The protection and appropriate use of human genetic and genomic information (HGI) are essential to respecting privacy and confidentiality while maintaining the trust of those who are considering, currently undergoing or who have previously had genetic and/or genomic testing. While acknowledging that all forms of medical and health information require protections to maintain privacy and confidentiality, this position statement aims to emphasize key considerations relevant specifically to HGI in healthcare settings. It explores the characteristics of HGI and how these contribute to unique considerations regarding protection and utilization. This position statement aims to highlight the interests of both the individual as well as the family.
As genetics and genomics become integrated into mainstream medicine, HGI is being generated, stored and accessed in a wide range of healthcare settings. This position statement provides background on the types of HGI and the contexts within which it may be used, together with guidance on appropriate usage and best practice for all healthcare professionals (HCPs; see Table 1) handling HGI.
Table 1. Terminology
This position statement seeks to outline the key considerations relevant to HGI in healthcare settings. This is important and necessary given the differing governance arrangements between Australian states and territories, between private and public sector entities, as well as between Australia and New Zealand. Also, as it is common for specimens or samples originating in Australasia to be sent overseas for genetic and/or genomic testing and/or research, it is difficult to have an intimate knowledge and understanding of the regulatory arrangements in every setting. Therefore, it is important to highlight the key considerations that are applicable to a range of scenarios.
The aim is to protect privacy and confidentiality while simultaneously balancing the interests of the individual with that of the broader family. This is done by educating individuals on the possible clinical and personal utility of HGI both for themselves as well as their family members. Individuals undergoing genetic and/or genomic testing should be provided with clear information, in an accessible format that they can understand, of how the data generated from their genetic and/or genomic test will be used, protected, stored and accessed, and in which circumstances and in what capacity the information may be shared. These protections will differ depending upon the setting in which the information is generated (clinical vs. research vs. consumer marketplace), and in accordance with the provisions in the specific consent provided by the consumer prior to testing.
Scope
This position statement applies to germline genetic information. It does not apply to somatic genetic information or infectious disease genomic testing. Box 1 outlines examples of the types of HGI commonly handled in healthcare settings. This information can originate from many different sources and settings. These settings may vary from clinical settings to research (Box 2), through to consumer-driven medical marketplaces (Savard et al., Reference Savard, Terrill, Dunlop, Samanek and Metcalfe2020).
Box 1. Examples of human genetic information
Clinical, research or consumer genetic/genomic results and reports
Medical records of a personal or family history of an inherited condition
Family tree (pedigree)
Consent documentation
Genomic data (raw and/or interpreted)
Entire genomic datasets
Medical correspondence
Medical photography of phenotypic features
Box 2. Research setting
The distinction between research and clinical HGI can be difficult to draw (National Health and Medical Research Council, 2015). Genomic results are often the product of information sharing between clinical and research HCPs. Clinical information is used to facilitate the interpretation of research findings. Translational research is increasingly informing clinical practice; clinical testing can be reinterpreted in research settings.
Genomic sequencing data may be generated initially in a clinical context, but further analysis of that data may be undertaken (with consent, if the data is identified) within a research setting. Alternatively, genomic sequencing data may be generated initially in a research context, and later validated in an accredited diagnostic laboratory.
Regardless of whether genomic data is considered research or clinical, data management needs to be secured to protect individual privacy, and the process for data sharing needs to be clearly stated and monitored by the data custodians. However, the setting in which HGI is generated will also influence how that data is regulated; reinforcing the importance of HCPs being aware of the required legal or regulatory oversight for the information they handle.
The boundaries between clinical and nonclinical contexts can be arbitrary, fluid or difficult to define. However, it is the potential for HGI to traverse the boundaries of these various settings that need to be recognized and acknowledged to ensure an appropriate level of protection.
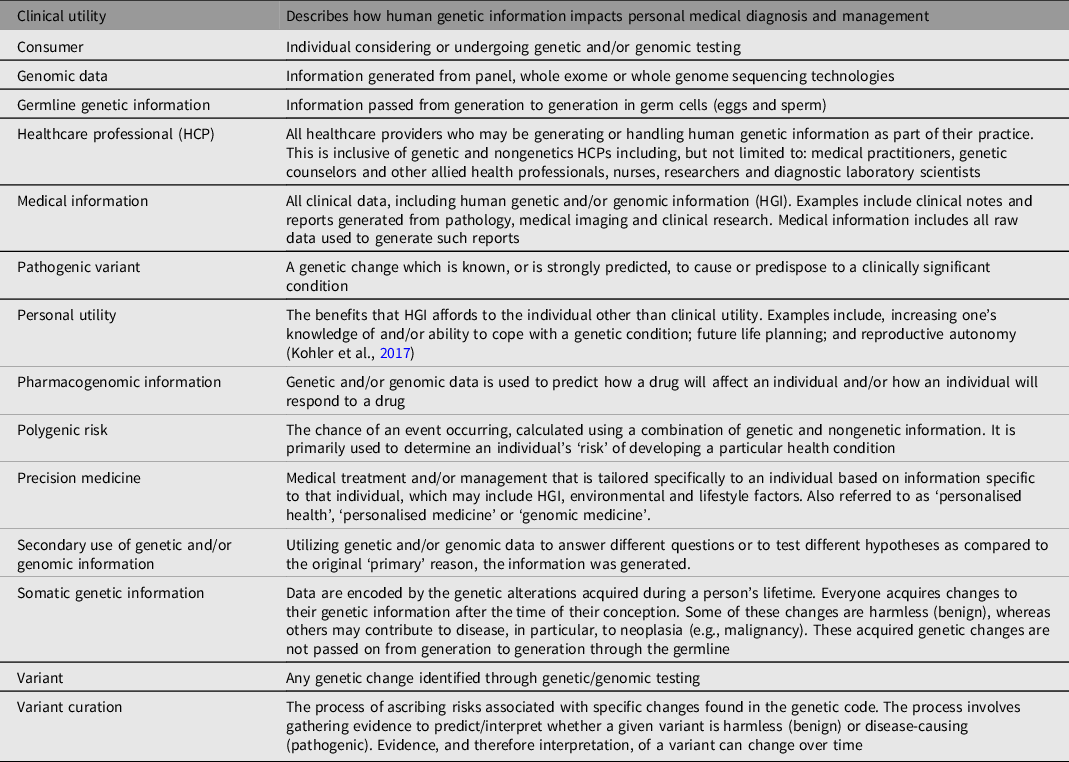
Figure 1 provides a visual framework of how this position statement was conceptualized and provides an overview of its scope.
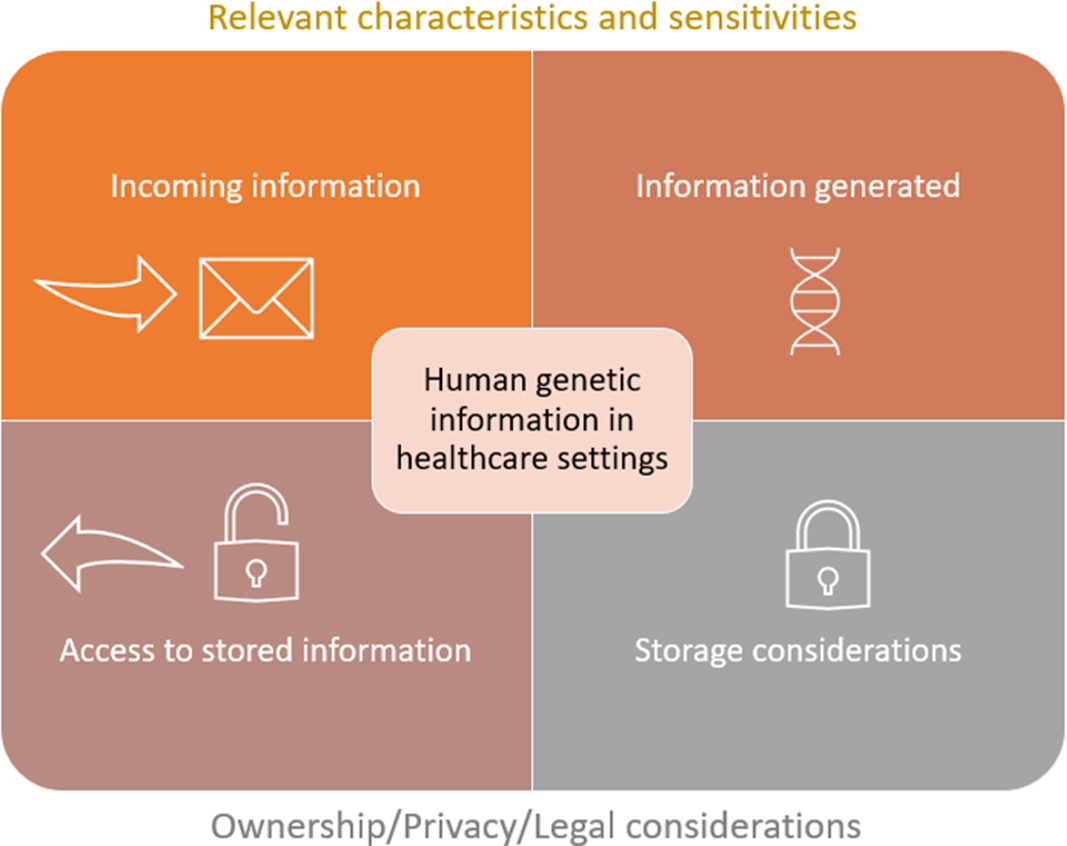
Fig. 1. Conceptual framework of the scope of this position statement. The upper section of the figure acknowledges that HGI may be received or requested to accompany a referral to a healthcare service, or that HGI may be generated as a result of a healthcare encounter. Regardless of how HGI is obtained, it is important for the HCP to recognize the relevant characteristics and sensitivities of the information at hand. It is these features that inform how the information should be protected. Protections are outlined in the lower section of the figure and include how the information is stored, utilized and accessed.
Notable Characteristics of Human Genetic Information
Historically, there has been debate about whether HGI is distinct from other forms of medical information and whether it requires a greater level of protection. This concept of ‘genetic exceptionalism’ stems, in part, from the historical misuse of genetic information (such as eugenics and genetic discrimination) as well as the characteristics of HGI that are viewed as distinct from other forms of personal medical information. This section outlines the characteristics of HGI that set it apart from other forms of health information in many circumstances.
Much HGI does not belong to a single individual. It is inherited from generation-to-generation and is therefore shared between genetic relatives. The inheritance patterns of many single gene conditions are well understood and allow for the identification of family members for which a particular genetic variant may be relevant. This shared nature of HGI may contribute to privacy and protection concerns within families. At the same time, it is this feature of HGI that highlights the importance of family communication and consensual sharing of genetic information to allow for accurate genetic counseling and risk assessment.
Some HGI has the unique potential to provide information about the chance of an individual developing a particular condition in the future (Vears et al., Reference Vears, Ayres, Boyle, Mansour and Newson2020). While this kind of predictive information may have significant clinical and/or personal utility for the individual as well as their family members, it can also raise fears of genetic discrimination (Newson et al., Reference Newson, Ayres, Boyle, Gabbett and Nisselle2018).
Every individual’s genetic information in its entirety is unique. Therefore, genomic data generated from exome and genome sequencing technologies are inherently reidentifiable. This means that these data can never be completely anonymized. This is relevant, as genomic data is often shared between research groups, on public databases to contribute to global knowledge of the human genome, and approaches to deidentification need to be considered in the context of the secondary reuse of HGI.
HGI comes in different forms; interpreted (curated) and uninterpreted (‘raw’ genomic data).
Interpretation can be highly nuanced and often relates to the likelihood of the variant contributing to a particular phenotype. Interpretation data is typically focused on a specific question. While this feature of HGI does not impact upon the protection of the information necessary, it does relate to how HGI is utilized in healthcare settings. Healthcare practitioners should be wary of the potential for reporting differences between laboratories, and importantly, the expectation that interpretations will change as knowledge evolves over time.
Uninterpreted raw data is often uploaded to online reference databases and sometimes supplied to the consumer from whom the data was generated. Raw genomic data has the potential to offer diagnostic, predictive and susceptibility information now and in the future. This potential exists both for the individual from whom the data was originally generated, as well as their biological relatives. Analysis and interpretation should only be undertaken by someone with the appropriate skills in variant curation.
These are some characteristics that define HGI. While these features may not be entirely unique to HGI (e.g., HGI shares properties with infectious disease), and while acknowledging that there are other forms of health data exceptionalism exist, it is important that HCPs are mindful of these properties as they have direct relevance to how HGI is protected and used.
Identifying the Appropriate Level of Protection
While the concept of genetic exceptionalism can still be applied to many forms of HGI, not all forms of genetic information are exceptional. The context in which HGI is generated and used needs to be considered when thinking about how it is to be protected. For example, with the advent of precision medicine, some genetic information is highly individual and will not generate sensitivities or implications for family members in the same way as other HGI might. Examples include certain types of pharmacogenomic information and polygenic risk scores.
Figure 2 illustrates the different sensitivities of human genetic information requiring different levels of protection.
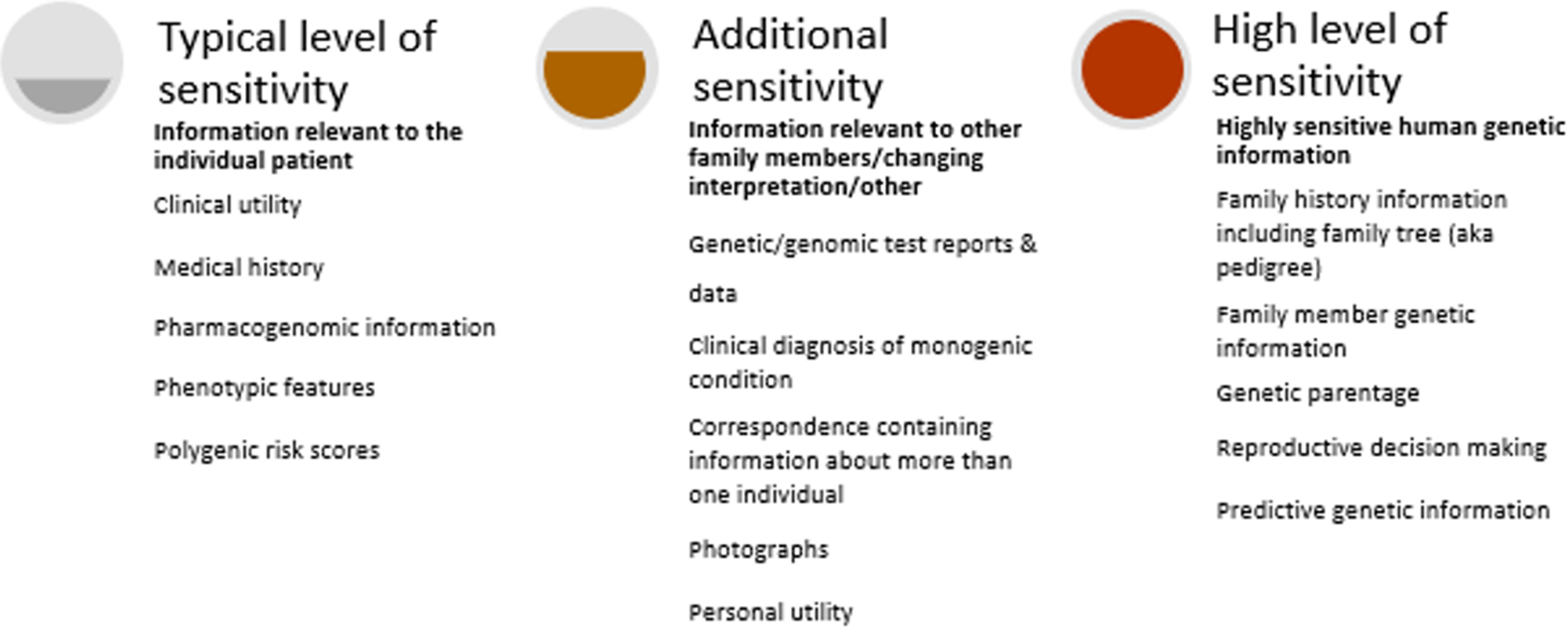
Fig. 2. Varying levels of sensitivity of human genetic information.
The following cases illustrate some of these sensitivities and how they may present in healthcare settings.
Sensitivity: Family Member Genetic Information
It can be important to seek genetic information about relatives to ensure accurate genetic counseling (e.g., inheritance patterns, risk assessment) and testing. The following illustrative case of Evelyn not only demonstrates the importance of sharing family genetic information, but also introduces how a HCP may end up handling genetic information that belongs to someone other than the individual seeking their care. Historically, HGI has been stored within genetic files grouped by family. While this remains a viable solution for HCP who have access to family-based storage systems, most electronic medical records (EMRs) are individual and episode focused, not family-oriented. This is important to consider, and HCPs should make every effort to ensure that HGI is afforded the necessary protections to maintain privacy and confidentiality.
Illustrative case 1
Evelyn, a 25-year-old female, is referred to a genetics clinic for a clinical assessment and consideration of genetic testing. The referral indicates that Evelyn’s brother, Jack, was recently diagnosed with Marfan syndrome, and that Evelyn would like to know whether she also has the condition. Marfan syndrome is a connective tissue disorder and can affect the heart, eyes, blood vessels and bones. Confirming or excluding a diagnosis of Marfan syndrome for Evelyn would have a direct impact on her medical care and surveillance recommendations.
To provide the most accurate assessment for Evelyn, it is necessary for the HCP seeing Evelyn gather further information about her brother. Specifically, it is necessary to understand whether Jack has a confirmed genetic diagnosis of Marfan syndrome, or whether his diagnosis is based on clinical features alone. Evelyn informs the HCP that Jack did have genetic testing as part of his diagnosis. Jack’s consent is required to access a copy of his genetic test report. This information is necessary for the HCP to determine which genetic variant to test Evelyn for, and therefore informs which test or technology is most appropriate.
Sensitivity: Differing Accounts of Family History Information
Due to the shared nature of HGI, detailed family health history information is often collected. As outlined in illustrative case 1, this helps to inform genetic counseling and the appropriate test to offer. Information collected often includes information about the health status of the individual’s relatives without their knowledge or permission. Unless verified by collecting the relevant medical records, it is important to note that the documented family history reflects one person’s knowledge and perception, which may not be entirely accurate or complete. For these reasons, family history information should be treated as highly sensitive, and requires special consideration regarding storage, access and sharing. The following illustrative case involving Mary, Adah and Iris explores this issue further.
Illustrative case 2: Mary
Mary’s brother, José, has recently been diagnosed with Huntington disease (HD). No other person in the family has been diagnosed with HD, although their father died with a neurological condition thought to be Parkinson disease. HD is usually an adult-onset condition that involves both cognitive and physical deterioration over time. There is no cure for HD. Mary would like to have a genetic test to find out if she is at risk of developing HD. This is a form of testing called ‘presymptomatic’ testing because Mary does not have any symptoms of HD, but the test can predict whether she will develop the condition in the future. Figure 3 displays a pedigree for Illustrative Case 2.
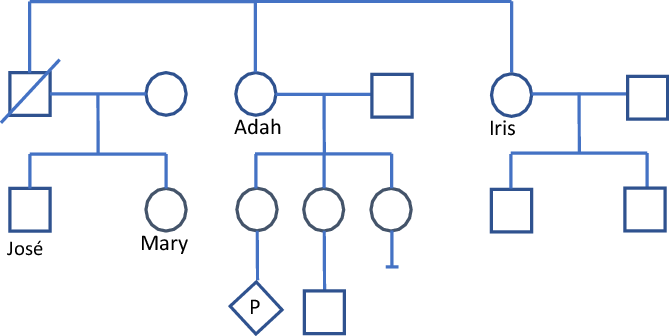
Fig. 3. Illustrative case 2 — Pedigree.
Adah
Adah has learned about the diagnosis of HD in her nephew José, her deceased brother’s son. Adah is in her late fifties and wants to have presymptomatic genetic testing. She has many health issues and wonders if she too has HD. Adah has the genetic test and learns that she has the pathogenic variant that causes HD. Because of her relationship to José, the result also indicates that the pathogenic variant in José inherited from his father (Adah’s brother). Adah chooses not to disclose her result to other family members. She has three daughters and a grandson. One of her daughters is currently pregnant. Adah’s nondisclosure is denying her daughters the opportunity to learn about their own health risks, and the risks for future generations. Adah insists that they have enough to worry about and insists that she does not wish to tell anyone in her family of her diagnosis.
Iris
Six months later, Iris seeks advice about presymptomatic testing for HD. Iris is Adah’s sister. Iris believes that her risk is small, as she is not aware of anyone else in her family having HD. She thinks it is more likely that her nephew José inherited HD from his mother’s side of the family. The HCP knows that Iris has a 50% chance of having the HD variant because the HCP knows Adah’s result and knows that the variant is in Iris’s side of the family. The HCP cannot disclose this, and so must manage the case carefully. Iris is 60 years of age and a retired nurse. She is well and healthy. She believes that due to her wellbeing, it is unlikely she has inherited the HD variant in her family, but feels it is important to have the test to inform her children of their risks and testing options. Iris has predictive testing and is found to have the familial pathogenic variant causing HD.
Sensitivity: Predictive Genetic Information
Illustrative case 2 demonstrates the sensitivities around HGI that predict the future health status of an individual. In most circumstances, this information should only be accessed with explicit consent from the individual themselves (or in the case of incapacity, their substitute decision maker, or next-of-kin). This case also highlights some of the challenges that can arise when family members are not willing to share or disclose their genetic information. This can create ethical and clinical challenges for HCPs who hold family information but do not have permission to share it. There is a tension between maintaining individual confidentiality and disclosure of information to a third party (someone the HCP does not have a relationship with, but the information could be useful and/or important to their health).
The issue of disclosure without consent and duty to warn is discussed further in this article in the section headed Ownership.
Sensitivity: Reproductive Decision Making
HGI may be used to inform the chances of passing on a genetic condition to future generations and thus can be used to inform reproductive decision-making. Decision-making around reproductive choices is highly personal and has the potential to be sensitive within families. HCPs should recognize this sensitivity and take extra precautions when documenting information and decisions about these matters.
For example, a couple may not wish for their child affected by a genetic condition to be aware of their decision to seek prenatal diagnostic testing in a subsequent pregnancy, or their decision not to continue a pregnancy confirmed to be affected by the same condition. This information should therefore not be stored within their child’s medical record, which they may access in the future. Furthermore, medical correspondence letters that document reproductive intentions should be handled with extra care, particularly around requests for release of information from other HCPs, or other third parties. Illustrative case 3 highlights this matter further.
Illustrative case 3
Akio is a 10-month-old male with moderate bilateral sensorineural hearing loss. After discussion, and upon obtaining consent from his parents, Saya and Ryuki, his pediatrician organizes genomic testing, which identifies an autosomal recessive cause for Akio’s hearing loss. These results are given to the family in a clinic appointment. Saya and Ryuki disclose during this appointment that they would like to have another baby and ask about the chance of having another child with hearing loss and their reproductive testing options. The pediatrician explains the 1 in 4 (25%) chance of recurrence if Saya and Ryuki are confirmed to be carriers of the variants identified in Akio, and goes on to provide information regarding the reproductive testing options available. This is a common scenario in pediatric genetics. It is important to pause here and consider the complexities and the different information being discussed in Akio’s clinic appointment.
Akio’s information
Akio’s genetic test result explains why he has hearing loss and precludes the need for other diagnostic investigations. It also helps to confirm that his hearing loss is expected to be isolated, in that it is not part of a genetic syndrome that has other associated health problems. All this information is relevant to Akio’s medical management and should accordingly be stored in Akio’s medical record.
Saya and Ryuki’s information
While it is commonplace for parents to inquire about the chance of recurrence in subsequent pregnancies and the reproductive testing options in a pediatric context, it is important to note that the details of these discussions belong to Saya and Ryuki. If documented in clinical notes or medical correspondence, this information needs to be stored in Saya and Ryuki’s medical records, not Akio’s. This information becomes particularly sensitive if Saya and Ryuki disclose how they intend to use the genetic information (i.e., prenatal diagnosis with a view to termination of an affected pregnancy). This information is not relevant to Akio’s medical management and does not belong in Akio’s medical record. This distinction is important to maintain privacy and confidentiality for all parties involved. When Akio is older, he will have the opportunity to access his medical records and the information that is relevant to his healthcare. He should not be privy to the details of his parent’s reproductive decision-making.
Sensitivity: Genetic Parentage
Genetic and genomic data have the potential to reveal misattributed genetic parentage. As the use of HGI in the healthcare setting continues to increase, it is of utmost importance that consumers are made aware of this possibility during the pretest consent process. Examples of situations where misattributed parentage may be revealed generally involve the testing of parents for variant(s) identified in a child, or when parentage needs to be confirmed as part of the variant curation process. If misattributed genetic parentage is identified, HCPs should treat this information as highly sensitive and should be extremely cautious about whether or how this information is documented, stored and protected. Each case of misattributed genetic parentage should be assessed on its own merits to determine how this information should be handled and protected.
Practicalities in the Australasian Context
Having considered different types of HGI and the various sensitivities that may apply when handling HGI in healthcare settings, we now shift focus to the appropriate (and inappropriate) use of HGI and how this information is protected in a practical and legal sense.
The Appropriate and Inappropriate Use of HGI
The Human Genetics Society of Australasia (HGSA) supports the use of HGI within the healthcare setting to diagnose, prevent, manage and/or inform risk of disease for an individual, a family or future generations.
When handling HGI in a healthcare setting, it is commonplace to ask about an individual’s ancestry as this may be relevant to the interpretation of their genetic information and/or determining the most appropriate test to offer. Like family health information, it needs to be acknowledged that self-reported ancestral information has the potential to be inaccurate or misinformed as it may be influenced by inaccurate, misinformed or incomplete information passed on from prior generations. Nevertheless, this information can provide invaluable insights into the worldviews, cultural relations, customs and beliefs of individuals and families. In no circumstance should this information be used to determine the level or quality of healthcare that is provided to an individual.
It cannot be ignored, however, that current knowledge and understanding of HGI is heavily biased toward Anglo-Saxon and European-centric descendant populations. It is critically important that work is done to expand current knowledge to be more representative of diverse ancestral backgrounds. This will help to diversify online databases of genomic information, which are commonly referenced during variant curation. Increased diversity is essential to ensure equal access not only to better serve individuals of all ancestral backgrounds but also to promote a future where HGI can be interpreted and utilized for individuals from a range of ancestral backgrounds and provide equitable healthcare to all. At the same time, it is important to be aware that particular care is needed in the secondary reuse of genetic and genomic data in combination with data that references race or ethnicity. Specific examples of this have already challenged practices in Australasia (Perbal, Reference Perbal2013).
While acknowledging that ethnicity and race can provide context and nuance to data, it is of critical importance to acknowledge that HGI does not define race or ethnicity. For this reason, the HGSA joins the American Society of Human Genetics and the European Society of Human Genetics in denouncing the use of HGI in forming, justifying or contributing to racial theories. This is not only dangerous, but not scientifically founded. Race cannot be defined by HGI (American Society of Human Genetics, 2018; European Society of Human Genetics, 2018).
Ownership
The concept of who owns a given set of genetic information can be complex. In legal terms, ‘ownership’ is considered in the context of property law, where the item, object or thing has certain qualities including the right to enjoyment or use; the right to sell; the right to exclude others and the right to give away (transfer ownership).
Genetic and genomic data has value to the individual in privacy terms and may have value in other contexts (e.g., contribution to research or in terms of family legacy). However, under Australian and New Zealand laws, these data are unlikely to have commercial value so that it can be sold. Human Tissue Acts (or equivalent) in Australian states and territories prohibit the trading (sale) of human tissue, both before or after death. Human tissue can be given away in the context of gametes (donor eggs or sperm), but not sold.
Some consumers request access to their raw genomic data following genomic sequencing. This raises the question of ownership, including whether an individual has a right (whether through ownership, or other rights) to this information. As previously described, a unique characteristic of HGI is that it may be shared with their family members. In some circumstances, it may be considered that people have a right to this information if they have paid for it. However, individual testing laboratories will also have policies about whether raw data is released directly to consumers, to whom, and in what format. These issues need to be considered carefully when obtaining consent for genomic testing.
In Australia, there is currently no clear legal position to answer these issues and therefore legal ownership of genetic information remains uncertain (Bonython & Arnold, Reference Bonython and Arnold2015). In New Zealand, it is not clear whether a biological sample has any legal status once separated an individual (Te Aka Matua o te Ture Law Commission, 2018). It may be possible to facilitate appropriate access to information without needing to address the question of ownership, but it is important that individuals understand they may not always be able to regulate the use of their genetic information (e.g., raw genomic data) when it is in the possession of external agencies, especially those based outside the country in which the individual resides.
The concept of ownership must also be considered in the context of customary and/or traditional rights of First Nation people, NZ Maori, Australian Aboriginal and Torres Straight Islanders. While a full exploration of this is outside of the scope of this document, NZ Māori view genetic information, specifically DNA, as Taonga (treasure). The implication of customary ownership and the place HGI has among Maori is that secondary use of HGI warrants careful community consultation.
Other Legal Considerations
Laws around privacy and protection of information are not uniform. Different countries, states and territories have different rules. Privacy laws provide some context for privacy arrangements in Australia and New Zealand; however, different levels of legal regulation (e.g., policies, guidelines, and legislation) apply in different Australian states and territories. HCPs need to know and adhere to the legal frameworks they are working within and seek advice if they are not aware of these.
It is also important to note that it is common practice for both clinical and research genetic and genomic testing to be undertaken in laboratories abroad. This means that the HGI generated will be subject to other laws and guidelines regarding the scope of protection, use and disclosure of genetic information. These rules may differ from those in the jurisdiction where the individual is receiving care. It is good practice to inform patient about where their data is being analyzed and stored as part of the informed consent process.
Storage
The practicalities of how information is stored and accessed are the primary way in which health information is protected. Health information is now stored using many different platforms, including but not limited to: paper-based filing, computer hard drives and external servers, EMRs, research databases and cloud-based or other online systems. HCPs need to be aware of and adhere to their institution’s storage platforms, and the relevant laws and policies in place to ensure the protection of privacy and confidentiality.
Special consideration must be given to how HGI is stored within the clinical setting. In the past, there has been a recommendation to store paper-based HGI (e.g., test results, reports and medical correspondence, notes) in a separate paper-based genetics file that is stored securely, typically within a clinical genetics department. The HGSA acknowledges, however, that with the mainstreaming of genomic medicine, genetic and/or genomic investigation will increasingly be ordered outside of the clinical genetics setting, and that paper-based medical records are becoming superseded by a range of electronic formats. Not only does this highlight the importance of educating HCPs about the sensitivities of genetic information outlined in this document, but also raises questions about how to responsibly store HGI within an EMR (Box 3).
Box 3. Considerations regarding EMRs

Box 4. The ABC case
The issue of a HCP having permission versus an obligation to disclose HGI without consent was recently examined in the English courts in ABC v St George’s Healthcare Trust [2020] EWHC 455 (QB). This case considered whether a HCP caring for an individual with psychiatric concerns who had HD had an obligation to disclose the individual’s genetic test results to a third party, ABC (the daughter of the individual) despite the individual’s express refusal to disclose.
The court held that HCPs have a duty to undertake a detailed and careful balancing exercise to consider individual confidentiality and the interests of any relatives with whom the HCPs have a ‘proximal’ relationship. Importantly, the court held that where the proximal relative’s interests should be prioritized over those of the individual, and the HCP acts reasonably, as informed by peer opinion and professional standards, disclosure without consent will be unlikely to result in legal liability for ‘breach of confidentiality’.
It is important to note that this decision from the English courts does not create a duty or obligation to disclose information in England. The duty, as described in this case, is to undertake a balancing exercise in circumstances where there may be a conflict of duties and interests (duty to maintain confidentiality versus a third party’s interest in learning of their genetic risk). Also, while this decision creates a new duty for English HCPs, this duty does not automatically apply to HCPs practising in Australia. A similar case would need to be considered in an Australian court to determine if the same obligation or duty exists in Australian law.
Sharing of Human Genetic Information
Due to the shared nature of HGI, it is common for information requests to be received in the healthcare setting from HCPs or family members to access HGI. HGI may also be generated and used outside of the healthcare setting (e.g., law enforcement, forms of direct to consumer/online genetic testing). While this is not the focus of this document, it needs to be acknowledged that there may be instances when third parties may request stored HGI from a HCP and vice versa. The sharing of HGI is supported and encouraged within families to facilitate informed decision making for all individuals, but only with appropriate consent, or in line with existing guidelines and legislation.
Sharing of HGI is important to progress universal knowledge. With appropriate consent, the sharing of HGI in its different forms promotes equity by enabling HCPs and researchers around the globe to collaborate and apply genetic and genomic knowledge to all individuals. Sharing may take many forms, such as contributing deidentified genomic data or specific variants to population databases of normal and pathogenic variants, or peer-reviewed publication in the medical literature. It should be noted that there may be circumstances where genetic and/or genomic data cannot be completely separated from the medium in which it is stored. This is particularly important to consider in the context of the secondary use of HGI and genomic data.
Healthcare Professionals
HGI often needs to be shared between HCPs of different specialties involved in an individual’s medical care to ensure coordinated and appropriate health management. This means HGI can be shared between that individual’s HCPs for reasons directly related to their care or treatment. Every effort should be made to ensure that individuals undergoing genetic and/or genomic testing are aware of this ‘continuity of care’ practice, as their consent to share their HGI for this purpose is usually implied.
The presence of variants predicting future disease, functional state or reproductive risks is particularly sensitive information. In addition, with an increase in precision medicine being targeted at specific genetic variants, it may be possible to indirectly learn of an individual’s genetic status based on their medication or treatment regime. Divulging this information in identified form to third parties will require the individual’s explicit consent in most instances.
Family Members
Where a HCP anticipates a situation in which HGI may be of interest or potential benefit to other family members, they should discuss this with the individual prior to treatment being commenced or as part of protocols for ordering tests (e.g., informed genomic consent). Through counseling, individuals should be encouraged to consider the interests of their genetic relatives regarding the value of the HGI for their own health.
Disclosure Without Consent and ‘Duty to Disclose’
Working with HGI and families raises the specific issue of whether a HCP has permission to disclose HGI from one individual to a relative of that individual, for the purposes of supporting or informing health decisions and, in particular, genetic risk.
Disclosure of genetic information to a third party without an individual’s express consent is permitted in certain circumstances; however, this differs slightly in each state and territory of Australia and different regulations apply depending on whether a person is working in the private sector or the public sector (McWhirter et al., Reference McWhirter, Johnston and Burke2019). New Zealand also has legislation regulating the circumstances in which medical information can be disclosed to a third party. This is outlined in the Health Act 1956 (NZ) which links with the Privacy Act 2020 (NZ) and the Information Privacy Principles within the Privacy Act NZ.
Overall, these arrangements provide permission for a HCP to disclose genetic information to a third party without consent in circumstances where the information could lessen or prevent a serious threat to the life, health or safety of a person.
There are two important factors to consider in this context: (1) these regulatory frameworks provide permission to disclose information in certain circumstances, but do not confer or create an obligation to disclose information; (2) there is no guidance about what constitutes a ‘serious threat’ to the life, health or safety of a person, so this is currently a subjective test which will be undertaken on a case-by-case basis. Further, in some Australian states and territories, it is necessary for the serious threat to be imminent to justify disclosure of personal information without consent.
Box 4 describes an international legal case relevant to the topic of disclosure of HGI without consent. While this case is not Australasian in origin, it does provide international context for the consideration of this topic in a Common law country.
Other Third Parties
If named or identifiable access to HGI is requested by third parties, such as insurers or employers, consent from the individual from whom the genetic information was generated must be sought prior to disclosure. Except if required by law or a court order, there is no obligation on a HCP to disclose information to such a third party in Australia or New Zealand. The existing legal framework provides permission to disclose information in certain circumstances, but not an obligation to disclose information.
Research and Public Databases
Genomic and variant data, along with relevant health and/or developmental information, are often shared in public online databases to support shared knowledge and transparency worldwide. Although this data were shared in a deidentified format (e.g., without that individual’s name or date of birth), it is important to acknowledge that some identifying details may be shared (such as country or State of origin, or detail of a specific genetic variant). It is important that individuals undergoing genomic sequencing are made aware of this practice, and are aware of the inherently identifiable nature of the data generated and that genomic data in its entirety is never truly deidentifiable on page 6). Individuals should be informed of this at the time of consent for genomic testing, and that sharing may also be possible under specific legal principles, such as the public interest.
Acknowledgments
The authors would like to thank the additional members of the Education, Ethics and Social Issues Committee of the HGSA: Amy Niselle, Heather Renton, Danya Vears, Michael Gabbett, Chris Jacobs, Dylan Mordaunt, Bronwyn Terrill and Aideen McInerney-Leo. This statement was reviewed and approved by the HGSA council in August 2021.
Financial support
The open access publication of this Position Statement has been approved by the Human Genetics Society of Australasia.
Conflict of interest
No conflicts of interest to declare.
Ethical standards
Not applicable.






