Monozygotic (MZ) twins represent a unique biological circumstance in which individuals who share all of their segregating genes are also raised contemporaneously, yet develop different observable characteristics over time. MZ twins who are discordant for a salient and potentially life-threatening disorder such as anorexia nervosa (AN) provide an opportunity to isolate environmental from genetic etiological factors (i.e., co-twin control method; Foley et al., Reference Foley, Neale and Kendler2000; Goldberg & Fischer, Reference Goldberg, Fischer, Everitt and Howell2005). We identified a sample of 22 rigorously discordant MZ twins for AN (i.e., the co-twin did not meet any of the criteria for AN) and explored differences on putative risk factors.
AN is heritable, with genetic factors accounting for approximately 50% of the variance in liability; unique environmental factors account for the remaining variance (for review, see, e.g., Yilmaz et al., Reference Yilmaz, Hardaway and Bulik2015). More specifically, observed differences between members of a MZ twin pair may result from the in utero environment or other genetic and epigenetic factors (Bruder et al., Reference Bruder, Piotrowski, Gijsbers, Andersson, Erickson, de Stahl and Dumanski2008; Czyz et al., Reference Czyz, Morahan, Ebers and Ramagopalan2012; Fraga et al., Reference Fraga, Ballestar, Paz, Ropero, Setien, Ballestar and Esteller2005) or may be the result of unique environmental factors that occur after birth (Martin et al., Reference Martin, Boomsma and Machin1997; Plomin et al., Reference Plomin, DeFries, McClearn and Rutter1997). Identifying environmental factors that increase the likelihood of expression of genetic vulnerability to AN, either directly or through G × E interactions, is of particular interest (Bulik et al., Reference Bulik, Sullivan, Wade and Kendler2000; Campbell et al., Reference Campbell, Mill, Uher and Schmidt2011; Fairburn et al., Reference Fairburn, Cowen and Harrison1999; Rutter & Silberg, Reference Rutter and Silberg2002) as these factors may be more readily identifiable and modifiable than genetic risk factors.
Two known investigations, using different waves of the Australian Twin Registry, have applied the co-twin control method to AN (Wade et al., Reference Wade, Treloar, Martin, Statham and Heath2004, Reference Wade, Gillespie and Martin2007). Wade et al. (Reference Wade, Treloar, Martin, Statham and Heath2004) compared nine pairs of female MZ twins discordant for lifetime AN on weight, current psychopathology, temperament and coping styles, and family functioning while growing up. Twins with AN had significantly higher birth weights and significantly lower current weights than their unaffected co-twins. No differences emerged on the other measures. A subsequent study compared 14 twin pairs discordant for lifetime AN on reported family life events (Wade et al., Reference Wade, Gillespie and Martin2007). Affected twins reported significantly higher paternal protection than their co-twins; however, no other differences emerged in reports of family comments about weight or shape, amount eaten, parental expectations, criticism and conflicts, or maternal protection.
These prior co-twin control investigations provide valuable preliminary information regarding factors associated with AN. Although they included a limited range of variables, they did not assess the majority of purported risk factors and correlates identified in the literature. Numerous cross-sectional and longitudinal studies have explored risk factors and correlates of eating disorders and revealed that female sex, internalization of the thin ideal, higher levels of acculturation, negative self-evaluation, personality traits (e.g., perfectionism), increased shape and weight concerns, early childhood eating and digestive problems, general psychiatric morbidity, and exposure to adverse life events are commonly observed (for reviews, see Culbert et al., Reference Culbert, Racine and Klump2015; Jacobi et al., Reference Jacobi, Hayward, de Zwaan, Kraemer and Agras2004; Striegel-Moore & Bulik, Reference Striegel-Moore and Bulik2007). Thus, the goal of this study was to build on the existing literature by examining a large sample of 22 female MZ twin pairs rigorously discordant for AN on variables including personality characteristics, adverse life events, psychiatric comorbidity, and other health factors that may influence risk for AN.
Materials and Methods
Participants
Twins were from the population-based study Swedish Twin study of Adults: Genes and Environment (STAGE; http://ki.se/ki/jsp/polopoly.jsp?d=9610&l=en), a sub-sample of the Swedish Twin Registry (http://ki.se/twinreg; Furberg et al., Reference Furberg, Lichtenstein, Pedersen, Thornton, Bulik, Lerman and Sullivan2008; Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006). STAGE includes approximately 25,000 twins born between 1959 and 1985 who were between the ages of 20 and 47 at the time of interview. Using web-based questionnaires or telephone interviews, individuals provided information on health and socio-demographic measures, life habits, and behaviors. The response rate was 59.6%. Zygosity was assigned using responses to two questions and a previously validated algorithm described elsewhere (Lichtenstein et al., Reference Lichtenstein, De Faire, Floderus, Svartengren, Svedberg and Pedersen2002). A detailed description of the study is provided elsewhere (Furberg et al., Reference Furberg, Lichtenstein, Pedersen, Thornton, Bulik, Lerman and Sullivan2008; Lichtenstein et al., Reference Lichtenstein, Sullivan, Cnattingius, Gatz, Johansson, Carlstrom and Pedersen2006).
STAGE was approved by the Regional Ethics Committee at Karolinska Institutet and the Biomedical Institutional Review Board at the University of North Carolina. All participants provided informed consent.
Eating Disorders Assessment
Lifetime eating disorders were assessed using a self-report questionnaire based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (SCID; American Psychiatric Association, 1994; First et al., Reference First, Gibbon, Williams, Spitzer and Staff1999). Twins with a lifetime history of AN endorsed the following criteria: a lowest body mass index (BMI) < 17.55, being very afraid or extremely afraid of gaining weight or becoming fat when at low weight, and feeling very or extremely fat when at low body weight. We used a rigorous definition of discordance—the unaffected co-twin met none of these AN criteria. This is a conservative approach as often the unaffected co-twin exhibits sub-threshold symptoms. Twenty-two MZ pairs discordant for AN were identified.
Personality Characteristics
All personality characteristics evaluated in STAGE were examined in this study. Specifically, self-directedness (α = 0.84) was measured using 10 items from the Temperament and Character Inventory (TCI; Cloninger et al., Reference Cloninger, Przybeck, Svrakic and Wetzel1994). The sub-scales concern over mistakes (α = 0.82), personal standards (α = 0.81), and doubts about actions (α = 0.90) from the Multidimensional Perfectionism Scale (MPS; Frost et al., Reference Frost, Marten, Lahart and Rosenblate1990) were each assessed with four items. Nine items for the extraversion (α = 0.78) and 18 items for neuroticism (α = 0.90) scales from the short form of the Eysenck Personality Inventory (EPI-Q; Eysenck & Eysenck, Reference Eysenck and Eysenck1964) were assessed. For each sub-scale, the items were scored and summed according to their respective criteria.
Adverse Life Events
Participants were asked to indicate which events they experienced at any point in their lives: witnessing family violence before the age of 18; being emotionally abused or neglected; being physically neglected; being physically abused; being assaulted (being robbed, mugged, or physically attacked by a stranger); witnessing a robbery, mugging, or attack; being stalked; being discriminated against in a way that was highly distressing; being a victim of a hate crime; being sexually harassed; being sexually assaulted; and feeling forced to have sex. If a twin indicated that they experienced a life event, they were asked their age at first event. Response options were: 0–6 years old; 7–12 years old; 13–15 years old; 16–18 years old; and after 18 years of age.
Psychiatric Comorbidity and Other Health Issues
Participants were evaluated for lifetime major depression and generalized anxiety disorder using self-report assessments based on the SCID (First et al., Reference First, Spitzer, Gibbon and Williams2002). Major depression was considered present if five symptoms of depression associated with a change of functioning were endorsed and these symptoms were associated with significant impairment (criteria A and C). Generalized anxiety disorder was considered present if the participant endorsed excessive anxiety and worry (criterion A) and at least three symptoms (criterion C) resulting from anxiety and worry. Participants were also asked, ‘Do you have or have you ever had any of the following problems?’ and were instructed to respond ‘yes’ or ‘no’ to each: panic disorder, phobias, and obsessive-compulsive disorder. An ‘any anxiety’ composite variable was created indicating whether the participant had generalized anxiety disorder, panic disorder, phobias, or obsessive–compulsive disorder. Alcohol problems were considered present if the participant met lifetime DSM-IV criteria for abuse or dependence.
Participants were asked about health-related issues including ‘stomach trouble’, Crohn's disease, ulcerative colitis, current BMI, self-reported birth weight, age at menarche, and age at first diet. Stomach trouble, Crohn's disease, and ulcerative colitis were combined into one variable: gastrointestinal problems. Current BMI was calculated from self-reported height and weight. To assess age of first diet, participants were first asked whether they had ever dieted or limited the amount of food they ate in order to lose weight. Individuals who endorsed this item were then asked to report their age at first diet.
Statistical Analyses
Age-of-onset was available for major depression and alcohol problems. Because we were interested in factors that might contribute to the expression of AN, we wanted to minimize those that might be a consequence of the illness. Thus, if major depression or alcohol problems were present in the affected twin, her age-of-onset of AN was compared with her age-of-onset of the respective comorbid disorder: major depression or alcohol problems. If the age at AN onset was before the age-of-onset of the comorbid disorder, the comorbid disorder could not contribute to the expression of AN so the score for the comorbid disorder was changed to absent for these analyses. Similar methods were applied to the life event data: age at first experience of each specific event was compared with the age-of-onset of AN. If the onset of AN was younger than the age endorsed for a specific event, then the event was scored as absent for these analyses. No adjustments to scoring of comorbid disorders or life events were made for the unaffected co-twins because we were evaluating events and characteristics that might contribute to the onset of AN at any time. Theoretically, unaffected twins could develop AN at a later point in time than affected twins; thus, all comorbid disorders and events recorded at assessment predate AN and no changes in scores were performed. Censoring was performed in this manner to minimize Type I error.
Not all twins had data available for all measures. Thus, the number of pairs used in each analysis is presented in text or tables. McNemar's tests are used with paired nominal data to determine whether row and column marginal frequencies are equal. They were applied to the dichotomous variables (i.e., adverse life events and comorbidity diagnoses) to assess differences between twins with AN and their unaffected co-twins. Due to the small number of pairs for each test (between 8 and 22), exact p values were computed. Paired t-tests were performed to compare means in two samples when the observations in one sample (e.g., affected twins) were paired with the observations in the second sample (e.g., the unaffected co-twin). Two-sided paired t-tests were applied to the continuous variables (i.e., personality variables from the TCI, MPS and EPI-Q; BMI; birth weight; age at menarche; and age at first diet). We conceptualized the present study as exploratory and hypothesis generating because we included a rich array of variables, many of which had not been explored in the previous co-twin control studies of AN. Thus, the reader should be aware that the p values were not corrected for multiple comparisons. Analyses were conducted in SAS v9.2 (SAS Institute, Inc., 2004).
Results
Sample Description
The mean (SD) age of participants was 31.7 (6.3) years; the mean (SD) age-of-onset of AN was 17.8 (4.4) years. Approximately 61% of participants indicated that they were married or cohabiting with a partner. Over 70% of the sample indicated that they were university graduates; 25% had some secondary education or had graduated from secondary school; two individuals reported another form of education. This smaller sample is comparable to the larger STAGE sample for age and civil status. However, the current study sample had a higher mean level of education than the total STAGE sample (χ 2 = 14.6, p < .001; Pisetsky et al., Reference Pisetsky, Thornton, Lichtenstein, Pedersen and Bulik2013).
Personality Characteristics
For personality (Table 1), significant differences between affected and unaffected twins were observed for all three MPS sub-scales with higher mean scores in affected twins. Further, over 80% of the affected twins in each pair had higher scores for each measure (data not shown). No other differences on personality measures were observed.
TABLE 1 Mean (SD) of Personality Measures for Twins Affected With Anorexia Nervosa and Unaffected Co-Twins; Results of the Paired t-Tests and Effect Size
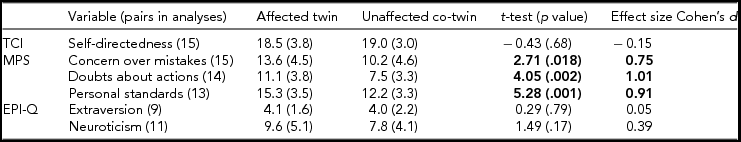
Note: TCI = Temperament and Character Inventory (Cloninger, Reference Cloninger, Przybeck, Svrakic and Wetzel1994), MPS = Multidimensional Perfectionism Scale (Frost et al., Reference Frost, Marten, Lahart and Rosenblate1990), EPI-Q = Eysenck Personality Inventory (Eysenck & Eysenck, Reference Eysenck and Eysenck1964). Bold type for t-test (p value) indicates significant results, and for the effect size Cohen's d, it indicates large effect sizes.
Adverse Life Events
No significant differences in life events were observed between affected and unaffected twins (Table 2). However, twice as many unaffected co-twins reported emotional neglect than affected twins (66.7% vs. 33.3%). Although this difference was not significantly different (p = .063), the magnitude of the difference between twins encourages further exploration. No affected twins who reported emotional neglect also had a co-twin who reported no emotional neglect; thus, odds ratios could not be computed.
TABLE 2 N (%) of Adverse Life Events, Psychiatric Comorbidity, and Health-Related Factors for Twins Affected With Anorexia Nervosa and Unaffected Co-Twins; Results of the McNemar's Tests

Psychiatric Comorbidity and Other Health Issues
No significant differences were observed between the affected and unaffected co-twins on comorbid psychiatric disorders, including the composite any anxiety variable, and other health-related factors (Table 2). Although small sample sizes precluded the detection of statistically significant differences between groups (p = .078), seven times as many affected twins reported gastrointestinal problems than unaffected twins (58.3% vs. 8.3%). Again, the numerical magnitude of the difference encourages further exploration of gastrointestinal problems and eating disorders. No affected twins who reported gastrointestinal problems also had a co-twin who reported gastrointestinal problems; thus, odds ratios could not be computed.
At the time of interview, all affected twins had BMI > 18.2 kg/m2; however, the mean (SD) BMI for affected twins, 20.9 (1.7) kg/m2, was significantly lower than for unaffected twins, 22.0 (2.5) kg/m2, t(21) = −2.49, p = .022. No significant differences were observed between affected and unaffected twins for birth weight, affected = 2,373.4 (640.4) grams, unaffected = 2,299.5 (496.5) grams, t(18) = 0.91, p = .38), or age at menarche, affected = 13.1 (1.3) years, unaffected = 13.1 (1.4) years, t(20) = 0.00, p = 1.00. All of the affected twins and 13 unaffected co-twins endorsed having ever dieted or limited the amount of food they ate to lose weight. Of those who endorsed ever dieting, all but one unaffected twin provided age of first diet. Of these individuals, affected twins reported a significantly younger age at first diet, 14.7 (2.6) years, than unaffected twins, 16.5 (3.9) years, t(11) = −2.42, p = .034.
Discussion
Consistent with a robust body of research on perfectionism in AN (Bardone-Cone et al., Reference Bardone-Cone, Wonderlich, Frost, Bulik, Mitchell, Uppala and Simonich2007; Lilenfeld et al., Reference Lilenfeld, Wonderlich, Riso, Crosby and Mitchell2006), affected twins displayed greater concern over mistakes, doubts about actions, and personal standards than unaffected co-twins. Our design cannot determine whether perfectionistic personality characteristics are a risk factor for developing AN or whether these features develop or intensify as a consequence of the illness (Wade et al., Reference Wade, Tiggemann, Bulik, Fairburn, Wray and Martin2008). Moreover, although the co-twin control method effectively identifies the environmental factors that might be of etiological relevance, it also detects differences in a number of other parameters that might be variably influenced by genetic and environmental factors and their interaction. The heritability of the MPS sub-scales concern over mistakes, personal standards, and doubts about actions has been estimated to be 0.29, 0.42, and 0.32, respectively (Tozzi et al., Reference Tozzi, Aggen, Neale, Anderson, Mazzeo, Neale and Bulik2004). Yet, despite sharing virtually all of their segregating genes, the affected co-twins scored significantly higher on these sub-scales than the unaffected twins. Thus, unmeasured environmental factors may have led to differential expression of perfectionistic traits. Such environmental factors may also have induced changes in gene expression (epigenetic changes), which could increase affected twins’ sensitivity to environmental factors (G × E interactions; Eaves et al., Reference Eaves, Last, Martin and Jinks1977; Plomin et al., Reference Plomin, DeFries and Loehlin1977). Alternatively, the high levels of perfectionism reported by the affected twins could reflect traits that were exacerbated by the experience of AN and be a sequela of the illness.
Affected twins engaged in dieting behavior earlier than unaffected co-twins who had dieted. There is a dearth of literature examining the association between age of first diet and lifetime prevalence of AN. However, dieting is an established risk factor for disordered eating (Polivy & Herman, Reference Polivy and Herman1985), and dieting during adolescence has been associated with greater risk for disordered eating during young adulthood (Neumark-Sztainer et al., Reference Neumark-Sztainer, Wall, Guo, Story, Haines and Eisenberg2006). Dieting may also represent a prodromal illness state (Grange & Loeb, Reference Grange and Loeb2007), reflecting increased weight concerns in the affected twin, which either predicts the development of eating disturbances or is an early manifestation of the disorder (e.g., Ghaderi & Scott, Reference Ghaderi and Scott2001; Patton et al., Reference Patton, Selzer, Coffey, Carlin and Wolfe1999).
Dieting at a younger age and closer to the pubertal transition may be particularly pernicious. Adolescence is a nutritionally vulnerable developmental stage due to accelerated growth, with total nutrient needs greater than at any other time in the lifecycle (Story & Stang, Reference Story, Stang, Stang and Story2005). Nutrient demands typically parallel adolescent growth, peaking on average between ages 11 and 12 in girls (Abbassi, Reference Abbassi1998) and gradually declining as adolescence progresses (e.g., Jacob & Nair, Reference Jacob and Nair2012; Siervogel et al., Reference Siervogel, Demerath, Schubert, Remsberg, Chumlea, Sun and Towne2003). Linear growth and pubertal development are highly correlated and may be influenced by similar underlying biological processes that are under genetic control (Gasser et al., Reference Gasser, Molinari and Largo2013).
Dieting may also disrupt the neuroendocrine system (Chial et al., Reference Chial, McAlpine and Camilleri2002), which controls puberty initiation and regulation (Ojeda et al., Reference Ojeda, Lomniczi, Mastronardi, Heger, Roth, Parent and Mungenast2006), and may lead to a cascade of negative downstream effects. For example, dieting during puberty has been associated with decreased levels of estrogen (Dorgan et al., Reference Dorgan, Hunsberger, McMahon, Kwiterovich, Lauer, Van Horn and Taylor2003), which has been positively associated with body dissatisfaction and drive for thinness (Racine et al., Reference Racine, Culbert, Keel, Sisk, Burt and Klump2012). Developmental twin studies also suggest that puberty moderates genetic effects on disordered eating (Klump et al., Reference Klump, McGue and Iacono2003, Reference Klump, Perkins, Burt, McGue and Iacono2007). Given the variety of maturational processes occurring during puberty, it is plausible that dieting during this time could adversely influence gene expression.
Consistent with a previous co-twin control study in AN (Wade et al., Reference Wade, Treloar, Martin, Statham and Heath2004), affected twins had lower current BMIs than their unaffected co-twin. This finding is consistent with several studies reporting that the mean BMI in women recovered from AN is lower than healthy control women (Dellava et al., Reference Dellava, Hamer, Kanodia, Reyes-Rodriguez and Bulik2011; Sullivan et al., Reference Sullivan, Bulik, Fear and Pickering1998). Alternatively, some affected twins could have been symptomatic.
That birth weight did not differ between affected and unaffected twins diverges from the findings of Wade et al. (Reference Wade, Treloar, Martin, Statham and Heath2004). Limited research in singletons has also suggested that birth weight is not associated with AN (Foley et al., Reference Foley, Neale and Kendler2000), except in the case of very preterm births (Cnattingius et al., Reference Cnattingius, Hultman, Dahl and Sparen1999).
The observation of increased gastrointestinal problems and AN is worth noting. Although findings were not significant due to low power, seven times as many affected twins reported gastrointestinal problems than unaffected co-twins. Reflux disorder, bloating, nausea, abdominal distention, constipation, and fullness are common gastrointestinal problems associated with AN (Chial et al., Reference Chial, McAlpine and Camilleri2002; Emmanuel et al., Reference Emmanuel, Stern, Treasure, Forbes and Kamm2004; Winstead & Willard, Reference Winstead and Willard2006). Individuals with AN also report a significantly greater prevalence of gastroesophageal reflux disorder than unaffected individuals (Winstead & Willard, Reference Winstead and Willard2006).
Maternal report of infant feeding problems has been linked to self-reported lifetime AN by age 30 (Nicholls & Viner, Reference Nicholls and Viner2009), and retrospective maternal report of early gastrointestinal problems has been associated with AN in adolescence (Råstam, Reference Råstam1992). Starvation has also been associated with neuroendocrine abnormalities that may contribute to gastrointestinal problems (Chial et al., Reference Chial, McAlpine and Camilleri2002). Finally, purging behaviors (in AN purging type) are known disruptors of gastrointestinal function (Brown & Mehler, Reference Brown and Mehler2013). Future prospective studies are required to further explore this relationship.
A novel suggestive finding from this investigation was that twice as many unaffected co-twins reported greater emotional abuse and neglect than affected twins, although we were underpowered to detect significance. This contrasts with other adverse life events, which were less frequent and did not differ between affected and unaffected twins. Chronic childhood illnesses, including AN (Garley & Johnson, Reference Garley and Johnson1994), are reported to have negative consequences on the emotional wellbeing of unaffected siblings (Howe, Reference Howe, Stoneman and Berman1993; Sharpe & Rossiter, Reference Sharpe and Rossiter2002). As MZ twins typically spend more time together than DZ twins or siblings (Horwitz et al., Reference Horwitz, Videon, Schmitz and Davis2003; Kendler & Gardner, Reference Kendler and Gardner1998), being the MZ co-twin of an individual with AN may be particularly challenging. Honey and Halse (Reference Honey and Halse2007) found that parents of children with AN believed, despite their best efforts, that their child's illness had a negative emotional impact on unaffected siblings, requiring additional parental attention for feelings of neglect and resentment. Similarly, Dimitropoulos et al. (Reference Dimitropoulos, Klopfer, Lazar and Schacter2009) conducted semistructured interviews with unaffected siblings to determine their perspective on caring for a sibling with AN and concluded that additional interventions for siblings should be developed to facilitate overall family functioning and improve sibling emotional wellbeing. Although our power was limited and the finding not statistically significant, the magnitude of the difference supports more detailed investigation of this variable and clinical vigilance for the impact of sibling AN on the wellbeing of other children in the family.
Even though this was a unique sample, limitations must be considered. First, zygosity was established via self-report. Second, lifetime weight, including birth weight, as well as diagnostic information, including AN diagnosis, were collected via self-report, and the accuracy of the data could not be verified through clinical interview or objective measurement. Third, the response rate of this population-based study was 59.6%; thus, undetected response biases may exist. Fourth, participants were women from Sweden; results may not generalize to men or other populations. Fifth, only the presence or absence of life events was reported. Information regarding number of occurrences and year of occurrence was unavailable. Consequently, one life event could have been reported under more than one heading. Sixth, STAGE was designed to assess many health and psychological variables in a large sample, thus not all of the constructs, including comorbid diagnoses, were assessed in depth. This potentially limits the validity of these diagnoses. Finally, given the sample size, we were not able to consider more complex analytic models to control for potential confounders, nor could we explore twins discordant for AN by sub-type (restricting vs. binge-purge).
Conclusions
Notable differences exist between MZ twins discordant for AN. Clinically, these findings highlight the importance of addressing dispositional traits in the context of AN treatment and prevention (Zucker et al., Reference Zucker, Herzog, Moskovich, Merwin and Lin2011). Perfectionism—whether a risk factor or sequela—is a personality signature for AN (Bulik et al., Reference Bulik, Tozzi, Anderson, Mazzeo, Aggen and Sullivan2003). The identification of early dieting is important—either as a risk factor for AN or as an early warning sign or prodrome of the illness. Vigilance for early dieting by parents, schools, and health care professionals may assist with detecting and redirecting individuals who are on a path to AN (Neumark-Sztainer et al., Reference Neumark-Sztainer, Friend, Flattum, Hannan, Story, Bauer and Petrich2010). Future studies of the epigenetic effects of dietary intake around the pubertal transition are warranted to inform biological models of AN risk. Last, the observation that twice as many unaffected co-twins reported emotional abuse and neglect as affected twins highlights that the impact of AN on all family members is substantial (Gilbert et al., Reference Gilbert, Shaw and Notar2000; Honey et al., Reference Honey, Boughtwood, Clarke, Halse, Kohn and Madden2008). Having an afflicted same aged sibling may be particularly pernicious. Additional research exploring the impact of AN on siblings, especially twin siblings, is warranted to inform future interventions incorporating sibling-based programs into family treatment paradigms for AN. Clinicians treating AN should actively explore the effects of a child with AN on the wellbeing of all family members. AN is a demanding illness that exacts a high emotional, financial, and time toll on caregivers (e.g., Ohara et al., Reference Ohara, Komaki, Yamagata, Hotta, Kamo and Ando2016; Toulany et al., Reference Toulany, Wong, Katzman, Akseer, Steinegger, Hancock-Howard and Coyte2015). Although preliminary, our results suggest that it may adversely affect not only primary caregivers, but also siblings of individuals with AN.
Acknowledgments
We thank all participants for their time and efforts. Dr. Thornton was supported by National Institute of Health grant 1K01AA 18719-01A1 (PI: Root). Dr. Trace was supported by National Institute of Health grant T32MH076694 (PI: Bulik) and 2012–2015 Hilda and Preston Davis Foundation Postdoctoral Fellowship Program in Eating Disorders Research Award. Dr. Bergin was supported by T322MH20030 (PI: Neale). Dr. Bulik acknowledges funding from the Swedish Research Council (VR Dnr: 538-2013-8864). Other support includes National Institutes of Health grants CA-085739 (PI: Sullivan) and AI-056014 (PI: Sullivan) and the Anorexia Nervosa Genetics Initiative (ANGI), an initiative of the Klarman Family Foundation. The Swedish Twin Registry is supported by grants from the Swedish Department of Higher Education and the Swedish Research Council.
Disclosure of Interests
Dr. Bulik is a grant recipient from Shire, and has served on a Shire Advisory Board. All other authors reported no biomedical financial interests or potential conflicts of interest.
Details of Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.




