Intrahepatic cholestasis of pregnancy (ICP) is a unique, pregnancy-related hepatic disorder characterized by pruritus with disturbed liver function tests and typically elevated serum levels of total bile acids (TBA; Williamson & Geenes, Reference Williamson and Geenes2014). The incidence of ICP varies around the globe, geographically between 0.1% and 15.6% (Ozkan et al., Reference Ozkan, Ceylan, Ozkan and Yildirim2015; Williamson & Geenes, Reference Williamson and Geenes2014), and in China, the incidence is about 1.0–4.0% along the Yangtze River (Li et al., Reference Li, Zhao, Ou and Jia2013). ICP is associated with increased risk for many fetal complications, such as sudden intrauterine fetal demise and stillbirth (Brouwers et al., Reference Brouwers, Koster, Page-Christiaens, Kemperman, Boon, Evers and Oudijk2015; Puljic et al., Reference Puljic, Kim, Page, Esakoff, Shaffer, LaCoursiere and Caughey2015; Williamson & Geenes, Reference Williamson and Geenes2014). The etiology of ICP is not fully understood; however, the contributing factors appear to be multifactorial, including ethnicity, diet, advanced maternal age, family history of biliary disease, ICP in previous pregnancy, and twin gestation (Diken et al., Reference Diken, Usta and Nassar2014; Williamson & Geenes, Reference Williamson and Geenes2014).
In comparison with singleton pregnancies, maternal complications, and perinatal sequelae are more frequent in multiple gestations. Multiple pregnancies tend to increase the risk of maternal mortality, morbidity, and pregnancy-associated complications, such as gestational diabetes mellitus (GDM), gestational hypertensive disorders, and postpartum hemorrhage (Obiechina et al., Reference Obiechina, Okolie, Eleje, Okechukwu and Anemeje2011; Rao et al., Reference Rao, Sairam and Shehata2004; Vogel et al., Reference Vogel, Torloni, Seuc, Betrán, Widmer, Souza and Merialdi2013). Multiple gestations have also been identified as linked to the development of ICP (Lausman et al., Reference Lausman, Al-Yaseen, Sam, Nitsch and Chan2006; Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008), but there are only a few studies focused on ICP with multiple gestations. One large retrospective cohort study demonstrated increased risks of adverse perinatal outcomes in ICP twin pregnancies (Liu et al., Reference Liu, Landon, Chen and Cheng2015). This study was carried out in a more economically dynamic area in China, and more attention was paid to the fetal outcomes. Sichuan is a populous province in the south-west part of China with a high incidence of ICP. Our study of multiple pregnancies of ICP in Sichuan is a reflection of this disease in China, and also serves to fill in the gap of ICP research in low-income regions.
Due to the development and increase in usage of assisted reproductive technology (ART), the incidence of multiple births has risen steadily over the past three decades (International Committee for Monitoring Assisted Reproductive Technology, 2013; Scholten et al., Reference Scholten, Chambers, van Loendersloot, van der Veen, Repping, Gianotten and Mol2015). Recent research found that ART multiple gestation infants were as high as 14.7–29.0% in different countries (Scholten et al., Reference Scholten, Chambers, van Loendersloot, van der Veen, Repping, Gianotten and Mol2015). The proportion of births from ART in mainland China was about 1% in 2011 (Yang et al., Reference Yang, Li, Li and Zhang2014). Despite the maternal and fetal problems rooted in multiple pregnancies, ART seems to be a single risk factor for substantial excess perinatal morbidities and many other maternal and neonatal adverse events. Complications of multiple pregnancies may be affected or compounded by the presence of ICP; the role of ART in these patients also needs to be explored. Given this background, we sought to ascertain the interaction effect of ICP and ART in twin pregnancies. We aim to investigate the association of ICP with maternal and neonatal complications in ART pregnancies as well as in spontaneous pregnancies (SP). Also, the influence of ART in ICP patients was analyzed.
Materials and Methods
This retrospective cohort study was undertaken at West China Second University Hospital of Sichuan University in Chengdu, China, following approval from the Ethics Committee. All twin pregnancies delivered above 28 gestational weeks in our hospital from January 2013 to May 2015 were included. International Classification of Diseases, 10th version (ICD-10) codes were used to identify twin pregnancies complicated by ICP. All the clinical data were obtained from the electronic medical records system.
All cases of ICP were confirmed by demonstration of serum TBA above 10 mmol/L, raised liver transaminase enzymes in association with pruritus, and no additional identifiable cause for their liver dysfunction (Royal College of Obstetricians and Gynaecologists, 2011; Williamson & Geenes, Reference Williamson and Geenes2014). Severe ICP was defined according to TBA levels as >40 mmol/L (Geenes et al., Reference Geenes, Chappell, Seed, Steer, Knight and Williamson2014; Glantz et al., Reference Glantz, Marschall and Mattsson2004; Raz et al., Reference Raz, Lavie, Vered, Goldiner, Skornick-Rapaport, Asher and Rimon2015; Royal College of Obstetricians and Gynaecologists, 2011; Williamson & Geenes, Reference Williamson and Geenes2014). Patients were excluded if their pruritus could be attributed to causes other than ICP, or if they had gallstones, cholecystitis, or liver cirrhosis. All patients were screened by lab tests for the infection of hepatitis virus, Epstein Barr virus, and cytomegalovirus. Methods of in vitro fertilization, intracytoplasmic sperm injection, and homologous or heterologous intrauterine insemination conceived twin pregnancies were all included in the ART group.
Data on maternal demographics, obstetric, and medical history were collected for all women, and data on serum biochemistry, management, and monitoring were collected for the ICP cases. Gestational age was calculated with reference to the first trimester ultrasound scan. First trimester ultrasound examination was also used to determine the chorionicity. Maternal complications such as preeclampsia, GDM, and premature rupture of membrane (PROM), placenta previa, placental abruption, postpartum hemorrhage, cesarean delivery rate, and meconium stained amniotic fluid (MSAF) were recorded. The perinatal outcomes studied were intrauterine fetal demise, preterm birth, birth weight, Apgar scores, neonatal intensive care unit (NICU) admission rate, and complications of respiratory tract of the newborns. Preeclampsia was defined according to the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin 2002 (ACOG, 2002). GDM was diagnosed by the 75 g oral glucose tolerance test (OGTT) recommended by the American Diabetes Association (2013). Postpartum hemorrhage was defined according to the ACOG practice bulletin 2006 (ACOG, 2006). Fetal growth restriction was defined <10th centile in either baby. A low Apgar score was defined as a score of <7 at 5 min.
Data were anonymized and double entered into a customized database. Statistical analysis was performed using SPSS version 19.0 (IBM, Amonki, NY, USA). We compared ICP with non-ICP twin pregnancies. Pregnancies conceived by ART and SP were analyzed separately in subgroup analysis. Comparisons of maternal and fetal outcomes on the difference of severity and onset time of ICP as well as conceiving methods were performed in ICP pregnancies. The effect of ART on maternal and fetal outcomes was also analyzed. A two-tailed t test and a χ2 test were used to compare the clinical characteristics between groups. Independent risk factors were analyzed and identified by univariate and multivariate logistic regression analyses, in which possible confounding factors (maternal age, gravidity, parity, pregestation, body mass index [BMI], chorionicity, and conceiving method) were taken into account. Multiple linear regression was also used in some perinatal outcomes. When analyzing ART effect, we also took socio-economic factors into the multivariate analysis. The unit of analysis was each pregnancy for both the maternal outcomes and the fetal outcomes. As for birth weight, average weight was calculated of the two live newborns; in the case of one deceased fetus, only the weight of the live one was used. p < .05 was considered statistically significant.
Results
There were a total of 1,543 patients with twin pregnancies delivered in this study: 71 twin gestations were excluded due to gestational age <28 weeks at delivery, unconfirmed chorionicity, and loss of data, and 1,472 twin gestations were included in the analysis. ICP was diagnosed in 362 patients and 677 patients conceived using ART (Figure 1). Maternal characteristics were compared between ICP and non-ICP twin sets. Women with ICP were more likely to be conceived by ART (52.8% vs. 43.8%; p = .003), and have dichorionic pregnancies (81.5% vs. 71.9%; p = .001). Maternal age, gravidity, parity, pregestation BMI, and socio-economic factors such as insurance, education, and area of residence did not differ significantly between the two groups (Table 1).
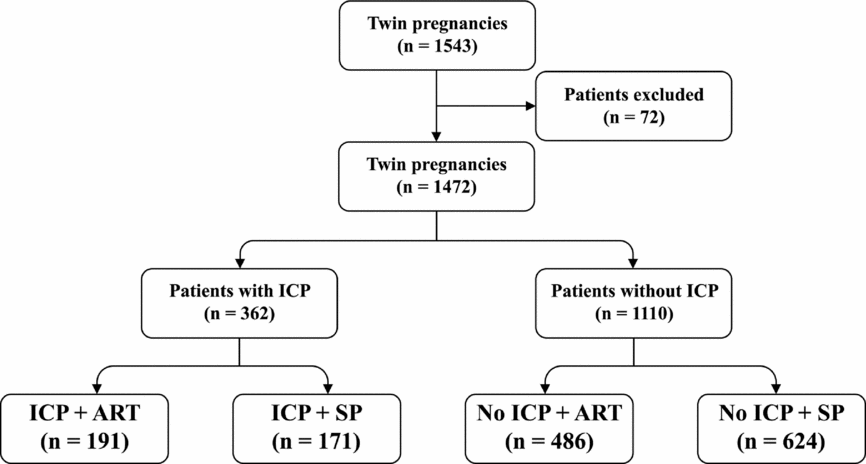
FIGURE 1 Flow diagram showing the cases ascertainment and groups of included patients.
TABLE 1 Baseline Maternal Characteristics
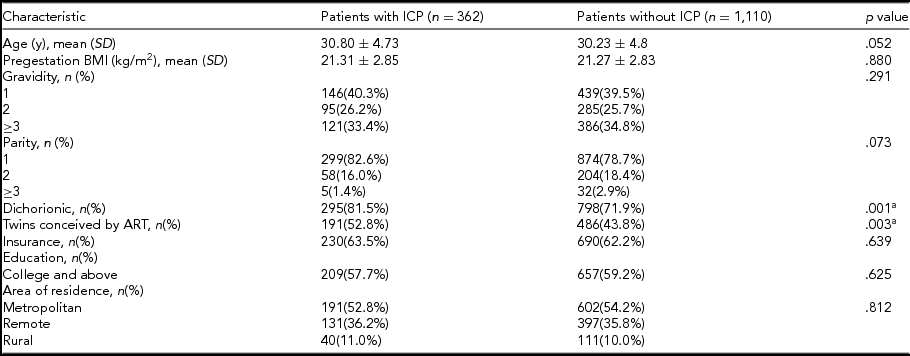
BMI = body mass index; ART = assisted reproductive techniques; ICP = intrahepatic cholestasis of pregnancy; SD = standard deviation.
a Denotes significant values if p < .05.
Except for lactate dehydrogenase measured at the time of diagnosis, there were no significant differences of liver enzymes and serum TBA between ART and SP of ICP. Serum TBA, alanine transaminase, and aspartate transaminase were not different at the time of diagnosis or at the time of delivery. The gestational weeks for the diagnosis of ICP (32.2 ± 3.6 vs. 32.4 ± 3.7 weeks in ART and SP, respectively; p = .75) or peak levels of serum TBA (35.9 ± 34.9 vs. 35.3 ± 33.1 μmol/l, respectively in ART and SP; p = .87) were not different, either. Ursodeoxycholic acid was given to all the ICP patients, and women with abnormal liver functions were also treated with S-adenosyl-L-methionine.
Regarding maternal complications, there were significantly higher rates of preeclampsia in the ICP population in our unadjusted analysis; the difference remained significant following adjustment for potential confounding factors (14.1% vs. 7.9%; aOR 1.96; 95% CI 1.35, 2.85). There was also an increased risk of MSAF (14.4% vs. 5.6%; aOR 3.10; 95% CI 2.10, 4.61). The incidence of PROM was lower in ICP patients (19.3% vs. 25.1%; aOR 0.71; 95% CI 0.53, 0.96). Regarding perinatal outcomes, preterm deliveries (83.4% vs. 64.8%; aOR 3.20; 95% CI 2.35, 4.37) were more likely in ICP twins. The risk of cesarean delivery was higher in ICP pregnancies in our unadjusted analysis; however, after controlling for the confounding factors, the difference has no statistical significance (88.4% vs. 83.4%; aOR 1.40; 95% CI 0.96, 2.02). No significant differences were found between groups in other maternal outcomes of rate of gestational diabetes, placental abruption, placental previa, or postpartum hemorrhage. Also, there were no significant differences between ICP and non-ICP twins in intrauterine fetal demise, fetal growth restriction, Apgar score at 5 min, NICU admission, or respiratory complications of neonates. To further preclude the influence of other gestational complications, we also performed multivariate analysis, taking preeclampsia as one of the controlling factors. The rate of delivery was still significantly different between ICP and non-ICP twin sets, as was the rate of MSAF and PROM (Table 2).
TABLE 2 Maternal and Fetal Outcomes in ICP and Non-ICP Pregnancies
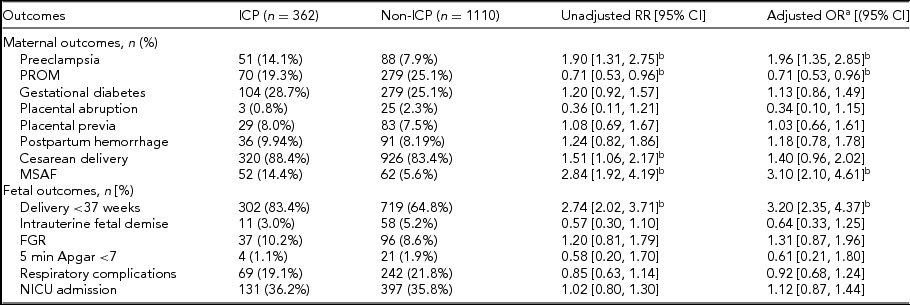
ICP = intrahepatic cholestasis of pregnancy; PROM = premature rupture of membranes; MSAF = meconium-stained amniotic fluid; FGR = Fetal growth restriction; BMI = body mass index; CI = confidence interval; OR = odds ratio; RR = risk ratio.
a Controlled for maternal age, gravidity, parity, pregestation BMI, chorionicity, conceiving method.
b Denotes significance with a CI that does not cross 1.
To better illustrate the differences in ART and SP twin sets, we further performed subgroup analysis. The rates of preeclampsia, MSAF, and preterm labor remained higher in ICP patients conceived by ART; the same differences were found in SP patients, with a slightly larger OR of preeclampsia and preterm delivery. These differences remained after adjustment for confounding factors. ICP and non-ICP population differed in mean newborn birthweight in subgroup analysis of ART twin pregnancies (2243.49 vs. 2327.13 in ICP and non-ICP, respectively; p = .037). Multivariate linear regression analysis demonstrated ICP was correlated with a lower birthweight after adjustment of the confounding factors (aOR -93.66; 95% CI -171.92, -15.40). This is probably due to the early gestational weeks at delivery in ICP twin pregnancies (Figure 2a and b).
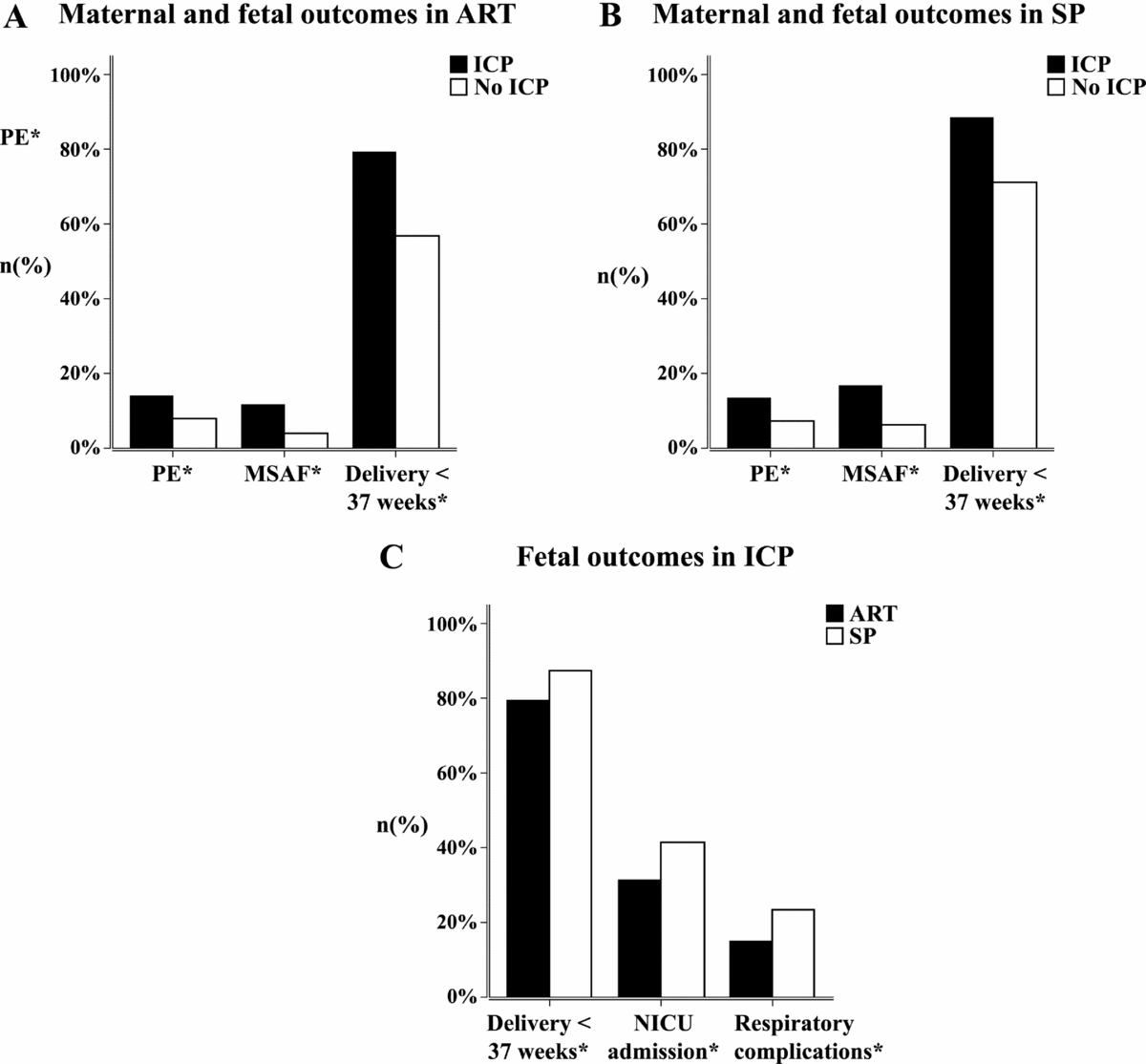
FIGURE 2 Maternal and fetal outcomes in pregnancies conceived by ART and spontaneous pregnancies (a and b); fetal outcomes in ICP pregnancies (c). (a) Different incidences of preeclampsia (PE), meconium-stained amniotic fluid (MSAF), and preterm delivery in ICP and non-ICP pregnancies conceived by ART; all these differences were statistically significant. (b) Different incidences of preeclampsia (PE), meconium stained amniotic fluid (MSAF), and preterm delivery in ICP and non-ICP spontaneous pregnancies; all these differences were statistically significant. (c) Different incidences of preterm labor, NICU admission, and respiratory complications in ART-conceived pregnancies and spontaneous pregnancies in ICP patients; all these differences were statistically significant.
Different maternal and fetal outcomes of ART pregnancies and SP are shown in Table 3. Gestational diabetes, placental previa, and postpartum hemorrhage were more common in ART pregnancies, while the incidence of fetal respiratory complications and NICU admission were lower. ART pregnancies also had lower rates of preterm deliveries at 34 weeks (Table 3). An analysis limited to pregnancies complicated by ICP did not show a difference in the risk of preeclampsia or MSAF based on conceiving methods. Incidence of gestational diabetes was not significantly different in ART versus SP pregnancies after adjustment for confounding factors (33.5% vs. 23.4%; aOR 1.17; 95% CI 0.66, 2.06). ART-conceived ICP twin pregnancies have a lower risk of delivery before 37 weeks (79.6% vs. 87.7%; aOR 0.48; 95% CI 0.24, 0.94), NICU admission of newborns (31.4% vs. 41.5%; aOR 0.61; 95% CI 0.38, 0.99), and neonatal respiratory complications (15.2% vs. 23.4%; aOR 0.53; 95% CI 0.29, 0.95); the significant difference remained even when adjusting for the gestational weeks at delivery (Figure 2c).
TABLE 3 Maternal and Fetal Outcomes in ART and SP Pregnancies
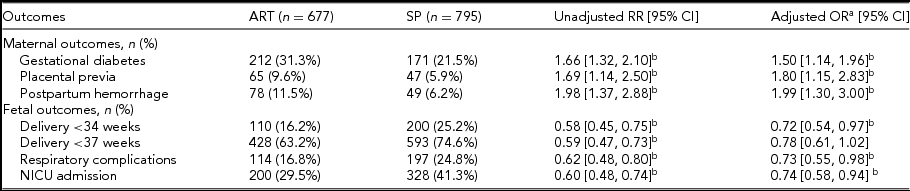
ART = assisted reproductive techniques; SP = spontaneous pregnancies; BMI = body mass index; CI = confidence interval; OR = odds ratio; RR = risk ratio; OR = odds ratio.
a Controlled for maternal age, gravidity, parity, pregestation BMI, chorionicity, insurance, education, and residence.
b Denotes significance with a CI that does not cross 1.
Significant relationships were found between the onset time of ICP and PROM, placenta previa, preterm delivery, NICU admission, and neonatal respiratory complications. We also performed analysis concerning the influence of ICP severity. Compared with the mild and moderate ICP cases, higher risks of MSAF (20.6% vs. 12.1%; aOR 1.92; 95% CI 1.03, 3.58) and preterm delivery (91.8% vs. 80.4%; aOR 2.80; 95% CI 1.26, 6.23) were observed in the severe group. Linear logistic regression indicates that the neonatal birthweight was influenced by the onset time and severity of ICP, which is lower in both the early onset ICP cases (aOR -211.83; 95% CI -294.39, -129.28) and the severe ICP cases (aOR -97.51; 95% CI -191.78, -3.23) (Table 4).
TABLE 4 Maternal and Fetal Outcomes in Early and Late Onset ICP Patients
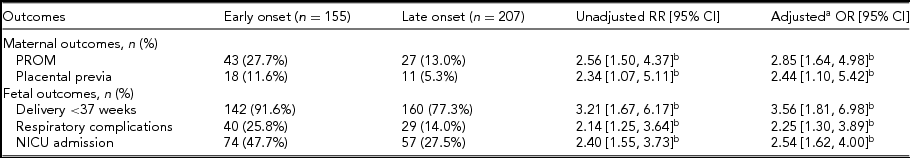
ART = assisted reproductive techniques; BMI = body mass index; CI = confidence interval; OR = odds ratio; RR = risk ratio; OR = odds ratio.
a Controlled for maternal age, gravidity, parity, pregestation BMI, chorionicity and conceiving method.
b Denotes significance with a confidence interval that does not cross 1.
Discussion
This study demonstrates the association of ICP and ART in twin pregnancies. Significant correlations between ICP and adverse maternal or neonatal outcomes were found. The present results are in accordance with several published studies that reported a higher risk of preeclampsia, preterm delivery, and MSAF (Brouwers et al., Reference Brouwers, Koster, Page-Christiaens, Kemperman, Boon, Evers and Oudijk2015; Geenes et al., Reference Geenes, Chappell, Seed, Steer, Knight and Williamson2014; Raz et al., Reference Raz, Lavie, Vered, Goldiner, Skornick-Rapaport, Asher and Rimon2015). Although the outcome of twin pregnancies resulting from ART has been the subject of controversy in the literature, our study demonstrated the impact of conceiving method on twin pregnancies. The findings indicate that patients with early-onset ICP or severe ICP had higher possibilities of preterm delivery, MSAF, and lower birth weight, and reveal the requirement of closer monitoring in these patients.
Epidemiologic surveys show significant regional differences of ICP prevalence. It varies from 0.1–1.5% in Europe to 9.2–15.6% in South American countries, such as Bolivia or Chile. It is particularly high in native Araucanians in Chile (28%) (Milkiewicz et al., Reference Milkiewicz, Elias, Williamson and Weaver2002; Wang et al., Reference Wang, Yao, Peng, Zhang, Ai, Ying and Liu2007; Williamson & Geenes, Reference Williamson and Geenes2014). A retrospective study analyzed ICP patients in our hospital from 1991 to 2000 and found the rate of ICP was as high as 5.2% in singleton pregnancies (Wang et al., Reference Wang, Yao, Peng, Zhang, Ai, Ying and Liu2007). Apart from regional factors, multiple gestations were considered to be another risk factor for ICP because of higher levels of estrogen and progesterone (Gonzalez et al., Reference Gonzalez, Reyes, Arrese, Figueroa, Lorca, Andresen and Arce1989; Lausman et al., Reference Lausman, Al-Yaseen, Sam, Nitsch and Chan2006; Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008; Ozkan et al., Reference Ozkan, Ceylan, Ozkan and Yildirim2015; Reyes & Sjovall, Reference Reyes and Sjovall2000).One study reported the incidence of ICP was 20.9% in twin pregnancies (Gonzalez et al., Reference Gonzalez, Reyes, Arrese, Figueroa, Lorca, Andresen and Arce1989). The incidence of ICP in our patients was as high as 24.6%. As an important medical center in the south-west part of China, the public acceptance and approval also contributes to this high rate of ICP.
The exaggeration of atherogenic-like response, including insulin resistance and dyslipidemia, is one of the basic adaptive changes in pregnant women, and may manifest itself as preeclampsia or GDM (Kaaja & Greer, Reference Kaaja and Greer2005). Some common pathogenetic pathways seem to be shared by preeclampsia and GDM (Kaaja & Greer, Reference Kaaja and Greer2005; Martineau et al., Reference Martineau, Raker, Powrie and Williamson2014; Reference Martineau, Raker, Dixon, Chambers, Machirori, King and Williamson2015), especially in women with higher BMI whose metabolic pathways are disturbed, which can cause adverse maternal or fetal outcomes (Cnattingius et al., Reference Cnattingius, Bergstrom, Lipworth and Kramer1998; Stothard et al., Reference Stothard, Tennant, Bell and Rankin2009). In our multivariate analysis, pregestation BMI is the common contributor to both preeclampsia (aOR 1.10) and GDM (aOR 1.12). The similar finding of obesity as a risk factor for preeclampsia or GDM was inconsistent with other studies (Al-Obaidly et al., Reference Al-Obaidly, Parrish, Murphy and Maxwell2014; Scifres et al., Reference Scifres, Feghali, Althouse, Caritis and Catov2015; Somprasit et al., Reference Somprasit, Tanprasertkul, Rattanasiri, Saksiriwutth, Wongkum, Kovavisarach and Wuthiwong2015). A retrospective study in Canada included 504 twin pregnancies. When participants were divided into four groups according to pregestation BMI, researchers found an increased risk of PE and GDM in the obese patients group (Al-Obaidly et al., Reference Al-Obaidly, Parrish, Murphy and Maxwell2014).
Except for pregestation BMI, the elevated TBA levels may act synergistically to cause preeclampsia or impaired glucose tolerance (Martineau et al., Reference Martineau, Raker, Powrie and Williamson2014; Reference Martineau, Raker, Dixon, Chambers, Machirori, King and Williamson2015; Ozkan et al., Reference Ozkan, Ceylan, Ozkan and Yildirim2015; Williamson & Geenes, Reference Williamson and Geenes2014). On the one hand, elevated serum bile acids may cause malfunction of endothelial cells and renal disturbances (Perez & Briz, Reference Perez and Briz2009; Smolarczyk et al., Reference Smolarczyk, Wojcicka-Jagodzinska, Piekarski, Romejko and Czajkowski2000). It can also trigger increased oxidative stress reaction, and leads to the up-regulation of soluble tyrosine kinasee1, which is considered to be a major contributor to the pathogenesis of preeclampsia (Anderson et al., Reference Anderson, Olsson, Kristensen, Akerstrom and Hansson2012; De Vivo et al., Reference De Vivo, Baviera, Giordano, Todarello, Corrado and D'Anna2008; Hu et al., Reference Hu, Liu and Xing2015; Li et al., Reference Li, Gu, Zhang, Lewis and Wang2005; Maynard et al., Reference Maynard, Min, Merchan, Lim, Li, Mondal and Karumanchi2003). The increased incidence of preeclampsia in ICP patients was proven in singleton pregnancies (Geenes et al., Reference Geenes, Chappell, Seed, Steer, Knight and Williamson2014; Goulis et al., Reference Goulis, Walker, de Swiet, Redman and Williamson2004; Lee et al., Reference Lee, Goodwin, Greenspoon and Incerpi2006; Marschall et al., Reference Marschall, Shemer, Ludvigsson and Stephansson2012; Wikstrom et al., Reference Wikstrom Shemer, Marschall, Ludvigsson and Stephansson2013), but studies reported in the literature are not consistent on the incidence of preeclampsia in ICP twin pregnancies. Lausman et al. (Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008) found no difference in the incidence of preeclampsia in ICP pregnancies in 263 multiple pregnancies in Canada (Lausman et al., Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008). Another study of 124 twin pregnancies revealed that the incidence of preeclampsia were almost five-fold in ICP patients compared to the controls (Raz et al., Reference Raz, Lavie, Vered, Goldiner, Skornick-Rapaport, Asher and Rimon2015). Moreover, Raz et al. (Reference Raz, Lavie, Vered, Goldiner, Skornick-Rapaport, Asher and Rimon2015) found preeclampsia was concomitant with ICP severity at initial diagnosis, and occurred most often 2–4 weeks after the diagnosis of ICP. In our study of 1,543 twin pregnancies, the correlation of diagnosis time of ICP and preeclampsia was not found, but incidence of preeclampsia was significantly higher (almost two-fold) in ICP twin sets.
On the other hand, bile acids were reported to activate the farnesoid X (FXR) receptor and promote insulin release (Ma et al., Reference Ma, Saha, Chan and Moore2006). Disruption in homeostatic pathways for glucose balance was observed in ICP pregnancies (Martineau et al., Reference Martineau, Raker, Powrie and Williamson2014; Reference Martineau, Raker, Dixon, Chambers, Machirori, King and Williamson2015). Studies targeting singletons indicated an elevated risk of GDM in ICP pregnancies with an OR of 1.6–2.8 (Diken et al., Reference Diken, Usta and Nassar2014; Martineau et al., Reference Martineau, Raker, Dixon, Chambers, Machirori, King and Williamson2015). However, the incidence of GDM was not different in women predisposed to ICP in our study. Multivariate analysis revealed the major contributors to GDM were pregestation BMI as well as the patient's age. This may indicate the possibility of the inability in glucose homeostasis regulation with advancing age, which may contribute more to the pathogenesis of GDM, in addition to the known effects upon bile acid metabolism. Interventions to control weight gain should be used to improve outcomes in these high-risk women.
Except for maternal complications, the accumulating data indicated higher risk of fetal complications relating to elevated circulating bile acids (Geenes et al., Reference Geenes, Chappell, Seed, Steer, Knight and Williamson2014; Lausman et al., Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008; Puljic et al., Reference Puljic, Kim, Page, Esakoff, Shaffer, LaCoursiere and Caughey2015; Shemer et al., Reference Shemer, Thorsell, Marschall and Kaijser2013; Wikstrom et al., Reference Wikstrom Shemer, Marschall, Ludvigsson and Stephansson2013). In our study, of the 362 ICP twin pregnancies after 28 weeks of gestation were included, we found that ICP was correlated with higher risks of preterm labor and MSAF, while early onset ICP had higher incidence of PROM. Other studies with smaller numbers of participants with twin pregnancies had different findings (Gonzalez et al., Reference Gonzalez, Reyes, Arrese, Figueroa, Lorca, Andresen and Arce1989; Lausman et al., Reference Lausman, Al-Yaseen, Sam, Nitsch, Barrett and Chan2008). In ICP pregnancies, the transplacental gradient for excretion of bile acids from the fetus to mother is reversed, leading to the accumulation of the toxic compounds in the fetal serum as well as amniotic fluid (Brites, Reference Brites2002; Reyes & Sjovall, Reference Reyes and Sjovall2000; Williamson & Geenes, Reference Williamson and Geenes2014). A dose-dependent bile acid effect on myometrial contractility was demonstrated in rodents (Campos et al., Reference Campos, Castillo and Toro1988). Bile acids can cause increased expression and response of the oxytocin receptor in human myometrial cells (Germain et al., Reference Germain, Kato, Carvajal, Valenzuela, Valdes and Glasinovic2003; Israel et al., Reference Israel, Guzman and Campos1986). Preterm labor may be explained by increased uterine contractility in the third trimester of ICP patients (Zhao et al., Reference Zhao, Zhang, Yao and Yang2014). Alterations of hepatic metabolites in amniotic fluid in ICP pregnancies were observed (Menon et al., Reference Menon, Jones, Gunst, Kacerovsky, Fortunato, Saade and Basraon2014). Bile salts could react with surface-active phospholipid and reduce the hydrophobicity and lubricity of amniotic and chorionic epithelium (Hills, Reference Hills1994). We found women with early-onset ICP had a higher risk of PROM compared with the late-onset group in the subanalysis focusing on ICP pregnancies. However, when compared with the non-ICP pregnancies, the incidence of PROM was not higher, but this correlation was not found when we took in gestational weeks at delivery in multivariate analysis. Over-activated gut motility stimulated by bile acids was found in animal studies (Campos et al., Reference Campos, Guerra and Israel1986), which may explain MSAF.
Although our study did not find the hypodevelopment of fetal lungs, other studies revealed the altered composition and structure of fetal pulmonary surfactant in ICP pregnancies, and the changes of fetal pulmonary surfactant were correlated with the bile acids in amniotic fluid as well as in fetal cord blood (Yu et al., Reference Yu, Ding and Wang2011; Zhang et al., Reference Zhang, Li, Wang, Pitre, Fang, Frank and Schuetz2015). Intratracheal injection of bile acids in rabbits can cause atelectasis, eosinophilic infiltration, and the formation of hyaline membrane in lung (Kaneko et al., Reference Kaneko, Sato, Katsuya and Miyauchi1990). Hypodevelopment of fetal lungs is the most common reason for NICU admission in ICP pregnancies, however, the most disastrous and unacceptable consequence of ICP pregnancies is stillbirth. The rate of stillbirth in women with ICP is reported to be 1.5–7.0% (Geenes et al., Reference Geenes, Chappell, Seed, Steer, Knight and Williamson2014; Jin et al., Reference Jin, Pan, Huang, Yu, Zhong and Zhang2015; Kawakita et al., Reference Kawakita, Parikh, Ramsey, Huang, Zeymo, Fernandez and Iqbal2015; Rioseco et al., Reference Rioseco, Ivankovic, Manzur, Hamed, Kato, Parer and Germain1994); especially in patients with a TBA level above 100 mmol/L, the rate of stillbirth was as high as 10–15% (Brouwers et al., Reference Brouwers, Koster, Page-Christiaens, Kemperman, Boon, Evers and Oudijk2015; Kawakita et al., Reference Kawakita, Parikh, Ramsey, Huang, Zeymo, Fernandez and Iqbal2015). In our study, the stillbirth rate was 3.0% in ICP twin pregnancies. Postmortem studies of stillborn neonates provided evidence of acute anoxia (Reid et al., Reference Reid, Ivey, Rencoret and Storey1976), which may be explained by sudden arrhythmias in neonates or marked vasoconstriction of placental chorionic vessels caused by bile acids (Al Inizi et al., Reference Al-Inizi, Gupta and Gale2006; Miragoli et al., Reference Miragoli, Kadir, Sheppard, Salvarani, Virta, Wells and Gorelik2011; Sepulveda et al., Reference Sepulveda, Gonzalez, Cruz and Rudolph1991). Different myocardial tissue velocities of both mitral and tricuspid valves in ICP fetuses were also found using tissue Doppler imaging (Ataalla et al., Reference Ataalla, Ziada, Gaber, Ossman, Bayomy and Elemary2016).
Like Raz et al. (Reference Raz, Lavie, Vered, Goldiner, Skornick-Rapaport, Asher and Rimon2015), we took 32 gestational weeks as the cut-off time point to determine the onset time of ICP. In our linear regression analysis, we did not find the correlation of ICP and birth weight, which is similar in previous reported results in singletons. However, after performing a subanalysis in ICP pregnancies, concentrating on severity and onset time, the differences were revealed. Patients from the severe ICP group had higher frequencies of preterm deliveries and MSAF than the mild group. The same results were found in the early-onset group compared with late-onset group. These findings indicate that the increased TBA correlates with poorer fetal outcomes, and the correlation is reinforced by time. In the present study, we also revealed a higher incidence of newborns in the early-onset group experienced respiratory problems and NICU admission. These findings indicate the possibility that it is the time of the disease, rather than the level of circulating TBA, that might be more disastrous to the fetal lungs.
The different incidences of ICP in ART pregnancies were rarely reported. In Finland, researchers included 225 IVF pregnancies (including multiple pregnancies) and 671 control pregnancies. The risk ratio of ICP for IVF singletons pregnancies was 3.8 (95% CI 1.0, 15.0; Koivurova et al., Reference Koivurova, Hartikainen, Karinen, Gissler, Hemminki, Martikainen and Jarvelin2002). The authors attributed this phenomenon to metabolic disturbances regarding infertility itself. Zamah et al. (Reference Zamah, El-Sayed and Milki2008) reported two patients with first-trimester cholestasis of pregnancy after IVF. The elevated serum bile acids level was associated with markedly elevated maternal serum estrogen levels caused by ovarian hyperstimulation syndrome (Zamah et al., Reference Zamah, El-Sayed and Milki2008). The abnormal liver function and higher incidence of ICP in ART pregnancies were also found in Kopylov's (Reference Kopylov, Avidan, Papageorgiou, Katz, Sivan, Zimlichman and Maor2013) study. They concluded that a potential pathogenetic mechanism for this correlation was rooted in the hormonal hyperstimulation syndrome occurring with ART treatments (Kopylov et al., Reference Kopylov, Avidan, Papageorgiou, Katz, Sivan, Zimlichman and Maor2013). In our study, the rate of ICP was higher in ART pregnancies. But in the separate analysis of ART and SP pregnancies, we did not find much difference in the influence of ICP between these two groups of patients. There were no huge differences of the aOR for preeclampsia, MSAF, and preterm delivery between ART and SP pregnancies.
Several studies comparing the neonatal and maternal outcome of ART and SP twin pregnancies have reported conflicting results (Bamberg et al., Reference Bamberg, Fotopoulou, Neissner, Slowinski, Dudenhausen, Proquitte and Henrich2012; Fan et al., Reference Fan, Sun, Yang, Ye and Wang2013; Moini et al., Reference Moini, Shiva, Arabipoor, Hosseini, Chehrazi and Sadeghi2012; Qin et al., Reference Qin, Wang, Sheng, Liang, Tan and Xia2015; Stern et al., Reference Stern, Luke, Tobias, Gopal, Hornstein and Diop2015; Szymusik et al., Reference Szymusik, Kosinska-Kaczynska, Bomba-Opon and Wielgos2012). Some studies reported that complications, such as GDM and preterm delivery were significantly more common in ART twins (Bamberg et al., Reference Bamberg, Fotopoulou, Neissner, Slowinski, Dudenhausen, Proquitte and Henrich2012; Moini et al., Reference Moini, Shiva, Arabipoor, Hosseini, Chehrazi and Sadeghi2012; Qin et al., Reference Qin, Wang, Sheng, Liang, Tan and Xia2015; Stern et al., Reference Stern, Luke, Tobias, Gopal, Hornstein and Diop2015). Similar to these studies, our research revealed higher risks for GDM, placental previa, and postpartum hemorrhage for ART pregnancies. But, as for fetal outcomes, the incidence of preterm deliveries, fetal respiratory complications, and NICU admission were lower in ART pregnancies, even when taking socio-economic factors into consideration. The same results were found in subgroup analysis of ICP pregnancies. Although many studies indicated a higher preterm delivery rate in ART pregnancies, there were still some studies with dissenting results. A Polish study found similar rates of preterm deliveries of ART and SP pregnancies (Szymusik et al., Reference Szymusik, Kosinska-Kaczynska, Bomba-Opon and Wielgos2012). Fan et al. (Reference Fan, Sun, Yang, Ye and Wang2013) included 162 ART dichorionic twin pregnancies together with 213 SP in Hubei province, China, and revealed a slightly lower incidence of preterm deliveries in the ART group. Our finding of lower rates in some adverse fetal outcomes might be explained by the larger gestational weeks at delivery in ART patients, which could lead to better development of fetal lungs (Gerber, Reference Gerber2015; Huddle et al., Reference Huddle, Tekes and Tunkel2016). The difference in the rate of monochorionic twin pregnancies also contributes to the different outcomes. Many studies have shown that dichorionic twin pregnancies have a better prognosis and lower rates of preterm deliveries than monochorionic twin pregnancies (Burgess et al., Reference Burgess, Unal, Nietert and Newman2014; Carter et al., Reference Carter, Bishop, Goetzinger, Tuuli and Cahill2015; D'Arpe et al., Reference D'Arpe, Franceschetti, De Stefano, D'Amelio, Maragno, Candelieri and Benedetti Panici2016; Masheer et al., Reference Masheer, Maheen and Munim2015). For ART pregnancies, the rate of monochorionic pregnancy was only 6.35%, while the rate of monochorionic pregnancy in spontaneous twin pregnancies was 42.26% in our study. Better compliance with doctors’ suggestions and more attention and care from family members might also lead to better outcomes for ART pregnant women. The long-term outcomes of ART twins are still largely unknown. Prospective studies are needed to explore the influence of ART.
This study addresses a significant gap in the literature on the outcomes of ICP twin pregnancies in Southwest China, where the incidence of ICP is higher compared with other places. The few existing studies on ICP twin pregnancies have been confined to high-income countries. To the best of our knowledge, the number of ICP twin pregnancies included in our study is the largest. Patients deriving from a single medical center ascertained the same diagnostic criteria, treatment, and prenatal monitoring. This study has limitations that should be noted. Medical records rather than direct patient interview were used to obtain the data. This resulted in lack of data on several maternal variables, such as educational level, family income, and smoking status. As a retrospective study in a large tertiary hospital, our results cannot be generalized to patients in community settings or to those delivering at home, who are the most common cases in low-income places in China.
In conclusion, our findings of a strong association of ICP and adverse maternal and fetal outcomes in twin pregnancies support the current practice of close antenatal monitoring of ICP twin pregnancies. More focus should also be paid on severe ICP and early-onset ICP to improve the prediction and treatment of these patients.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Number: 81200452) and supported by the Science and Technology Department of Sichuan Province (Number: 2015SZ0139). The authors declare no conflict of interest.








