The DP is an ultimate exit from the labor market due to permanent incapacity to work caused by disease or injury. High rates of DP are considered a major public health problem with negative consequences for the individual, employers, and society as a whole (Alexanderson & Norlund, Reference Alexanderson and Norlund2004). During the last decades several European countries have seen a steady rise in DP primarily accounted for by an increase in individuals with mental health diagnoses (OECD, 2013). Currently this is the largest DP-diagnosis group in Sweden and the United Kingdom. Understanding the pathways — involving both heritable and environmental risk factors — in the development of DP is crucial for improving prevention strategies targeting early exclusion from the labor market.
Although the decision on granting DP is based on medical grounds, a number of factors other than those related to the underlying diagnosis have been associated with DP (Dekkers-Sanchez et al., Reference Dekkers-Sanchez, Hoving, Sluiter and Frings-Dresen2008). A variety of socio-demographic, work-related, and health-related factors have been reported to increase the risk of DP (Alexanderson & Norlund, Reference Alexanderson and Norlund2004; Bultmann et al., Reference Bultmann, Christensen, Burr, Lund and Rugulies2008; Knudsen et al., Reference Knudsen, Overland, Aakvaag, Harvey, Hotopf and Mykletun2010; Mykletun et al., Reference Mykletun, Overland, Dahl, Krokstad, Bjerkeset, Glozier and Prince2006). Specifically, the risk for DP is inflated in the presence of psychiatric comorbidity compared to DPs without comorbid psychiatric conditions (Ervasti et al., Reference Ervasti, Vahtera, Pentti, Oksanen, Ahola, Kivekas and Virtanen2014; Wedegaertner et al., Reference Wedegaertner, Arnhold-Kerri, Sittaro, Bleich, Geyer and Lee2013). However, more knowledge is needed regarding to what extent DP and comorbid psychiatric disorders share common genetic and/or environmental factors.
Previous twin studies have shown that a large proportion of the variance in DP is explained by heritable factors. For example, 45% of the liability to DP due to mental diagnoses was explained by genetic factors (Harkonmaki et al., Reference Harkonmaki, Silventoinen, Levalahti, Pitkaniemi, Huunan-Seppala, Klaukka and Kaprio2008; Narusyte et al., Reference Narusyte, Ropponen, Silventoinen, Alexanderson, Kaprio, Samuelsson and Svedberg2011). In a similar manner, genetic factors have been found to explain approximately 40% and 20% of the variation in depression and anxiety respectively, the two most common mental diagnoses underlying DP (Bienvenu et al., Reference Bienvenu, Davydow and Kendler2011). It is possible that the genetic liability to DP due to depressive or anxiety diagnoses might be explained by genetic factors contributing to medical conditions that are comorbid with the disorder underlying DP. One of such conditions may be CF, which has previously been reported to frequently co-occur with depressive and anxiety disorders (Fischler et al., Reference Fischler, Cluydts, De Gucht, Kaufman and De Meirleir1997; Hickie et al., Reference Hickie, Lloyd, Wakefield and Parker1990; Kroenke et al., Reference Kroenke, Spitzer, Williams, Monahan and Lowe2007) as well as to predict DP or work disability (Knudsen et al., Reference Knudsen, Henderson, Harvey and Chalder2011; Leone et al., Reference Leone, Huibers, Kant, Van Schayck, Bleijenberg and Andre Knottnerus2006; Markkula et al., Reference Markkula, Kalso, Huunan-Seppälä, Koskenvuo, Koskenvuo, Leino-Arjas and Kaprio2011; van Amelsvoort et al., Reference Van Amelsvoort, Kant, Beurskens, Schroer and Swaen2002). The co-occurrence of depression, anxiety, and CF has partly a common genetic influence (Kato et al., Reference Kato, Sullivan, Evengard and Pedersen2009) as well as unique genetic and environmental influences on CF (Hickie et al., Reference Hickie, Kirk and Martin1999). Thus, genetic susceptibility to CF may also contribute to genetic liability to DP and explain the developmental pathway to DP due to depressive or anxiety diagnoses.
The present study aimed to investigate to what extent liability to DP due to mental diagnoses among women can be explained by genetic and environmental factors contributing to MD and GAD as well as to CF.
Materials and Methods
Participants
The data were derived from the population-based Swedish Twin project of Disability Pension and Sickness Absence (STODS), which includes all twins from the Swedish Twin Registry (STR) born in Sweden between 1925 and 1958 (n = 59,598 individuals; Lichtenstein et al., Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen2002). STODS includes survey data from STR, information on old-age retirement and year of emigration from Statistics Sweden, data on deaths from the National Board of Health and Welfare, and data on sick leave and DP from the National Social Insurance Agency. The data from these various population-based registries were linked to the twins by using the unique 10-digit personal identification number assigned to all residents in Sweden.
The study sample included all women who have participated in the Screening Across the Lifespan Twin Study (SALT). SALT was conducted with the purpose to screen twins for common complex diseases and is described in more detail elsewhere (Lichtenstein et al., Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen2002; Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006). Twins were interviewed by telephone between January 1998 and March 2003. In the present study, we included participants from the SALT study who were at risk of DP, younger than 65 years of age, lived in Sweden, and who were not on old-age retirement or DP at baseline. Baseline was defined as the date of the SALT interview. Also, twin pairs with unknown zygosity or missing interview data were excluded. Assignment of zygosity was based on questions about twin intra-pair similarity in childhood. This method was validated with DNA, and showed at least 99% accuracy (Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006).
The final study sample included 9,985 female twin individuals, comprised of 1,776 monozygotic (MZ) and 2,358 dizygotic (DZ) twin pairs as well as 1,717 females from same-sex twin pairs where information on the co-twin was missing. The mean age of the participants at baseline was 53.2 ± 5.7 years.
Measures
DP due to mood and neurotic diagnoses
In Sweden, adults younger than 65 years of age with a medically confirmed disease or injury that has led to permanent work incapacity can be granted DP. For all twins, information on date of and main DP diagnosis were obtained from the National Social Insurance Agency's database MicroData for Analyses of the Social insurance (MiDAS) for the years 1998–2010. DP diagnoses were based on the 9th and 10th revisions of the International Classification of Diseases (ICD; WHO, 1993). For the purposes of this study, ICD-9 diagnoses were recoded to their ICD-10 equivalents. Mood and neurotic DP diagnoses included ICD-10 sections F30–39 and F40–48.
Major depression (MD) and generalized anxiety disorder (GAD)
In SALT, the presence of MD and GAD were assessed using the computerized Composite International Diagnostic Interview-Short Form (CIDI-SF), adapted from its original design for 12-month prevalence of DSM-IV disorders (Kessler et al., Reference Kessler, Andrews, Mroczek, Üstün and Wittchen1998). CIDI-SF criteria for MD were validated against 1-year prevalence data with the full Composite International Diagnostic Interview (Kendler et al., Reference Kendler, Gatz, Gardner and Pedersen2006). Twins were considered as positive for a history of MD if they either met the criteria for MD or reported that they used or had used antidepressant medication (Kendler et al., Reference Kendler, Gatz, Gardner and Pedersen2006). Individuals were classified as having GAD if they reported excess worry and anxiety that had lasted for at least six months (DSM-IV criterion A) and at least three of five symptoms (except ‘difficulty concentrating or mind going blank’) that were associated with worry and anxiety and had lasted for at least six months (criterion C). The assessment of MD and GAD in SALT is described in more detail elsewhere (Kendler et al., Reference Kendler, Gatz, Gardner and Pedersen2006; Reference Kendler, Gardner, Gatz and Pedersen2007). For the purpose of the present study, a binary variable combining MD and GAD was created to indicate the presence or absence of MD or GAD. That is, individuals were treated as affected (MD/GAD = 1) if they had a positive history of MD or of GAD.
Chronic fatigue
Presence of CF was evaluated by using criteria that were based on the Centers for Disease Control and Prevention consensus criteria for CF syndrome (Fukuda et al., Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff1994). The stem question was ‘Have you felt abnormally tired during the last six months?’, and was used to code CF if any of the exclusionary conditions were absent as well as if CF implied impairment for the individual. Information on exclusionary conditions was obtained from the SALT interview and included data on presence of a number of disorders and conditions (e.g., morbid obesity, alcohol abuse, or multiple sclerosis; Evengård et al., Reference Evengård, Jacks, Pedersen and Sullivan2005). In the present study, the presence of impairment related to CF was defined if the woman found herself being ‘too tired to live a normal life’, or that fatigue was the reason behind her social problems or reduced work capacity. A binary variable was created and individuals were treated as affected (CF = 1) in the presence of CF. For further details on the CF-screening procedure, see (Evengård et al., Reference Evengård, Jacks, Pedersen and Sullivan2005).
Statistical Analyses
Prevalence rates for MD/GAD, CF, and DP due to neurotic or mood diagnoses were calculated for each zygosity group. First estimates of genetic and environmental influences on MD/GAD, CF, and DP were obtained by comparing how similar twins are in a pair. Within-pair similarity for the measures was assessed by calculating intraclass tetrachoric correlations for each zygosity group. The twin method relies on the fact that MZ twins share approximately all (100%) of their genes while DZ twins share on average half (50%) of all their segregating genes. Correlations among MZ twins that are twice DZ correlations would, therefore, suggest genetic influences, while more equal correlations between both zygosity groups indicate shared environmental influences. Unique environmental influences are suggested by MZ twin correlations less than 1.0 and also include measurement error. Cross-twin, cross-trait tetrachoric correlations were calculated and compared between MZ and DZ twins to provide initial estimates of the genetic and environmental influences on the covariation between the phenotypes.
Descriptive statistics, intraclass, and cross-twin cross-trait correlations were computed using SAS statistical software (SAS Institute Inc., 2013).
Multivariate Genetic Analyses
A multivariate genetic model was applied to decompose the covariance between MD/GAD and DP into additive genetic (A), shared environmental (C), or unique environmental (E) components. The purpose of the multivariate genetic analyses was to examine to what extent the total variation in DP due to mood and neurotic diagnoses was explained by factors contributing to MD/GAD and CF (Figure 1).
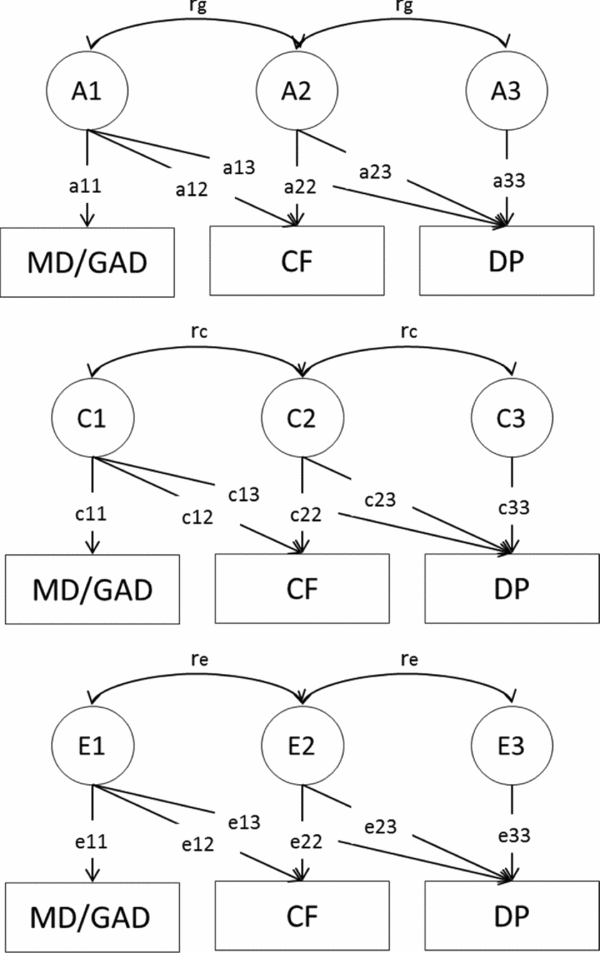
FIGURE 1 Cholesky decomposition of the covariance between major depression/general anxiety disorder (MD/GAD), chronic fatigue (CF), and disability pension (DP) due to mood and neurotic diagnoses.
Genetic (rg ) and environmental (re ) correlations between MD/GAD and DP as well as CF and DP were estimated. A genetic correlation indicates the extent to which genetic effects on DP overlap with genetic effects on MD/GAD or CF. Similarly, the shared and non-shared environmental correlations indicate to what extent environmental effects on DP overlap with environmental effects on MD/GAD or CF. In addition, we calculated the extent to which the covariance between the phenotypes could be explained by common genetic or environmental effects, that is, whether the genetic or environmental effects in MD/GAD and/or CF were contributing to the genetic or environmental effects in DP.
First, we estimated the full model, which included all variance components (A, C, and E). Then, we fit two constrained models. In the first one, the shared environmental component (C) was constrained to be zero for MD/GAD, DP, and CF, and in the second model the genetic component (A) was constrained to be zero. Model fit was determined using Akaike's information criterion (AIC) where a lower AIC indicates a better fitting model.
Genetic analyses were performed using the software package Mx, which applies maximum-likelihood approach (Neale et al., Reference Neale, Boker, Xie and Maes2006).
The study was approved by the Regional Ethical Review Board of Stockholm, Sweden.
Results
Descriptive statistics are presented in Table 1. Approximately 3% of the cohort was granted DP due to mood and neurotic diagnoses during the follow-up period between 1998 and 2010. The prevalence of MD/GAD was 30% while the prevalence of CF was 7% in both zygosity groups.
TABLE 1 Prevalence of Major Depression/General Anxiety Disorder (MD/GAD), Chronic Fatigue (CF), and Disability Pension (DP) Due to Mood And Neurotic Diagnoses Among 9,985 Monozygotic (MZ) and Dizygotic (DZ) Female Twins

Intraclass and cross-twin cross-trait correlations are presented in Table 2. For all measures, the intraclass correlations among MZ twins were at least twice the correlations among DZ twins, suggesting that additive genetic influences are of importance. Dominant genetic effects seemed to also contribute to the variance of MD/GAD and CF as the intraclass correlations among MZ twins were at least twice the DZ correlations. Cross-twin, cross-trait correlations among MZ twins exceeded those of the DZ twins, which indicate the importance of genetic influences on the covariation between the measures. Correlation between DP and MD/GAD among MZ twins was less than twice the correlation between DZ twins, suggesting the presence of shared environmental factors. The phenotypic correlation between MD/GAD and DP was 0.37 and 0.41 between CF and DP.
TABLE 2 Intraclass and Cross-Twin Cross-Trait Tetrachoric Correlations With 95% Confidence Intervals (CI) for Major Depression/General Anxiety Disorder (MD/GAD), Chronic Fatigue (CF), and Disability Pension (DP) Due to Mood and Neurotic Diagnoses

aIntraclass correlations; Phenotypic correlations were: DP-MD/GAD: 0.37 (0.34–0.40); DP-CF: 0.41 (0.37–0.45); MD/GAD-CF: 0.32 (0.30–0.34).
Multivariate Genetic Analyses of MD/GAD, CF, and DP
First, we fit a full model including A, C, and E variance components for all phenotypes (Table 3). Also, a full A, D, E model was fit to estimate dominant genetic effects, as suggested by the intraclass correlations for MD/GAD and CF. Next, models where the C or D components were set to zero were fit. This resulted in an improvement in model fit (Δχ2 = 3.88, Δdf = 6, ΔAIC = -8.12). Omitting the A component from the ACE model resulted in deterioration in model fit (Δχ2 = 3.88, Δdf = 6, AIC = -11.03). Thus, the final best-fitting model included A and E components for MD/GAD, CF, and DP.
TABLE 3 Multivariate Model-Fitting Results for Major Depression/General Anxiety Disorder (MD/GAD), Chronic Fatigue (CF), and Disability Pension (DP) Due to Mood and Neurotic Diagnoses

AIC = Akaike's information criterion, χ2 = difference in log-likelihoods between models, df = degrees of freedom, A = additive genetic, C = shared environment, E = non-shared environment; best-fitting and parsimonious model in bold.
The standardized and unsquared estimates of the best-fitting model are presented in Figure 2. The total estimated genetic variance of DP was 0.562 + 0.072 + 0.382 = 0.46. Genetic influences unique to DP explained 68% (0.562/0.46 = 0.68) of the total genetic variance in DP. Approximately 31% of the genetic variance in DP was explained by genetic influences on MD/GAD. Genetic contributions unique to CF explained 1% of the variance in DP and were not significant.

FIGURE 2 Estimates of the best-fitting multivariate model of major depression/general anxiety disorder (MD/GAD), chronic fatigue (CF), and disability pension (DP) due to mood and neurotic diagnoses.
The total estimated non-shared environmental variance of DP was 0.682 + 0.242 + 0.162 = 0.54. Non-shared environmental influences on DP, MD/GAD, and CF explained 85% (0.682/0.54 = 0.86.), 5% (0.162/0.64 = 0.05), and 10% (0.242/0.54 = 0.11), respectively, of the total non-shared environmental variance in DP.
Genetic factors explained 67% (0.65*0.38/0.65*0.38 + 0.76*0.16) of the total covariance (i.e., estimated phenotypic correlation) between MD/GAD and DP and 49% (0.52*0.07 + 0.38*0.39/0.52*0.07 + 0.38*0.39+0.76*0.24 + 0.10*0.16) of the total covariance between CF and DP. The majority (80%) of the genetic factors contributing to the covariance between CF and DP were in common with MD/GAD (0.38*0.39/0.52*0.07 + 0.38*0.39). The genetic correlation (rg ) between MD/GAD and DP and between CF and DP, were 0.56 and 0.42, respectively.
Non-shared environmental factors explained 33% (0.76*0.16/0.65*0.38 + 0.76*0.16) of the total covariance between MD/GAD and DP and 51% of the total covariance between CF and DP. The majority (92%) of the non-shared environmental contributions to the covariance between CF and DP were in common with CF (0.76*0.24/0.76*0.24 + 0.10*0.16). The non-shared environmental correlation (re ) between MD/GAD and DP was 0.21 and between CF and DP it was 0.35.
Discussion
In the present study, we investigated the genetic and environmental contributions from MD/GAD and CF to DP due to mood and neurotic diagnoses among women. Approximately 30% of the total genetic variance in DP was explained by the genetic factors contributing from MD/GAD. There was a small and non-significant genetic contribution from CF to DP. Non-shared environmental influences in common with MD/GAD or CF played a minor role for the variance in DP.
Of the genetic factors contributing to DP due to mood and neurotic diagnoses, 68% were not explained by genetic influences common with MD/GAD or CF. The results are in line with the hypothesis that DP is a multifactorial phenomenon and that factors other than those related to the DP diagnosis are of importance (Alexanderson and Norlund, Reference Alexanderson and Norlund2004). For example, personality disorders were reported to predict DP in young adults (Ostby, Reference Ostby, Czajkowski, Knudsen, Ystrom, Gjerde, Kendler and Reichborn-Kjennerud2014) and the association between personality disorders and long-term sick leave was at least partly explained by an overlapping genetic liability (Gjerde et al., Reference Gjerde, Roysamb, Czajkowski, Knudsen, Ostby, Tambs and Orstavik2014). Previous research has also reported a positive association between personality traits and DP. For example, a study of DP due to low-back diagnoses showed that those with neuroticism (heritable to 43%) had higher risk for future DP (Ropponen et al., Reference Ropponen, Silventoinen, Svedberg, Alexanderson, Huunan-Seppala, Koskenvuo and Kaprio2012). Another study reported that individuals with Distressed Personality type (Type D, heritable to 52%) were more often on DP as compared to non-Type D individuals (Kupper et al., Reference Kupper, Denollet, DeGeus, Boomsma and Willemsen2007; Mommersteeg et al., Reference Mommersteeg, Denollet and Martens2012). Finally, a few previous studies of DP irrespective of diagnoses suggested that several biological and early childhood factors (e.g., chronic childhood disease, abnormal birth weight, or early deviant behavior) were associated with a higher risk of future DP; factors that have been shown to be heritable in women (Upmark & Thundal, Reference Upmark and Thundal2002).
A major part of the variation in DP due to neurotic and mood diagnoses was explained by non-shared environmental factors. That is, the majority of the environmental factors contributing to DP due to neurotic and mood diagnoses was unique to DP and not shared with MD/GAD or CF. Previous studies have identified a wide range of factors that are associated with future DP due to various diagnoses including, specific occupational groups, physical and psychosocial work environment, educational level, family situation, social class, severity of disorders, and comorbidity (Allebeck & Mastekaasa, Reference Allebeck and Mastekaasa2004; Hannerz et al., Reference Hannerz, Tuchsen, Spangenberg and Albertsen2004; Karlsson et al., Reference Karlsson, Carstensen, Gjesdal and Alexanderson2007; Leinonen et al., Reference Leinonen, Pietilainen, Laaksonen, Rahkonen, Lahelma and Martikainen2011; Pietilainen et al., Reference Pietilainen, Laaksonen, Rahkonen and Lahelma2011; Reinholdt et al., Reference Reinholdt, Upmark and Alexanderson2010; Stattin, Reference Stattin2005).
The covariance between MD/GAD and DP due to neurotic and mood diagnoses was largely attributable to genetic effects, whereas non-shared environmental influences were predominant in the covariance between CF and DP. This suggests that the liability to DP due to neurotic and mood diagnoses may be partly explained by MD/GAD through genetic and by CF through environmental mechanisms. If replicated, this knowledge may help improving strategies used to prevent DP.
Almost half of the covariance between CF and DP due to mood and neurotic diagnoses could be attributed to genetic effects. The major part of the covariance was explained by genetic contributions to MD/GAD. Whereas the covariance between CF and DP was primarily attributable to non-shared environmental effects that were in common with CF. These findings are in line with the results of a previous study which suggested a shared genetic predisposition for MD, GAD, and functional somatic syndromes (including CF; Kato et al., Reference Kato, Sullivan, Evengard and Pedersen2009). Also, environmental influences on these studied functional somatic syndromes were not shared with MD and GAD (Kato et al., Reference Kato, Sullivan, Evengard and Pedersen2009).
Genetic and environmental correlations between two phenotypes may imply both an overlap and a causal relationship between the phenotypes (De Moor et al., Reference De Moor, Boomsma, Stubbe, Willemsen and De Geus2008). Our results of moderate genetic and environmental correlations between MD/GAD and DP as well as CF and DP suggest that MD/GAD and CF may have a direct (causal) effect on DP. However, this hypothesis needs to be further tested by applying causal models (Neale & Kendler, Reference Neale and Kendler1995).
Strengths and Limitations
The strengths of this study include the large population-based twin sample, the use of register data on DP of high quality, and the comprehensive diagnostic interview data. In this study, the number of DPs due to mood and neurotic diagnoses was insufficient among men (i.e., there were no twin pairs where both twins had DP) to perform genetic analyses. Thus, only women were included. A similar study including men would be of importance as previous research showed that different genetic factors among women and men were influential on depression (Kendler et al., Reference Kendler, Gatz, Gardner and Pedersen2006). Further, as this study included twins in the age range between 40 and 65 years, the results cannot be generalized to younger ages. Another potential limitation concerns the DP diagnoses. An individual may have had several diagnoses in which one was the main diagnosis for being granted DP. In this study, the main DP diagnoses were used. Hence, individuals that were granted DP due to somatic diagnoses but had a mental diagnosis as a secondary diagnosis were not included in the study. The reported overlap of genetic and environmental factors between the phenotypes might therefore be underestimated.
In conclusion, a large part of the genetic liability to DP due to mood or neurotic diagnoses was explained by genetic effects not in common with depression and anxiety disorders or CF at baseline. The results suggest that the process leading to DP is complex and influenced by factors other than those related to the disorder underlying DP.
Acknowledgments
The study was supported by the Swedish Research Council (P.S., grant number 521-2008-3054), Swedish Research Council for Health, Working Life and Welfare (K.A., grant number 2007-1762), Karolinska Institutet: Strategic Research Program in Epidemiology, and the Swedish Society of Medicine (J.N., grant number SLS-173211; P.S., grant numbers SLS-330341, SLS-171611, SLS-250931).







