In the past two decades, genetic epidemiological research (the study of the role of genetic factors, and the interplay between genetic and environmental factors, in determining health and disease in families and in populations) in eating disorders has begun to inform a literature that had been predominantly focused on environmentally oriented risk factors. Reference Mazzeo and Bulik1 The now substantial body of research using quantitative genetic models with twin samples reveals a substantial heritable influence upon the expression disordered eating phenotypes, a median estimate of 52%. Reference Culbert, Racine and Klump2 Recently, more sophisticated analyses, such as ‘full quantitative gene–environment interplay models’, Reference Purcell3 have permitted researchers to statistically investigate, first, how an individual's genotype may render them more prone to environmental risk exposure (gene–environment correlation); and second, how an individual's genotype may vary their levels of vulnerability to the influence of environmental risk (gene–environment interaction). Reference Johnson4 To date, five studies have addressed gene–environment interplay related to disordered eating in twin samples (see online Table DS1), Reference Racine, Burt, Iacono, McGue and Klump5–Reference O'Connor, Klump, VanHuysse, McGue and Iacono9 showing that the impact of parental divorce, puberty and dietary restraint are moderated by genetic vulnerability for body dissatisfaction, disordered eating and binge eating, respectively. Arguably, in order to protect people against this vulnerability, it is most profitable to identify pervasive risk factors that are modifiable. Weight-related peer-teasing/bullying is one such risk factor, highly prevalent among children and adolescents, reported to occur in approximately one of every four youths. Reference Libbey, Story, Neumark-Sztainer and Boutelle10 It is characterised by ‘targeted negative commentary’ specifically relating to the victim's weight, Reference Libbey, Story, Neumark-Sztainer and Boutelle10 including jokes, name-calling and socially aggressive behaviour such as exclusion, singling out or being laughed at. Aside from increasing the likelihood of psychological morbidity encompassing anxiety, clinical levels of depression and suicidality, Reference Eisenberg, Neumark-Sztainer and Story11 experience of weight-related peer-teasing is significantly associated with disordered eating. Reference Menzel, Schaefer, Burke, Mayhew, Brannick and Thompson12 Despite weight-related peer-teasing being relatively common, not all of those targeted develop symptoms of disordered eating or clinical eating psychopathology. This scenario is suggestive of a mechanism underpinned by gene–environment interplay. Therefore, the objective of the current study is to examine quantitative gene–environment interplay in order to investigate whether peer-teasing may vary the contribution of genetic and environmental influences on disordered eating.
Method
Participants
Three waves of twin adolescent data include interview-based measures of disordered eating, described previously. Reference Wilksch and Wade13 At wave 1, the parents of female–female twins aged between 12 and 15 years, registered with the Australian Twin Registry (ATR), were approached to consent to the involvement of their daughters in the study. Families were predominantly White. Of the 719 families approached by the ATR, 411 (57.2%) agreed to participate, 237 (33%) said no, and 71 (9.9%) did not reply. Self-report questionnaires were sent to both parents, including those families where the parents did not live together. In total 595 parents returned questionnaires, constituting 351 families. Upon return of at least one of the parents' questionnaires, twins were contacted and interviewed over the telephone with the Eating Disorder Examination (EDE). Reference Fairburn, Cooper, Fairburn and Wilson14 Interviews were completed with 699 children, representing 377 monozygotic twins (187 complete pairs and 3 incomplete pairs, where 2 children had cerebral palsy and had difficulties talking and 1 withdrew), 308 dizygotic twins (154 complete pairs), with 7 pairs where zygosity was unknown (permission was not granted to access a biological sample for testing) i.e. 348 complete and 3 incomplete pairs. This sample represents 48.8% of those families who were initially approached.
At wave 2 every twin, including non-responders, were contacted again, where the mean time between first and second assessment was 1.15 years (s.d. = 0.17). A total of 514 parents completed questionnaires (86% of wave 1) and 669 twins completed interviews (96% of wave 1). All twins were recontacted at wave 3, and 499 were reinterviewed (71% of wave 1). Mean ages at each of the three waves were 13.96 years (s.d. = 0.80, range 12.70–16.28), 15.10 years, (s.d. = 0.83, range 13.76–17.56), and 16.90 years (s.d. = 0.70, range 15.49–19.84). Elapsed time between wave 1 and wave 3 ranged from 1.91 to 4.65, with a mean of 2.96 years (s.d. = 0.27). There was no relationship between attrition at wave 3 and EDE scores at wave 1 (odds ratio (OR) = 1.16, 95% CI 0.89–1.51).
Zygosity assignment was based on parental responses to standard questions about physical similarity and confusion of twins by parents, teachers and strangers, methods that give better than 95% agreement with genotyping. Reference Eaves, Eysenck, Martin, Jardine, Heath and Feingold15 Where there was uncertainty (n = 46 pairs), DNA testing was used to assign zygosity. The Flinders University Clinical Research Ethics Committee approved the data collection process and written informed consent from parents and written assent from the twins was obtained after the procedures had been fully explained.
Assessment protocol
Twins were interviewed over the telephone at each of the three waves. Each twin in the pair had a different interviewer. At each interview the EDE (12th edition) Reference Fairburn, Cooper, Fairburn and Wilson14 was administered, which subsequently provided the measure for disordered eating (i.e. EDE global). At wave 1 and wave 2 twins also responded verbally to various self-report questionnaires, Reference Wilksch and Wade13 including weight-related peer-teasing. 16 There was opportunity for clarification where any questions were not understood. Postgraduate clinical psychology trainees (n = 16) trained in use of the EDE conducted the interviews. The influence of weight and shape questions from the EDE was modified slightly to adapt to the younger age of the twin sample relative to the original EDE population. Reference Wilksch and Wade13
Disordered eating
The EDE addresses issues including weight concern, shape concern, eating concern and dietary restraint over the previous 28 days using 22 items that sum to provide a global measure of eating psychopathology. The EDE global measure is used extensively across a number of treatment studies to assess outcome related to disordered eating, with established validity and reliability. Reference Berg, Peterson, Frazier and Crow17 It has good convergence with subscale scores of the self-reported version, EDE-Q. Reference Berg, Peterson, Frazier and Crow18 Existing studies report test–retest reliability in clinical populations over 2–7 days and over 6–14 days to range from 0.50 to 0.88 for the scores of the four subscales constituting the EDE: restraint, eating concern, shape concern, weight concern. Reference Berg, Peterson, Frazier and Crow17 Internal consistency and interrater reliability for the EDE subscales has been previously found to range from 0.51 to 0.85, and 0.65 to 0.99, respectively. Reference Berg, Peterson, Frazier and Crow17 With the present data, the global EDE Reference Fairburn, Cooper, Fairburn and Wilson14 has previously been found to possess construct and convergent validly, in addition to factorial invariance, medium–large cross-wave correlations and high internal reliability. Reference Fairweather-Schmidt and Wade19 To encapsulate the trajectory of disordered eating over three time points, the slope (b) of the EDE global was calculated using the unstandardised regression formula (Formula 1).
Formula 1: derivation of slope using unstandardised regression formula
Where: x = wave; y = EDE global mean score; n = number of waves.
Weight-related peer-teasing
Weight-related peer-teasing was assessed at wave 1 and wave 2 with eight items, rated on a five-point Likert-type scale (1, never and 5, always), and was derived from the Weight Teasing–Peers subscale of the McKnight Risk Factor Survey IV. 16 Four items of the Weight Teasing–Peers subscale referred to teasing from girls, whereas the remaining four items referred to teasing from boys. Examples of these items included, ‘In the past year, how often have girls (including sisters) made fun of you because of your weight?’ and ‘In the past year, how often have boys (including brothers) made fun of you because of your weight?’ Higher scores indicated more peer weight-related teasing. Only wave 1 teasing was used in the moderation model, and in this sample, reliability statistics for the subscale were good (Cronbach's α = 0.87).
Body mass index
We adopted the Center for Disease Control recommendation to use body mass index (BMI)-for-age (or BMI centile) in this sample, as it is considered to be more accurate for children than BMI. Weight and height of the twins were reported by both the mother and father separately at wave 1. These reports were highly correlated and so the mother's report was used or the father's report if the mother's was missing.
Statistical analyses
For the purpose of the following analyses, all data were treated as being continuous. A full information maximum likelihood (FIML) approach using the statistical package Mx was used, Reference Neale20 which uses the raw data and incorporates complete and incomplete pairs of twins and those with missing data across the waves of data collection. Therefore 685 twins were included in the analyses. Initial analyses were undertaken in Mx to determine the strength of the phenotypic relationship between peer-teasing and disordered eating. The estimator used by Mx accounts for the non-independence of twin–twin pairs producing adjusted confidence intervals.
Prior to performing the biometric analyses, preliminary data screening revealed that the potential moderating variable, peer-teasing, was not normally distributed, but notably skewed (skew 1.60). This indicates that relatively few people in the sample experienced peer-teasing. To address this skew, normal weights of the raw scores were derived (i.e. using the liability threshold model). The threshold liability model is widely adopted in genetic research, and provides the underlying continuous distribution for the maximum likelihood estimation of data. Here, thresholds for the liability distribution are derived, and a normal distribution is assumed (mean, 0 and variance, 1) thus, standardised scores were analysed in Mx. In accordance with prior research our models did not control for BMI as the genetic influences that have an impact on both disordered eating and BMI have been shown to be predominantly independent. Reference Slof-Op 't Landt, Bartels, Van Furth, Van Beijsterveldt, Meulenbelt and Slagboom21 Analyses undertaken with this particular data-set Reference Fairweather-Schmidt and Wade19 are consistent with this independence.
Use of a full quantitative gene–environment interplay model (FQGEIM, Fig. 1) Reference Purcell3 is vital to this study as it permits capture of the multivariate and interactive relationships between genes and environments. Although the FQGEIM still divides the A (additive genetic), C (common/shared environment) and E (non-shared environment) effects as per models for single phenotypes, the FQGEIM additionally allows us to investigate how one phenotype (for example peer-teasing) moderates the expression of another phenotype (for example disordered eating). This is achieved by incorporating the relationship between these phenotypes (i.e. ACE ‘common’ effects: Ac, Cc and Ec, where c is the common pathway). The FQGEIM also accommodates the possibility that the peer-teasing phenotype moderates (M, moderator) all ACE paths of disordered eating (T, trait), denoting moderating effects common to both M and T with a ‘c’ (see central area of Fig. 1), and moderating effects unique to disordered eating are labelled ‘u’ (see right hand side of Fig. 1). Both of these ACE paths influence disordered eating (T), expressed by ‘effect + (moderated effect×level of moderator)’. Thus, each path leading to disordered eating (T) will have an A, C or E coefficient for both common and unique effects (six paths in total: Ac, Cc, Ec, Au, Cu and Eu). Each individual A, C or E coefficient is then summed with the product of the degree to which disordered eating is modified by the level of peer-teasing (for example Amc) and the peer-teasing coefficient. It is clear, then, that each A, C or E influence on disordered eating is not set, but varies in accordance with the action of peer-teasing. Further, the FQGEIM can produce estimates of gene–environment correlations by looking at the relationship between peer-teasing (M) and disordered eating (T). Reference Johnson4,Reference Krueger, South, Johnson and Iacono22
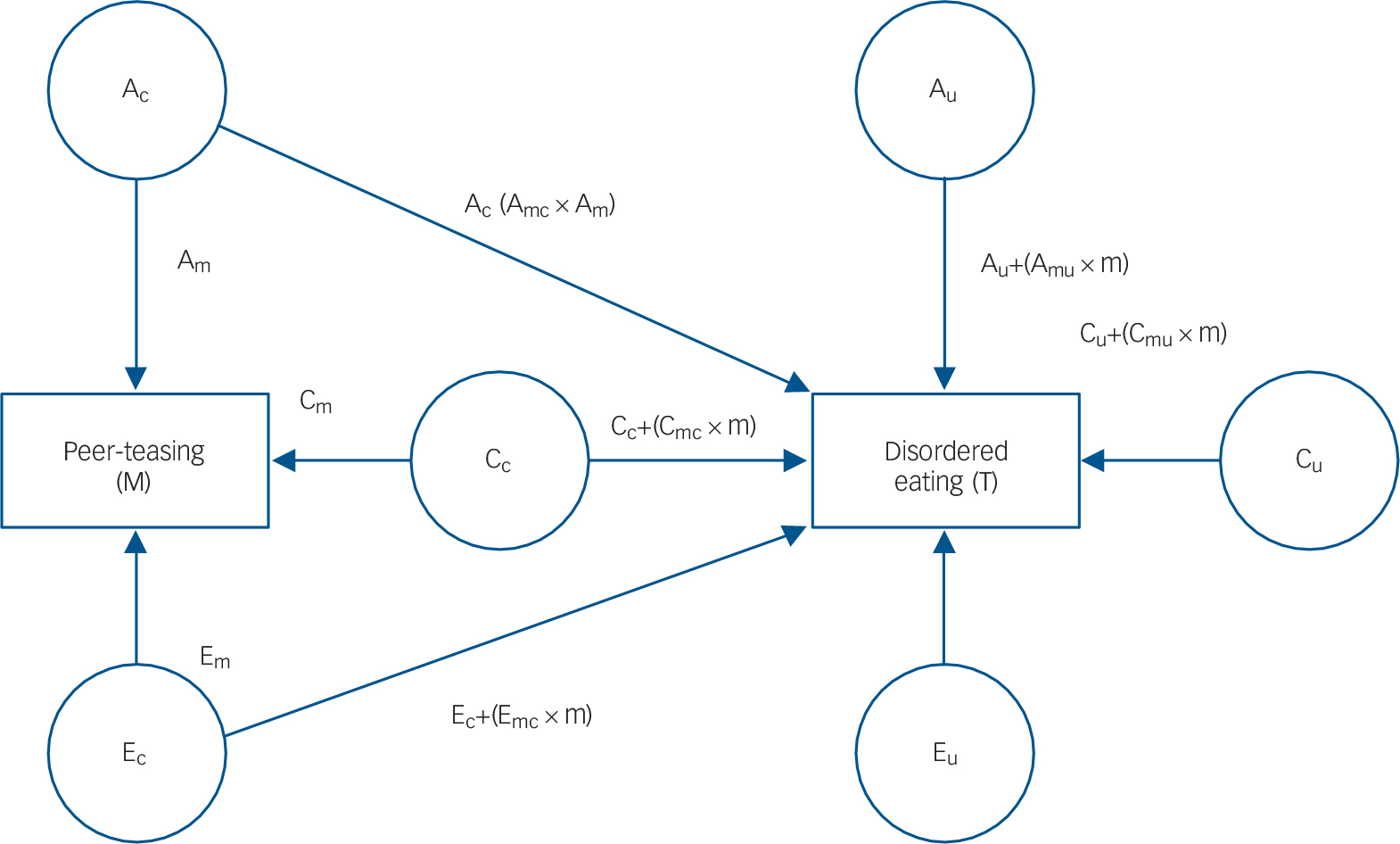
Fig. 1 Path diagram representing the full quantitative gene–environment interplay model for one twin.
A, additive genetic effects; C, common/shared environmental effects; c, common pathway; E, non-shared environmental effects; M, moderator; T, trait; u, unique pathway; m, moderator pathway.
Thus, to establish whether moderation improved the fit of our data, an initial comparison was made between FQGEIM with all parameters constrained (all Amc, Cmc, Emc, Amu, Cmu and Emu fixed at zero, therefore the contribution of a given parameter is not affected by varying levels of the moderator), and an alternative FQGEIM in which full moderation (no parameter constraints) was permitted. Model fit was assessed with the likelihood-ratio test (LRT; chi-square distribution), which addresses the difference in the −2 log likelihood (−2lnL) between the unmoderated and fully moderated model; and, the Akaike's information criterion (AIC). The AIC recognises the most parsimonious fit of data to the model and produces a metric where lower AIC values are preferred.
The analogous process is undertaken to determine which source(s) of variance were being influenced by the moderating variable. Beginning with the no moderation model, each source of variance in turn (for example only Amc and Amu moderation; shared environment Cmc and Cmu, and non-shared environment Emc and Emu, are fixed) was free to be influenced by the moderating variable (three models). Subsequently, a further three models were run that considered moderation of a combination of sources (for example genetic, A, and, non-shared environment, E; shared environment parameters Cmc and Cmu are fixed). As before, model fit was assessed using LRT and AIC.
Finally, once the best-fitting model was identified, models were run that specified the moderating variable at five standardised deviation levels (−2, −1, 0, 1, 2) around and including the moderator mean. Variance component estimates for A, C and E were produced; A, C and E estimates as a proportion of the total variance were calculated (for example, E% = E/(A+C+E)); and lastly, correlations between the moderator and the three sources of variance (rA, rC and rE) were also derived. We primarily report the unstandardised parameter estimates, as these estimates more accurately depict absolute changes in genetic and environmental influences than standardised estimates, which represent these changes as proportions of the total variance, Reference Purcell3 and also make our results more comparable with previous investigations in this field. Reference Klump, Burt, McGue and Iacono7
Results
Phenotypic correlations
Wave 1 peer-teasing was significantly correlated with wave 1 EDE (r = 0.62, 95% CI 0.57–0.66), wave 2 EDE (r = 0.36, 95% CI 0.29–0.42), wave 3 EDE, (r = 0.27, 95% CI 0.18–0.34) and wave 1 BMI centile (r = 0.36, 95% CI 0.29–0.42). These relationships show that a higher level of reported peer-teasing is associated with more symptoms of disordered eating and a higher BMI centile.
Biometric moderator analyses
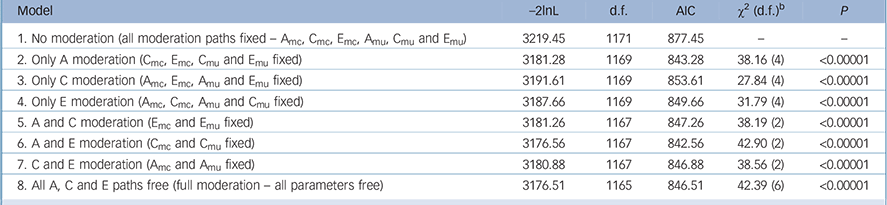
To investigate how the relationship between disordered eating and peer-teasing are influenced by genetic and environmental components, we fit a FQGEIM continuous moderator model Reference Purcell3,Reference Johnson4 to these data (Fig. 1). Table 1 reports model fit information where fit indices suggest that the full moderation model (Model 1, all A, C and E, and moderation linked paths are free) provides a better fit to these data than the model fixing all paths (Model 2, assuming no moderation). Subsequently, a further six models were run to identify which parameters were likely to be producing this effect. That is, whether all or, if not, which source(s) of the genetic, shared environment, or unique environment variance was moderated by peer-teasing. Thus, three subsequent models (Models 3–5) only permitted moderation of genetic (Amc and Amu), shared environment (Cmc and Cmu) or unique environment (Emc and Emu); −2lnL values were compared to the no moderation model (baseline model). A final three models (models 6–8) allowed moderation of two sources of variance: either A (Amc and Amu) and C (Cmc and Cmu), A (Amc and Amu) and E (Emc and Emu), or C (Cmc and Cmu) and E (Emc and Emu). LRTs (and associated χ2 and P-values) identified any significant differences between the fit of the baseline no moderation model and each subsequent moderation model. As reported in Table 1, the model permitting moderation of the genetic (Amc and Amu) and non-shared environmental sources (Emc and Emu) was identified as the best-fitting model according to the LRT and AIC (χ2 = 42.90, d.f. = 2, P<0.00001).
Table 1 Fit statistics for biometric models without moderation, with full moderation and models with specific paths fixed a
| Model | –2lnL | d.f. | AIC | χ2 (d.f.) b | P |
|---|---|---|---|---|---|
| 1. No moderation (all moderation paths fixed – Amc, Cmc, Emc, Amu, Cmu and Emu) | 3219.45 | 1171 | 877.45 | – | – |
| 2. Only A moderation (Cmc, Emc, Cmu and Emu fixed) | 3181.28 | 1169 | 843.28 | 38.16 (4) | <0.00001 |
| 3. Only C moderation (Amc, Emc, Amu and Emu fixed) | 3191.61 | 1169 | 853.61 | 27.84 (4) | <0.00001 |
| 4. Only E moderation (Amc, Cmc, Amu and Cmu fixed) | 3187.66 | 1169 | 849.66 | 31.79 (4) | <0.00001 |
| 5. A and C moderation (Emc and Emu fixed) | 3181.26 | 1167 | 847.26 | 38.19 (2) | <0.00001 |
| 6. A and E moderation (Cmc and Cmu fixed) | 3176.56 | 1167 | 842.56 | 42.90 (2) | <0.00001 |
| 7. C and E moderation (Amc and Amu fixed) | 3180.88 | 1167 | 846.88 | 38.56 (2) | <0.00001 |
| 8. All A, C and E paths free (full moderation – all parameters free) | 3176.51 | 1165 | 846.51 | 42.39 (6) | <0.00001 |
2lnL, −2 log likelihood; AIC, Akaike's Information Criterion.
a. The best-fitting model is Model 6.
b. χ2 (d.f.) represents likelihood-ratio test of difference between no moderation and moderation models.
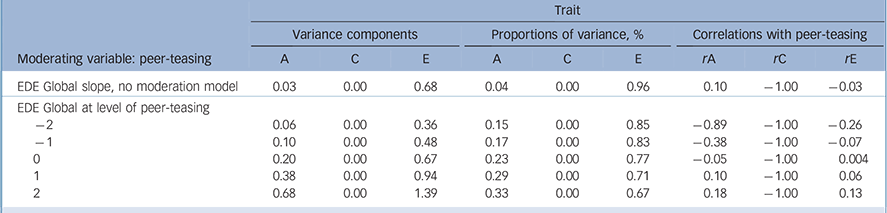
Table 2 reports the genetic, shared and non-shared environment estimates pertaining to the no moderation model, and a model allowing moderation of the genetic and non-shared environmental sources, identified above as the best-fitting model (AE). The no moderation model, analogous to the ‘standard’ twin modelling approach, identifies disordered eating is predominantly influenced (i.e. 96%) by peer-teasing through non-shared (E) sources (Fig. 2(a)), but there is a very modest heritable component (i.e. 4%). In contrast, the best-fitting model for moderation of disordered eating by peer-teasing, including the AE paths (Fig. 2(b)), showed the impact of heritable and environmental sources at varying levels of peer-teasing (i.e. −2 to +2 standard deviation units from mean level of peer-teasing). Confidence intervals (95%) for model pathways (see Fig. 1) were as follows: Am (0.0036 to 0.4044) Cm (0.00 to 0.3389), Em (0.4690 to 0.6572), Ac (−0.23 to 0.15), Cc (0.00 to 0.00), Ec (−0.15 to 0.16), Au (0.11 to 0.81), Cu (0.00 to 0.00), Eu (0.58 to 1.17). The unstandardised parameter estimates indicate that as peer-teasing increased, the contribution of both heritability and the non-shared environment increased. Although a low genetic correlation suggests that the aetiologies of disordered eating and peer-teasing are essentially unrelated at a genetic level, varying levels of peer-teasing reveal that trait disordered eating and environmental sources are less influential at low levels of peer-teasing (for example at −2s.d. rA = −0.89, rE = −0.29), whereas environmental sources become more important when higher levels of peer-teasing are experienced (for example at +2s.d. rA = 0.18, rE = 0.13).
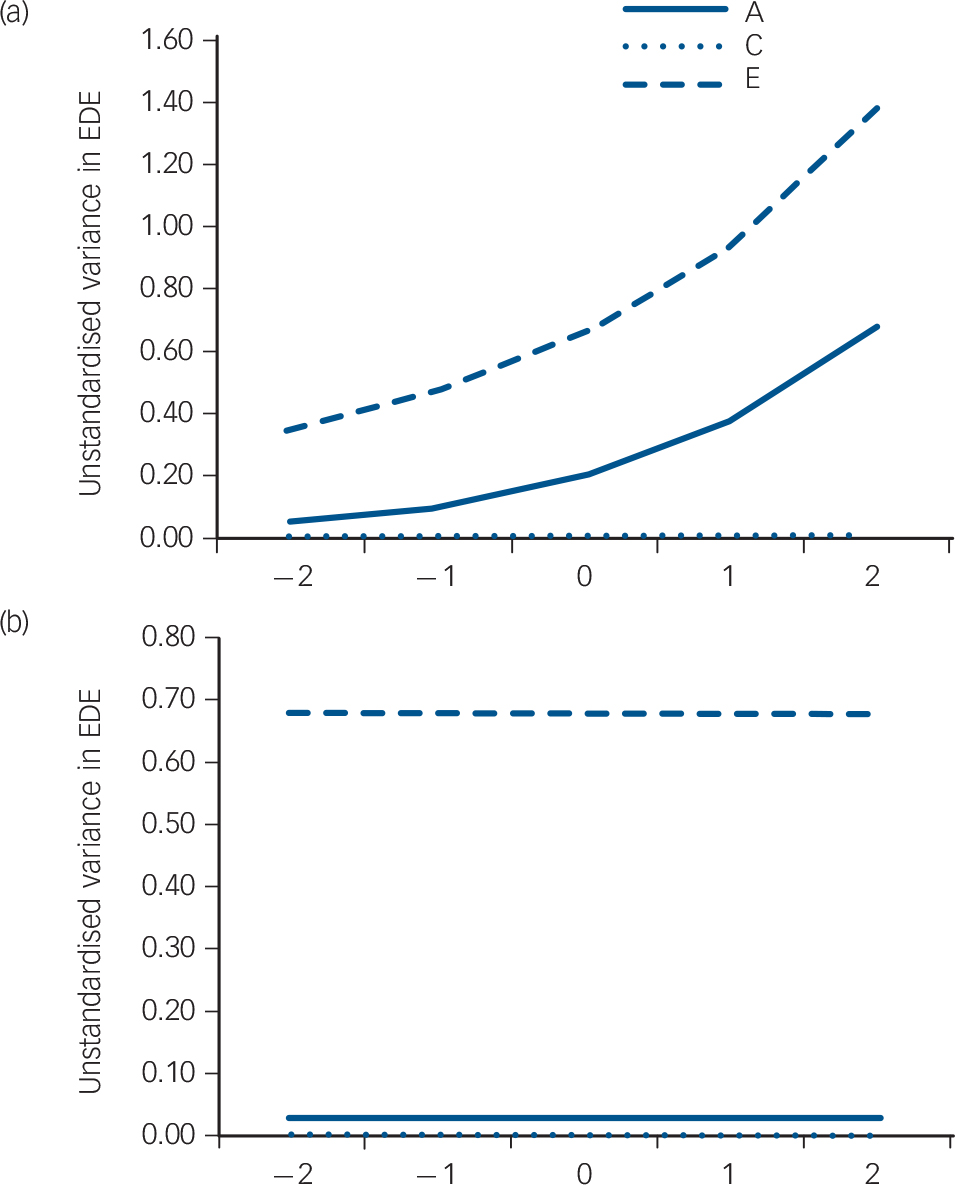
Fig. 2 Unstandardised variance in disordered eating across fixed (a) and between −2 to +2 standard deviation units of peer-teasing (b).
A, additive genetic variance; C, shared environmental variance (fixed); E, non-shared environmental variance; EDE, Eating Disorder Examination.
Table 2 Standardised estimates of variance components and proportions of variance in Eating Disorder Examination (EDE) and peer-teasing and their genetic and environmental correlations for AE moderation model a
| Trait | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variance components | Proportions of variance, % | Correlations with peer-teasing | |||||||
| Moderating variable: peer-teasing | A | C | E | A | C | E | rA | rC | rE |
| EDE Global slope, no moderation model | 0.03 | 0.00 | 0.68 | 0.04 | 0.00 | 0.96 | 0.10 | −1.00 | −0.03 |
| EDE Global at level of peer-teasing | |||||||||
| −2 | 0.06 | 0.00 | 0.36 | 0.15 | 0.00 | 0.85 | −0.89 | −1.00 | −0.26 |
| −1 | 0.10 | 0.00 | 0.48 | 0.17 | 0.00 | 0.83 | −0.38 | −1.00 | −0.07 |
| 0 | 0.20 | 0.00 | 0.67 | 0.23 | 0.00 | 0.77 | −0.05 | −1.00 | 0.004 |
| 1 | 0.38 | 0.00 | 0.94 | 0.29 | 0.00 | 0.71 | 0.10 | −1.00 | 0.06 |
| 2 | 0.68 | 0.00 | 1.39 | 0.33 | 0.00 | 0.67 | 0.18 | −1.00 | 0.13 |
A, additive genetic; C, common/shared environment; E, non-shared environment.
a. Variance components may not necessarily sum to 1.00; proportions of variance sum to 1.00.
Discussion
Main findings
This represents only the third study to identify a risk factor that moderates genetic vulnerability for disordered eating, in addition to puberty and dietary restraint. Reference Racine, Burt, Iacono, McGue and Klump5,Reference Klump, Burt, McGue and Iacono7 The results of this research show that weight-related peer-teasing influences disordered eating over adolescence through increases in both genetic and non-shared environmental factors, with unstandardised estimates indicating that genetic factors increased 11-fold and proportionally more than environmental sources (a 4-fold increase). In other words, vulnerability to peer-teasing varies primarily according to the individual's genotype. This accords with clinical observations, where a client recounts a single, but salient, episode of teasing related to weight or size that triggers a catastrophic response in the form of strict and unrelenting dietary restraint that develops into anorexia nervosa or bulimia nervosa. We also found that as peer-teasing increased, its correlation with genetic risk for disordered eating increased as did the correlation with non-shared environmental factors. In other words, it seems possible that genetic vulnerability to disordered eating evokes peer-teasing in the environment. However, peer-teasing is so prevalent, Reference Libbey, Story, Neumark-Sztainer and Boutelle10 with increased reach through the emergence of widely available social media in adolescent populations, it is still possible that risk is activated with little or any genetic trigger of the teasing. This includes BMI, a very heritable trait, Reference Nan, Guo, Warner, Fowler, Barrett and Boomsma23 which had a correlation of 0.36 with weight-related peer-teasing, or a 13% overlap in variance – in other words, such teasing may occur in the absence of objective overweight.
Implications
A multiplicity of risk factors for eating disorders and disordered eating exist, and a better understanding of the complex interplay between genes and the environment enables us to identify those risk factors that potentially afford interventions ‘more bang for bucks’. Recent work has confirmed a genetic sensitivity to the environment where adverse contexts not only trigger adverse responses in the genetically vulnerable, but also trigger stronger adaptive behaviour within protective environments in this same group. For example, studies show that the effect of physical activity on weight loss is greater for those with greater genetic susceptibility to obesity than those with low susceptibility. Reference Ahmad, Rukh, Varga, Ali, Kurbasic and Shungin24 Although such work is yet to be carried out with respect to disordered eating, this suggests that a focus on developing protective environments may be of great value in preventing eating disorders development among those with a genetic liability.
The implications of the current research for developing protective environments for weight-related peer-teasing are manifold. First, although larger effect sizes can be obtained from the use of selective prevention, where body image concerns already exist in older adolescents or young women, this research suggests that intervention in early adolescence may be optimal with respect to tackling peer-teasing, in the context of a universal prevention framework. Second, given the public health importance of decreasing peer-teasing in order to reduce the incidence of disordered eating, further research to investigate the impact of policies that support the elimination of appearance commentary related to adolescent girls is required. Such policies may be implemented at school level, with the inclusion of parental education, and supplemented by class-based programmes targeting appearance-based commentary from peers. One such example is Happy Being Me, which improved body dissatisfaction, media internalisation, dieting, appearance conversations, appearance teasing and self-esteem at 3-month follow-up with 12-year-old girls, Reference Richardson and Paxton25 and weight and concerns in 13-year-old girls at 12-month follow-up. Reference Wilksch, Paxton, Byrne, Austin, McLean and Thompson26 The third broad strategy is to equip adolescent girls with the skills required to neutralise the impact of such commentary. For example, the use of media literacy programmes such as Media Smart have been shown to reduce eating concerns and perceived pressure to be thin in 13-year-old girls at 6-month follow-up. Reference Wilksch, Paxton, Byrne, Austin, McLean and Thompson26
Strengths and limitations
The strengths of the current research include examination of the longitudinal trajectory of disordered eating over adolescence, the use of psychometrically strong measures (including the first use of an interview-based measure of disordered eating in examination of gene–environment interactions), and use of more comprehensive full quantitative gene–environment interplay models. However, results should be interpreted in the context of the following limitations. First, baseline response rate was 49%, but this equates with other large longitudinal epidemiological studies of twins and singletons. Reference Wade, Bergin, Tiggemann, Bulik and Fairburn27 Second, participants reporting disordered eating at wave 1 were offered referrals, which may have influenced subsequent levels of disordered eating. Third, a larger sample would have enhanced our ability to more definitely reject certain models, and to model inclusion of potential confounding variables such as BMI.
Future directions for research
Future research should investigate a variety of modifiable risk factors that moderate genetic vulnerability for disordered eating. In this way we can begin making more informed choices about targets for interventions. Future research should also test aetiological models that more explicitly investigate the relations between peer-teasing and other variables such as perfectionism, internalisation of the thin ideal and difficulties with emotion regulation, providing more intervention opportunities to decrease the impact of peer-teasing on disordered eating.
Funding
Grants and from the National Health and Medical Research Council (NHMRC) to T.D.W. supported this work. Administrative support for data collection was received from the Australian Twin Registry, which is supported by an Enabling Grant () from the NHMRC administered by the University of Melbourne.
Acknowledgements
We would like to thank the twins and their families for their participation in this research, Ms Judith Slater for coordinating the data collection, and Associate Professor Susan South and Dr Gu Zhu for their advice relating to statistical modelling







eLetters
No eLetters have been published for this article.