking status, nicotine dependence, age, gender, social situation, and family structure. Our results were compared with results from other well-known studies. This type of advanced epidemiological research can provide important information for understanding smoking behavior and nicotine dependence and suggestions for clinicians on finding possible ways to prevent nicotine dependence.
Materials and Methods
Design of the Sample
The study was carried out at Kırıkkale University, in Kırıkkale, Turkey. We created a list of 1,000 twin pairs using different sources by asking schools, using available municipality and health center records, and by inquiring in neighborhoods. A subset of 325 pairs (650 individuals) was selected randomly from individuals over the age of 15 years; 626 individuals were interviewed in face-to-face interviews. Four individuals refused the interview and we removed those pairs from the list. The final sample consisted of 618 individuals (for a brief report about this study, see Öncel & Aliev, Reference Öncel and Aliev2013).
Data Collection
Data collection was performed using multiple methods to contact twins. The primary method of data collection was face-to-face interview using two interviewers to ensure data quality, although we also interviewed some individuals via phone if they lived in other cities. Because research participation is less commonplace in Turkey, interviewers carried paperwork from the university and from the ethics review board explaining the study and attesting to its authenticity. Municipal authorities also helped interviewers in rural regions to prevent misunderstandings during data collection. Participants were given the opportunity to discuss participation with their family before making a decision. All twins provided consent, either oral or written, as per Turkey regulations before being interviewed. Interview completion took an average of 25 min (see the Appendix for an English translation of the interview). Answers to some questions about their family/parents were determined by cross checking both twins’ responses. In conflicting cases, we re-interviewed both twins in an effort to remedy the discrepancy, or we defined the data as missing. Almost all individuals agreed to contribute in the future to follow-up analyses and DNA collection. From the initial list of potential participants, four federal employees refused to participate in our research, referring to security agreements in their jobs as the reason for their refusal. The final sample consisted of 618 respondents.
Measures
The interview included questions about age, gender, smoking status, smoking status of parents, education status of parents, income, daily sports activities, smoking history (age when started or quit smoking, daily average number of cigarettes smoked, attempts to quit smoking, and reasons for starting to smoke), alcohol use, sporting activities, and behavioral problems. Alcohol use reflects current use. We also asked about lifetime feelings of depression. It was hard to identify the income level in Turkey because of the high inflation rate. A binary ‘income’ variable was defined with income <US$1,200/month and ≥US$1,200/month. The education level of parents was defined using a two-level group variable: illiterate, primary school, and secondary school were coded 0; high school, university, and graduate school were coded 1.
A categorical variable Body Mass Index (BMI) was based on self-reported weight and height and was defined using a three-level variable: normal (<25), overweight (25–30), and obese (>30), coded as 1, 2, and 3 respectively. We defined smokers as those who had a period of more than 1 month at some point in their lifetime during which they smoked cigarettes or used tobacco products every day. This definition is culture-based, as the smoking behavior of the Turkish population is such that smokers use tobacco products every day. We note that all interviewers were trained in how to interpret and code respondent answers and none of the interviewers mentioned that they encountered twins who smoked, but not on a regular basis. Former smokers were defined as those who reported a quit age of less than their current age. The binary smoking status variable used in regression analyses reflects both current and former smokers as smokers. Age of onset of smoking was defined as the age when the twin started smoking regularly. We defined the age of onset by using a four-level variable (<14 years, 14–17 years, 17–20 years, and ≥20 years). The number of cigarettes smoked per day by parents and twins was also changed to categorical variables having four levels: 1–10, 11–20, 21—30, and ≥31 cigarettes/day, defining non-smokers as missing. A confirmed Turkish translation of the Fagerström Test for Nicotine Dependence (FTND) was used to assess nicotine dependence (Uysal et al., Reference Uysal, Kadakal, Karşidağ, Bayram, Uysal and Yilmaz2004). Current smokers answered based on current smoking status, while former smokers answered for the time before they made a decision to quit. We created a binary nicotine dependence variable for FTND corresponding to the total FTND score as follows: 0–3 (not nicotine-dependent, coded as 0) and 4 and higher (nicotine-dependent, coded as 1). For zygosity determination, we asked standard questions for assessing zygosity via questions such as ‘Do you look as alike as two peas in a pod?’, if it was difficult to tell them apart for parents, sibs, school mates, and strangers, and if they used any signs or marks to distinguish themselves. Zygosity was determined based on the information provided by both twins (Goldsmith, Reference Goldsmith1991; Kaprio et al., Reference Kaprio, Sarna, Koskenvuo and Rantasalo1978; Öncel & Aliev, Reference Öncel and Aliev2013; Sarna et al., Reference Sarna, Kaprio, Sistonen and Koskenvuo1978).
Statistical Analyses
Descriptive statistics, cross tabulation, and correlation analyses were performed with IBM SPSS-20. Assumptions about the normal distribution of variables were tested using the Kolmogorov–Smirnov tests. We performed t-tests for equality of means of non-categorical variables (number of cigarettes per day, and FTND score) between genders. In analyses, including both twins, we used family ID as a clustering variable. Risk factors for smoking were determined and assessed first by univariate clustered logistic regression, and then by multivariate clustered logistic regression involving significant predictors from the univariate logistic regression using SAS software (Hosmer & Lemeshow, Reference Hosmer and Lemeshow2000).
Chi-square tests were used to determine whether there were significant associations between categorical variables. The Cramer's V for nominal-by-nominal variables and the Gamma for ordinal by nominal variables provided information about the strength of the association between two categorical variables.
Structural Equation Modeling (SEM) was performed using the OpenMx software (Neale et al., Reference Neale, Boker, Xie and Maes2003) in R. Zygosity determination was performed based on interview results (Goldsmith, Reference Goldsmith1991; Kaprio et al., Reference Kaprio, Sarna, Koskenvuo and Rantasalo1978; Öncel & Aliev, Reference Öncel and Aliev2013; Sarna et al., Reference Sarna, Kaprio, Sistonen and Koskenvuo1978). The classical twin study allows us to partition the variance in a trait into additive genetic (A), common environmental (C), and unique environmental (E) factors by comparing the similarity between monozygotic (MZ) and dizygotic (DZ) twins (see, e.g., Neale & Maes, Reference Neale and Maes2002 for a more detailed description of the (ACE) model). To partition the variance in liability to nicotine dependence, it is necessary to take smoking initiation (SI) into account as nicotine dependence is conditional on smoking initiation. This is modeled using the Causal Contingent Common Pathway model (CCC) model (Kendler et al., Reference Kendler, Neale, Sullivan, Corey, Gardner and Prescott1999) with a pathway connecting both phenotypes. Estimates can be obtained for the A, C, and E sources of variance in nicotine dependence that are shared with smoking initiation through the causal pathway as well as for A, C, and E factors contributing to nicotine dependence independent of smoking initiation. Analyses proceed with fitting a series of alternative models, testing the significance of the contributions of different sources of variance using a maximum likelihood approach in which the best model is chosen using a combination of goodness-of-fit and parsimony.
Results
Mean values and standard deviations (SD) of number of cigarettes per day, age at onset of smoking, and FTND score were 17.49 (SD = 9.478), 16.48 (SD = 4.237), and 4.36 (SD = 2.443) respectively in males, and 8.51 (SD = 6.154), 21.35 (SD = 7.2), and 2.86 (SD = 2.328) respectively in females. There was a significant positive correlation between the FTND score and number of cigarettes per day (r = 0.695, p < .0001), a significant negative correlation between the FTND score and age at onset of smoking (r = -0.159, p =.038), and a significant negative correlation between number of cigarettes per day and age at onset of smoking (r = -0.176, p = .019).
Table 1 provides frequency distributions of measured variables by zygosity, including all individuals. Table 2 presents the count, mean values, and SD of age, BMI, FTND, age at onset of smoking, and number of cigarettes per day for MZ males (MZM), DZ males (DZM), MZ females (MZF), DZ females (DZF), and opposite sex DZ (DOS) pairs. No gender difference was observed for BMI (p = .288). Age at onset of smoking, number of cigarettes per day, and FTND were different between genders (p = .000, p = .000, p = .001 respectively). Assumptions about equality of variances in Table 2 were made according to the Levene test, which compares variance differences between groups.
TABLE 1 Characteristics of Study Population From the Turkish Twin Study
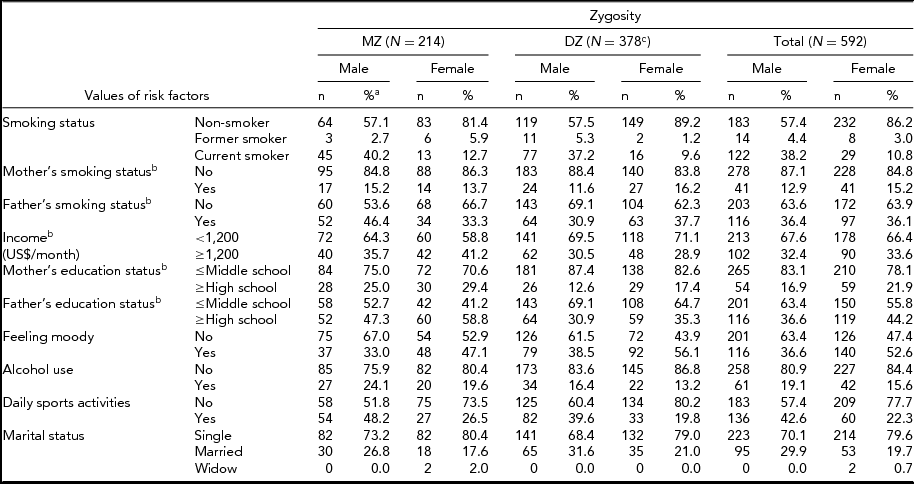
aPercentage of individuals within the same gender.
bBased on the first twin's response.
cFour DZ twins had missing data.
TABLE 2 Statistics of Twin Pairs by Zygosity
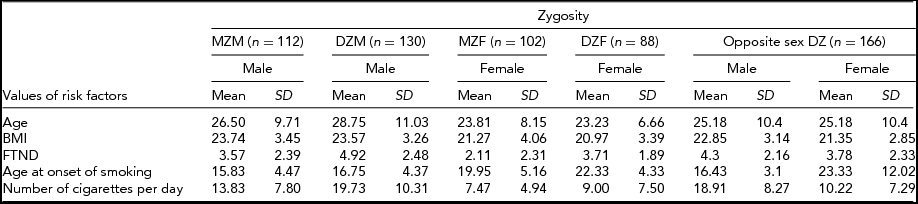
Table 3 presents tetrachoric correlations for univariate nominal variables and polychoric correlations for ordered categorical variables for MZ and DZ twin pairs. DZ pairs are divided into the same-sex and opposite-sex pairs. Tetrachoric and polychoric correlations of all variables for MZ twins were higher than those for DZ twins, suggesting that genetic factors may contribute to the variance of liability to smoking variables. In addition, asymptotic standard errors (ASE) were lower for MZ twins compared with DZ twins.
TABLE 3 Within Twin Tetrachoric and Polychoric Correlations
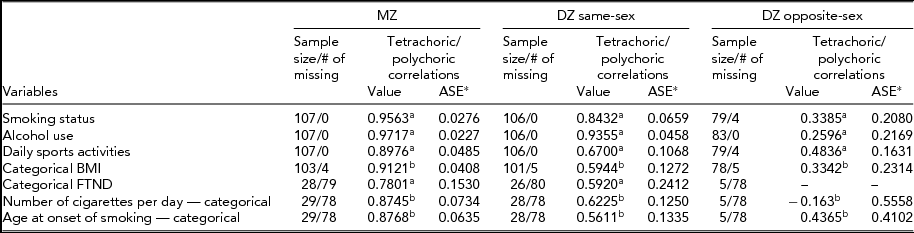
aTetrachoric correlations.
bPolychoric correlations.
*ASE: Asymptotic Standard Error; FTND, Fagerström Test for Nicotine Dependence.
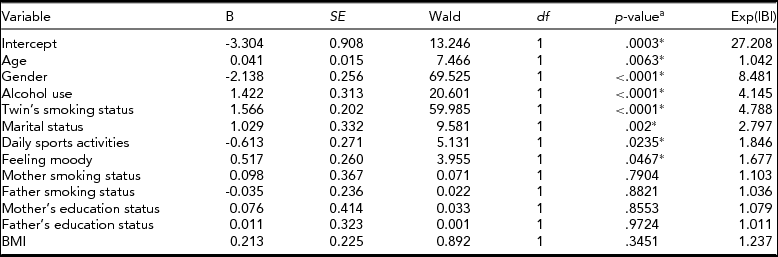
To determine the significant risk factors for smoking status, we performed separate bivariate clustered logistic regression analyses with 13 phenotypes: age, gender, alcohol use, twin's smoking status, marital status, daily sports activities, feeling moody, mother smoking status, father smoking status, mother's education status, father's education status, income, and BMI (Table 4). Then we selected significant variables and used them in multivariate clustered logistic regression (Table 5).
TABLE 4 Univariate Binary Clustered Logistic Regression Models for Predicting Smoking Status
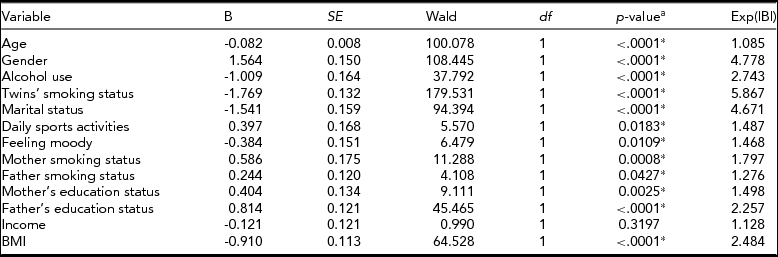
a p-values are based on logistic regression.
*p < .05 is significant.
TABLE 5 Binary Multivariate Clustered Logistic Regression Results for Predicting Smoking Status
a p-values are based on logistic regression.
*p < .05 is significant.
The risk of smoking was 8.5 times higher in males than in females. Having a smoking twin increased the risk of smoking 4.8 times, and alcohol use increased the risk 4.2 times. The study also showed that age, marital status, daily sports activities, and feeling depressed, all played a significant role in smoking behavior among twins (Table 5). Daily sport activities reduced the risk of smoking 1.85 times, whereas ever feeling moody increased the risk 1.68 times.
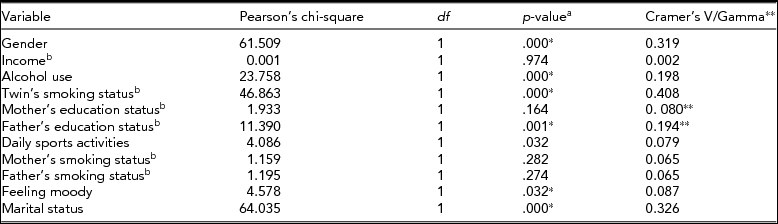
As can be seen from Table 6, gender, alcohol use, twin's smoking status, father's education status, marital status, daily sports activities, and feeling depressed were significantly related to smoking status. Smoking status showed a non-significant correlation to income, mother's education status, and smoking status of both parents. Although BMI, both parents’ smoking statuses, and education levels were significant in univariate analyses, they were not significant in multivariate analysis.
TABLE 6 Associations Between Smoking Status and Categorical Variables by Cross Tables
a p-values are based on chi-square test.
bBased on the first twin's response.
*p < .05 is significant.
**Gamma value for ordinal variables.
To assess latent genetic and environmental factors affecting smoking initiation and FTND, we used the CCC model for different zygosity groups (Maes & Neale, Reference Maes, Neale, Swan, Baker, Chassin, Conti, Lerman and Perkins2009, Ch. 6, pp. 245–288). Figure 1 shows a path diagram of FTND regressed onto initiation (smoking initiation) for this model. For simplicity, Figure 1 reflects only paths for Twin 1. Each variable has its own A, C, and E components and all covariance between smoking initiation and FTND is assumed to arise via the regression path.
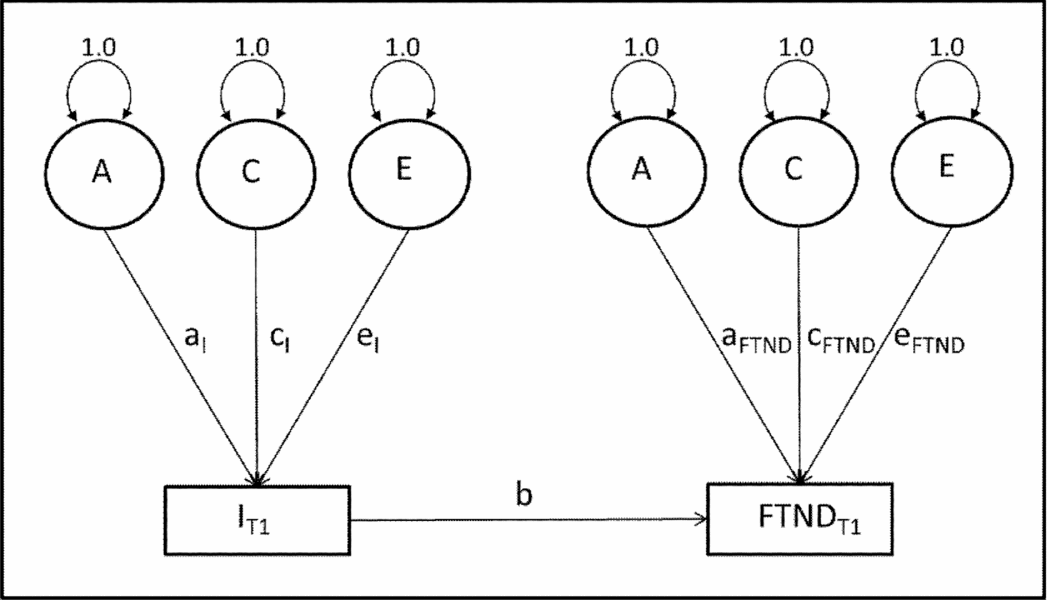
FIGURE 1 Causal contingent common pathway model. Note: A = additive genetics; C = common environment; E = unique environment; a, c, e, and b = regression path coefficients; SI = smoking initiation, T1 = twin 1.
Full model fitting results and comparisons with nested models are provided in Table 7. The fit of nested models was assessed as a function of change in the value of twice the log likelihood of the data, which is distributed as a chi-square statistic with degrees of freedom (df) equal to the difference in the number of parameters estimated between models. The model with dropped rg/rc paths and with the same male and female parameters, which tests sex differences in genetic and environmental factors, did not fit significantly worse. Next, dropping the causal b paths from smoking initiation (model 3) to FTND also fitted better than the full model. When testing the significance of A and C paths at the two stages, two models fitted the data almost equally well. The first one was a CE model where genetic components for both smoking initiation and FTND were dropped (model 6). The second was a model where genetic components for smoking initiation and common environmental components for FTND were dropped (model 9). Results suggested that familial resemblance for smoking initiation was entirely influenced by shared environmental factors for both males and females (c 2 I = 0.888). Familial resemblance for FTND could be accounted for by common environmental or genetic factors. Heritability estimate for males and females in the last model was h 2 FTND = 0.795. Since the aI paths could be dropped, heritability reflected genetic factors specific to FTND. In the full model, the b path was estimated to be negative for females and positive for males. Negative values of the b path could be explained by cultural specification. Even if woman try smoking at early ages or at high school ages, only a small percentage of them become regular smoker at later ages because it is less acceptable for women to smoke in Anatolian culture than it is for men (Table 7).
TABLE 7 Model Fitting Results for Fagerström Test for Nicotine Dependence
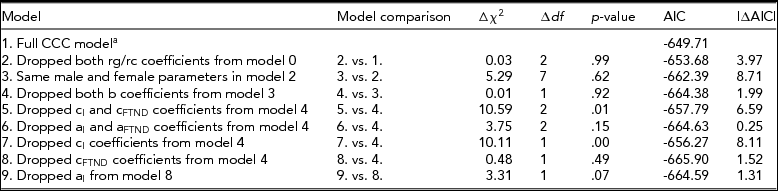
aFull model: Estimated parameters = 20, -2LL = 632.29, df = 641, AIC = −649.71.
Discussion and Conclusions
To the best of our knowledge, this study represents the first detailed data analysis on smoking patterns among twins in Turkey. Parallel to studies in Western cultures, we find that both genetic and environmental factors have a big effect on smoking. Twin and adoption studies have demonstrated that heritability is at least 50% for both smoking initiation and smoking persistence (Li, Reference Li2003a). The heritability of the FTND scores of 80% found in our study of Turkish twins is considerably higher (h 2 = 0.795) than that reported in previous studies in other cultures, as presented in a meta-analysis by Li et al. (Reference Li, Cheng, Ma and Swan2003b; h 2 = 0.37 for males and h 2 = 0.55 for females). Shared environmental components for smoking initiation are higher (c 2 = 0.887) than in other studies for both males and females (Li et al., Reference Li, Cheng, Ma and Swan2003b; c 2 = 0.49 for males and c 2 = 0.24 for females). Maes et al. (Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004) extended previous studies by examining the relationship among tobacco initiation, regular tobacco use, and nicotine dependence simultaneously. The heritabilities were estimated at 75%, 80%, and 60% respectively for tobacco initiation, regular tobacco use, and nicotine dependence. The variance specific to liability to regular tobacco was entirely accounted for by additive genetic factors for Virginia Twins. Lessov et al. (Reference Lessov, Martin, Statham, Todorov, Slutske, Bucholz and Madden2004) analyzed the most heritable items of nicotine dependence in young Australian twins and found that heritabilities for both males and females were similar. Heritability estimate for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV) nicotine dependence was 56% (95% CI, 40–63) for regular smokers, which is considerably lower than our estimates.
We find that gender and having a twin who smokes play a significant role in smoking behavior among Turkish twins. A multivariate clustered logistic regression model found that presence of a smoking twin in the family, age, alcohol use, marital status, daily sports activities, and feeling moody played a significant role in smoking behavior among twins.
This study provides valuable information regarding factors related to nicotine dependence, their relation to twin zygosity, gender, social situation, and family structure among a Turkish population. We are planning to genotype twins using saliva to compare actual and interview-based zygosities and provide genome-wide analysis. A new grant application from The Scientific and Technological Research Council of Turkey (TÜBİTAK) is under way. This will enable us to better understand genetic and environmental influences on behavioral outcomes across diverse cultures.
This study is not without limitations. The sample size for these initial analyses was small (<600 pairs), limiting our power to fit more complex models about genetic and environmental influences on smoking outcomes. In addition, our interview was very brief, as this study was intended to be a feasibility project to assess potential for twin studies in the Turkish culture. Accordingly, we are limited in our ability to study substance use and psychiatric factors that may be comorbid with smoking. We also note that our measure of regular smoking was defined to be relevant to the Turkish culture, and varies from other standard definitions of regular smoking. Cultural smoking practices in Turkey vary from other European nations in that traditional cigarette smokers in the Anatolian region of Turkey smoke on a regular basis (every day) rather than having a gradient of frequency of smoking, as is more common among smokers from other parts of the world. We believe this further underscores the need to do cross-cultural research.
Acknowledgments
We are grateful to all twins for participating in this study, their families, and municipal officials for their help with data collection in this study. The authors are also grateful to an anonymous referee for valuable comments and suggestions, which helped to considerably improve the quality of the manuscript. This research was supported by Kırıkkale University Research Grant: KKU, 2009/43. Danielle M. Dick was supported by K02 AA018755.
Appendix
Turkish Twin Study: English Translation of Interview Questions
-
1. Occupation (educational status): . . . . . .
-
2. Job title: . . .

-
5. Your family's location (where you grew up): . . .
-
6. Height: . . . (cm)
-
7. Weight: . . . (kg)
-
8. Who raised you?
-
(a) Mother and father (b) Only the mother (c) Only the father (d) Grandma, Grandpa
-
(e) Other relatives (f) In dorms
-
-
9. How many people are/were in your family(where you grew up)?: . . .
-
10. Who in your family is a smoker? Please indicate the number of cigarettes per day.
-
(a) Mother (. . .) (b) Father (. . .) (c) Sister (. . .) (d) Other (. . .) (e) None
-
11. What is the total income of your family? (TL — Turkish lira)
-
(a) -500 (b) 501 to 1000 (c) 1001 to 1500 (d) 1501 to 2000 (e) 2001–. . .
-
12. Mother's education level?
-
(a) Illiterate (b) Primary school (c) Secondary school (d) High school (e) University (f) Graduate
-
13. Father's education level?
-
(a) Illiterate (b) Primary school (c) Secondary school (d) High school (e) University (f) Graduate
-
14. Do you do sports regularly?
-
(a) No (b) Yes
-
15. How do you rate your performance in your school/job?
-
(a) Successful (b) Medium (c) Unsuccessful
-
16. What is the frequency with which you consume alcoholic beverages?
-
(a) Daily (b) Once a week (c) Once a month (d) Once a year (e) Never used
-
17. If you have used alcohol, what is the maximum number of drinks you have had in a 24-hour period? One drink is a regular glass of raki (raki is mostly used Turkish alcoholic drink with 40% alc. volume consumed with added water), a half liter bottle of beer, a glass of wine, or 100 grams of vodka, cognac, etc.: . . .
-
18. If you used alcohol, what is the age when you first used alcohol: . . .
-
19. Have you had alcohol-related problems in your lifetime, like feeling bad because you were drunk, or creating a disturbance? What is the age when this happened to you the first time . . .
-
20. Did you ever use thinner, marijuana, cocaine, opioids, or similar substances?
-
(a) No (b) Yes
-
21. If you used thinner, marijuana, cocaine, opioids, or similar substances, how old were you when you used them the first time? . . .
-
22. How often do you use thinner, marijuana, cocaine, opioids, or similar substances?
-
(a) Daily (b) Once a week (c) Once a month (d) Once a year (e) Never used
-
23. Have you felt depressed, moody, uninterested in anything, or like you didn't enjoy anything for a certain period in your life?
-
(a) No (b) Yes
-
24. Which of the following television channels do you watch? (You can choose more than one)
-
(a) ATV (b) Any of the TRT channels (c) Samanyolu (d) Star (e) Show TV (f) Kanal 7 (g) Kanal D (h) Others . . .
-
25. Which of the following newspapers do you read? (You can choose more than one)
-
(a) Cumhuriyet (b) Hürriyet (c) HaberTürk (d) Milliyet (e) Radikal (f) Sabah (g) Posta (h) Vakit (i) Zaman (j) Local newspaper (k) Other . . .
-
26. Would you support future genetic research related to nicotine dependence by providing a saliva sample and answering new interview questions?
-
(a) No (b) Yes
-
27. Questions about your twin:
-
(a) Are your twin (or you are one of triplets or quadruple)?
-
(1) No (go to question (l) (2) Yes
-
(b) Your twin (twin brother or sister):
-
(1) lives in Turkey (2) lives abroad (3) died in . . . (year)
-
(c) Your twin’s:
-
Name. . .
-
Current surname . . .
-
Current address . . .. . .. . .. . .. . .. . .. . .. . .. . .. . .
-
(d) Twin has the same gender as you?
-
(1) No — go to question (i) (2) Yes
-
(e) Were you and your twin identical or just as alike as two normal siblings?
-
(1) Were identical (2) Were like as two siblings (3) Do not know
-
(f) Were you and your twin so identical that people could not tell you apart?
-
(1) No (2)Yes (3) Do not remember
-
(g) Who could tell you apart when you were school age?

-
(h) Have you used any emblem, sign, or mark to distinguish from your twin?
-
(1) No (2) Yes (3) Do not remember
-
(i) How long have you lived with your twin?
-
Currently, we live together
-
We lived together until the age of . . .
-
(j) How often you see or call your twin?
-
(1) Every day or almost every day (2) About once a week (3) About once a month (4) Occasionally (5) Never
-
(k) Which one of you was born first?
-
(1) My twin was born first (2) I was born first (3) Do not know
-
(l) Are you one of a triple or quadruplet?
-
(1) No (2) Yes
-
(m) Your marital status:
-
(1) Single (2) Married (3) Remarried (4) Live with someone, but not married (5) Divorced or separated (6) Widowed
-
-
28. Did you have more than a month period in your lifetime during which you smoked cigarettes or used tobacco products every day?
-
Yes (continue) No (stop interview)
-
29. Questions regarding tobacco use:
-
(a) How many months or years did you continuously used cigarettes/tobacco products?
. . . years . . . months
-
(b) The average daily number of cigarettes/cigars/pipes used . . .
-
(c) Age when you started to smoke regularly? . . .
-
(d) If you are not a current smoker, how old were you when you quit? . . .
-
(e) What was the reason for starting to smoke?

-
(f) Did you try to quit smoking?
-
(a) No (b) Yes
-
(g) How many times have you tried to quit smoking? . . .
-
(h) What is the longest time that you stopped smoking? . . .
-
(i) If you tried to quit and experienced some problems, please provide

-
(j) Did the warnings on the cigarette pack affect you? (a) No (b) Yes
-
(k) Have you found any of the following methods for quitting to be effective?

-
(l) How soon after you wake up do you smoke your first cigarette?
-
(1) Within 5 minutes (2) Within 6–30 minutes (3) Within 31–60 minutes (4) After 60 minutes
-
(m) Do you find it difficult to refrain from smoking in places where it is forbidden
-
(a) No (b) Yes
-
(n) Which cigarette would you hate most to give up?
-
(1) The first one in the morning (2) Any other
-
(o) How many cigarettes do you smoke per day?
-
≥31; 21–30; 11–20; 1–10
-
(p) Do you smoke more frequently during the first hours of waking than during the rest of the day?
-
(a) No (b) Yes
-
(r) Do you smoke if you are so ill that you are in bed most of the day?
-
(a) No (b) Yes
-
(h) When do you smoke the most, generally?

-
(t) Reasons for smoking:

-
(u) By your knowledge, which diseases are caused by smoking? (check more than one if needed)

-

Interviewer's name: . . .
Date: . . .
-



















