Conventional volumetric magnetic resonance imaging (MRI) studies suggest that at least a proportion of people with schizophrenia, particularly men and those with a first psychotic episode, Reference Sim, DeWitt, Ditman, Zalesak, Greenhouse and Goff1,Reference Narr, Thompson, Szeszko, Robinson, Jang and Woods2 have smaller hippocampal volumes than controls, as shown by systematic review and meta-analysis of regional morphometry. Reference Chakos, Schobel, Gu, Gerig, Bradford and Charles3,Reference Steen, Mull, McClure, Hamer and Lieberman4 Decreased hippocampal size may be accompanied by reductions in N-acetylaspartate levels, Reference Steen, Hamer and Lieberman5 and by hyperactivity of excitatory cortical pathways. Reference Wang, Mamah, Harms, Karnik, Price and Gado6 One meta-analysis reported slightly lower hippocampal volumes in non-psychotic first-degree relatives of patients with schizophrenia. The effect size was small to moderate, and not consistently found – especially for the right hippocampus – making the results hard to interpret. Reference Boos, Aleman, Cahn, Hulshoff Pol and Kahn7 Interestingly, a study investigating people at high risk of psychosis, patients with first-episode psychosis and individuals with chronic schizophrenia found hippocampal structural differences after the onset of the disease, Reference Velakoulis, Wood, Wong, McGorry, Yung and Phillips8 as also confirmed by a meta-analysis of voxel-based structural MRI studies. Reference Ellison-Wright, Glahn, Laird, Thelen and Bullmore9 The same group showed, counterintuitively, that larger rather than smaller hippocampal volumes in the high-risk cohort predicted subsequent development of acute psychosis, i.e. people who later developed a psychotic disorder had larger volumes than the high-risk non-psychotic subgroup, with no difference from the normal group. Reference Phillips, Yung, Yuen, Pantelis and McGorry10 In addition, prior findings in schizophrenia have been inconsistent, reporting preserved volumes, Reference Staal, Hulshoff Pol, Schnack, Hoogendoorn, Jellema and Kahn11–Reference Frazier, Hodge, Breeze, Giuliano, Terry and Moore14 as also shown by a voxel-wise meta-analysis using anatomic likelihood estimation to identify consistently implicated brain areas across studies, Reference Glahn, Laird, Ellison-Wright, Thelen, Robinson and Lancaster15 or minimal volume reductions of about 4%. Reference Nelson, Saykin, Flashman and Riordan16 In this regard, in our prior region of interest study we found no hippocampal volume abnormality in schizophrenia. Reference Baiano, Perlini, Rambaldelli, Cerini, Dusi and Bellani17 Some post-mortem studies agree with imaging findings showing hippocampal atrophy in schizophrenia, Reference Jeste and Lohr18,Reference Heckers, Stone, Walsh, Shick, Koul and Benes19 but others report no neuropathological abnormality. Reference Hurlemann, Tepest, Maier, Falkai and Vogeley20,Reference Walker, Highley, Esiri, McDonald, Roberts and Evans21
From these findings it is not yet fully understood whether the hippocampus is consistently altered in schizophrenia; however, at least during the onset phase of the disease, dynamic processes in the brain may change its morphology. It is indeed a plastic structure sensitive to environmental, clinical and psychosocial phenomena. As such, it is of interest to investigate further whether potential hippocampal changes reflect neurodevelopmental anomalies, an aspect of disease progression or structural sequelae of psychosocial changes that accompany the illness. The role of such dimensions in affecting, or associating with, hippocampus volumes in schizophrenia has been underinvestigated. To this extent, schizophrenia is a heterogeneous syndrome including people with a relatively positive course of illness and others with a far poorer long-term prognosis. One strategy for better understanding the pathophysiology of the disease would be to relate specific clinical and social variables to deviations from normal brain anatomy. In this context different outcome measures have been used, such as duration and severity of positive symptoms, recovery from the first episode, psychopathological indicators of the Brief Psychiatric Rating Scale or functioning as detected with the Global Assessment of Functioning scale (see Bellani et al for a review). Reference Bellani, Dusi and Brambilla22
We report results of the first study (to our knowledge) to use three-dimensional (3D) surface mesh models to evaluate statistical effects of clinical and psychosocial variables on the structural anatomy of the hippocampus in schizophrenia. This advanced computational imaging technique examines, at high spatial resolution, neuroanatomical hippocampal differences with more spatial detail than conventional manual tracing methods, allowing calculation of the local radial shrinkage. Reference Thompson, Hayashi, De Zubicaray, Janke, Rose and Semple23 It can detect subtle or localised alterations in hippocampal structure, even when differences in overall hippocampal volumes are not detectable, as in our prior study. Reference Baiano, Perlini, Rambaldelli, Cerini, Dusi and Bellani17
Method
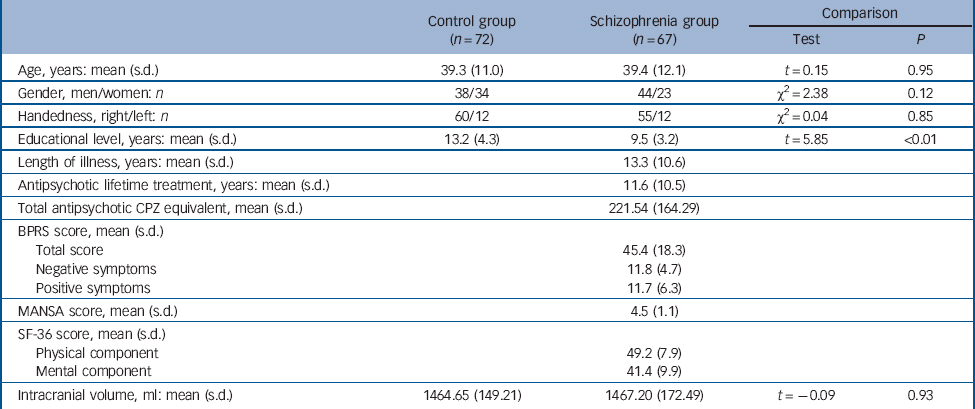
A total of 67 patients with a DSM-IV diagnosis of schizophrenia and a control group of 72 healthy individuals were studied (see Table 1). 24 Patients were recruited from the geographically defined catchment area of South Verona (around 100 000 inhabitants). They were being treated by the South Verona community-based mental health service and by other clinics reporting to the South Verona Psychiatric Care Register. Diagnoses of schizophrenia were obtained using the Item Group Checklist of the Schedule for Clinical Assessment in Neuropsychiatry (IGC-SCAN), Reference Wing25 administered by two trained research clinical psychologists with extensive experience in this measure; they had already administered at least ten previous IGC-SCAN assessments with a trained senior investigator. The Italian version of the SCAN was used, edited by our group. Reference Tansella and Nardini26 The psychopathological item groups completed by the two raters were compared, to discuss any discrepancies in the assessment of major symptoms. Diagnoses of schizophrenia were corroborated by the clinical consensus of two staff psychiatrists according to DSM-IV criteria. We also ensured the reliability of the IGC-SCAN diagnoses by holding regular consensus meetings with the psychiatrists treating the patients and a senior investigator. Patients with comorbid psychiatric disorders, alcohol or substance misuse within the 6 months preceding the study, history of traumatic head injury with loss of consciousness, epilepsy or other neurological disease were excluded. The patients' antipsychotic medication was recorded and chlorpromazine equivalent dosages were calculated (Table 1). Patients' clinical information was retrieved from psychiatric interviews, the attending psychiatrist and medical charts. Clinical symptoms were characterised using the 24-item Brief Psychiatric Rating Scale (BPRS) and psychosocial functioning with the 36-item Short Form Health Questionnaire (SF-36) and the Manchester Short Assessment of Quality of Life (MANSA). Reference Overall and Gorham27–Reference Priebe, Huxley, Knight and Evans29 These scales were administered by two trained clinical research psychologists. The reliability of the BPRS was established and monitored using procedures similar to those for the IGC-SCAN.
Participants in the control group had no DSM-IV Axis I disorder, determined using a brief modified version of the Structured Clinical Interview for DSM-IV – Non-Patient Version, Reference First, Gibbon and Williams30 no history of psychiatric disorder among first-degree relatives, no history of alcohol or substance misuse and no current major medical illness. All participants gave signed informed consent, after an explanation of all issues involved in participation in the research. The research was approved by the biomedical ethics committee of the Azienda Ospedaliera of Verona.
Psychosocial scales
MANSA
The MANSA is a clinical interview originally developed as a brief modified version of the Lancashire Quality of Life Profile. Reference Priebe, Huxley, Knight and Evans29 The scale encompasses three sections: the first describes stable personal patient details, such as personal information and diagnosis; the second records personal details that may change over time, such as education, employment, income, accommodation and family members living with the patient; and the third section consists of 16 questions that must be asked every time the assessment is applied. In this section, 4 questions address objective items (having a close friend, number of contacts with friends per week, judicial proceedings and physical violence experienced) and require a ‘yes’ or ‘no’ answer; the remaining 12 questions address subjective domains (satisfaction with life, employment or training, financial situation, quality of friendships, hobbies, satisfaction with accommodation, personal safety, sexual life, relationship with family, physical and mental health) and are rated on a seven-point scale, from ‘extremely negative’ (score 1) to ‘extremely positive’ (score 7).
SF-36
The SF-36 is one of the most widely used generic health status measures. Reference Ware and Sherbourne28 It is composed of 36 questions and standardised response choices, divided into eight multi-item scales: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitation due to emotional problems, and general mental health. It is applied to assess the previous 4 weeks.
Image acquisition
Magnetic resonance imaging scans were acquired with a 1.5 T Siemens Magnetom Symphony Maestro Class scanner, Syngo MR, 2002B (Siemens, Erlangen, Germany). A standard head coil was used for radio-frequency transmission and reception of the magnetic resonance signal; restraining foam pads were used to minimise head motion. First, T 1-weighted images were obtained to verify each participant's head position and the image quality, with acquisition parameters repetition time (TR) 450 ms, time to echo (TE) 14 ms, flip angle 90°, field of view (FOV) 230×230 mm2, 18 slices, slice thickness 5 mm, matrix size 384×512, number of excitations (NEX) 2. Proton density and T 2-weighted images were then acquired (TR = 2500 ms, TE = 24/121 ms, flip angle 180°, FOV = 230×230 mm2, 20 slices, slice thickness 5 mm, matrix size 410×512, NEX 2) according to an axial plane parallel to the anterior–posterior commissures to exclude focal lesions. Subsequently, a coronal 3D magnetisation prepared rapid gradient echo (MP-RAGE) sequence was acquired (TR = 2060 ms, TE = 3.9 ms, flip angle 15°, FOV = 176×235 mm2, slice thickness 1.25 mm, matrix size 270×512, inversion time 1100 ms) to obtain 144 images covering the entire brain.
Image processing and analysis
Anatomical imaging data were transferred to a personal computer workstation and analysed using the BRAINS2 software developed at the University of Iowa (http://www.nitrc.org/search/?type-of-search=group&cat=498:BRAINS+License). The hippocampus was manually traced on the T 1-weighted (MP-RAGE) images in the coronal plane as previously described by our group. Reference Brambilla, Harenski, Nicoletti, Sassi, Mallinger and Frank31 Specifically, the corona radiata and the ambient cistern were used as the superior border, the white matter served as the inferior border and the inferior horn of the lateral ventricle as the lateral one. The parahippocampal gyrus and other surrounding areas, such as entorhinal and perirhinal regions, were not included in the tracing. The hippocampus for this study was rigidly aligned and re-sliced for tracing, i.e. only a rigid body transform was used to align the brains for tracing, to avoid re-scaling the anatomy. Intracranial volume was traced in the coronal plane along the border of the brain and included the cerebrospinal fluid, dura mater, sinus, optic chiasm, brainstem, cerebral and cerebellar matter. The inferior border did not extend below the base of the cerebellum. The first and last ten slices including brain matter were traced, then one slice in every five was traced. Tracing was performed by two raters masked to the participant's identity and to sociodemographic and clinical variables. They achieved high reliability, as defined by intraclass correlation coefficients of 0.96 for both left and right hippocampal volumes and 0.97 for intracranial volume, obtained by masking on ten scans. The volumes were obtained by summing the volumes of all relevant slices and were expressed in ml. Raw measures were obtained.
Statistical analysis
Three-dimensional parametric surface maps were created from the automatically generated segmentations of each individual's hippocampus using anatomical surface modelling software. Reference Thompson, Hayashi, De Zubicaray, Janke, Rose and Semple23 A 3D medial curve was calculated from the hippocampal traces, computed separately for each individual. Local radial shape deflation was calculated by measuring the radial distances from each hippocampal surface point on the 3D surface mesh to the medial axis. Based on computed point-wise correspondences, surface models for all individuals in each diagnostic group were geometrically averaged. Statistical maps indicating local volumetric differences between groups were generated from the surface models. A regression was also run at each surface point to compare differences between diagnostic groups and determine the association of clinical factors (chronicity, illness severity and medication) and psychosocial scores (quality of life and health status) with radial shrinkage. All statistical maps were directionally divided to indicate a positive or negative association; the maps shown are one-sided statistical maps in the hypothesised direction for each clinical measure (with lower volumes associated with poorer performance). Colour-coded probability values were assigned to the average surface models and their overall (corrected) significance was assessed using a permutation test. The P values describe the significance of differences in radial distances between diagnostic groups or their association with clinical values. We used permutation tests to provide an overall significance value (a corrected P value) for the statistical whole maps. The overall permutations P was derived for left top, left bottom, right top and right bottom hippocampi. The primary threshold of P = 0.05 was established for all statistical maps and the suprathreshold area was compared with its (non-parametric) null distribution based on randomised data. Correlations with clinical variables (length of illness, positive symptoms, negative symptoms, antipsychotic lifetime treatment) and psychosocial scores (MANSA, SF-36 physical component, SF-36 mental component) were separately corrected by applying Bonferroni correction.
Table 1 Demographic and clinical characteristics of the sample
| Control group (n = 72) |
Schizophrenia group (n = 67) |
Comparison | ||
|---|---|---|---|---|
| Test | P | |||
| Age, years: mean (s.d.) | 39.3 (11.0) | 39.4 (12.1) | t = 0.15 | 0.95 |
| Gender, men/women: n | 38/34 | 44/23 | χ2 = 2.38 | 0.12 |
| Handedness, right/left: n | 60/12 | 55/12 | χ2 = 0.04 | 0.85 |
| Educational level, years: mean (s.d.) | 13.2 (4.3) | 9.5 (3.2) | t = 5.85 | <0.01 |
| Length of illness, years: mean (s.d.) | 13.3 (10.6) | |||
| Antipsychotic lifetime treatment, years: mean (s.d.) | 11.6 (10.5) | |||
| Total antipsychotic CPZ equivalent, mean (s.d.) | 221.54 (164.29) | |||
| BPRS score, mean (s.d.) | ||||
| Total score | 45.4 (18.3) | |||
| Negative symptoms | 11.8 (4.7) | |||
| Positive symptoms | 11.7 (6.3) | |||
| MANSA score, mean (s.d.) | 4.5 (1.1) | |||
| SF-36 score, mean (s.d.) | ||||
| Physical component | 49.2 (7.9) | |||
| Mental component | 41.4 (9.9) | |||
| Intracranial volume, ml: mean (s.d.) | 1464.65 (149.21) | 1467.20 (172.49) | t = −0.09 | 0.93 |
BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine; MANSA, Manchester Short Assessment of Quality of Life; SF-36, 36-item Short Form Health Survey.
Results
The demographic characteristics of the sample are listed in Table 1. All participants were White. All but one patient were receiving antipsychotic medication at the time of scanning: specifically, 25 patients were prescribed first-generation antipsychotic drugs (haloperidol n = 19, fluphenazine n = 4, zuclopenthixol n =2) and 41 patients were prescribed second-generation drugs (olanzapine n = 22, clozapine n = 9, risperidone n = 7, quetiapine n = 3). Five of these patients were taking another antipsychotic at the time of imaging (clotiapine n = 2, thioridazine n =1, quetiapine n = 1, olanzapine n = 1).
Group difference maps
We created 3D maps showing the mean level of hippocampal deflation in the schizophrenia group v. the control group. Separate maps were created for the left and right hippocampus. The results indicated no significant difference in either radial shrinkage or intracranial volume between the two groups (Table 1).
Sociodemographic variables
Significant associations were found in the maps for gender (left P<0.001; right P<0.001) and handedness (left P<0.001), with, as expected, a smaller hippocampus in women than in men and a larger left hippocampus in right-handed individuals. Next, we examined statistical effects of educational level in the schizophrenia group. Bilateral shape deflation was associated with lower levels of education in the schizophrenia group (left P = 0.001; right P<0.001) but not in the control group (P>0.05); see online Fig. DS1.
Clinical variables
We created maps of associations between regional shape deflation and clinical scores. Bilateral hippocampal deflation was associated with greater duration of illness (left P = 0.01, right P = 0.003; after Bonferroni correction: left P = 0.04, right P = 0.012) and higher levels of positive symptoms (left P = 0.05, right P = 0.005; after Bonferroni correction: left P = 0.20, right P = 0.02) and negative symptoms (left P = 0.01, right P = 0.008; after Bonferroni correction: left P = 0.04, right P = 0.032); see online Fig. DS2. Controlling the analyses for intracranial volume, gender and handedness, negative symptoms and left hippocampal deflation showed a trend for significant correlation (P = 0.09). Finally, the effect of antipsychotic medication on the hippocampal size of the group with schizophrenia was examined, showing that more years of antipsychotic drug intake were associated with greater right hippocampal deflation (right P = 0.04), which did not survive Bonferroni correction (P = 0.16). No significant correlation was observed between bilateral hippocampal region of interest volumes or intracranial volume and clinical measures (partial correlation analyses controlled for age, P>0.05, Bonferroni corrected).
Psychosocial variables
Examining generic health status with the SF-36, we found that lower levels of both physical health (left P = 0.01, right P = 0.01; after Bonferroni correction: left P = 0.03, right P = 0.03) and mental health (left P = 0.007, right P = 0.01; after Bonferroni correction: left P = 0.021, right P = 0.03) significantly correlated with bilateral deflation of the hippocampus. A similar association was found between a lower MANSA score and decreased hippocampal size (left P = 0.002, right P<0.001; after Bonferroni correction: left P = 0.006, right P<0.003); see online Fig. DS3. Controlling the analyses for intracranial volume, gender and handedness, SF-36 mental health scores and bilateral hippocampal deflation still correlated (right side P = 0.029, left side P = 0.09). The SF-36 and MANSA scores were not significantly associated with right and left hippocampal region of interest volumes or with intracranial volume (partial correlation analyses controlled for age, P>0.05, Bonferroni corrected).
Discussion
Using relatively novel methods for 3D mapping of the hippocampus, we did not detect significant hippocampal surface morphological differences at the group level in schizophrenia relative to age-matched controls. This confirms our earlier study, Reference Baiano, Perlini, Rambaldelli, Cerini, Dusi and Bellani17 and is in line with the somewhat controversial structural MRI literature summarised in the introduction, with some studies reporting hippocampal volume shrinkage and others reporting no abnormality. Notably, a preserved number and size of hippocampal neurons have been reported in schizophrenia. Reference Walker, Highley, Esiri, McDonald, Roberts and Evans21 In addition, positron emission tomography and single photon emission computed tomography studies yielded a small effect size for hippocampal metabolism in differentiating schizophrenia and control groups, with an almost complete overlap (>92%) between the two distributions. Reference Davidson and Heinrichs32 This suggests that hippocampal differences in schizophrenia, if present, might relate to local disturbances in cytoarchitectural organisation, Reference Heckers and Konradi33 with only partial or minimal overall effects on hippocampal morphology and metabolism. However, we investigated mainly patients with chronic disorder receiving long-term antipsychotic maintenance treatment, which may potentially have preserved or normalised the size of the hippocampus; decreased hippocampal volumes might be present in drug-naive people with a first episode of psychosis. Reference Vita, De Peri, Silenzi and Dieci34 Indeed, several human and animal studies suggest that some antipsychotic drugs may have neuroprotective effects on the hippocampus. Reference Pillai and Mahadik35,Reference Bearden, Thompson, Avedissian, Klunder, Nicoletti and Dierschke36
Interestingly, with surface-based anatomical maps we found that greater hippocampal deflation was correlated with disease severity (duration of illness, positive and negative symptoms) and with poor social outcome identified in terms of lower levels of education, quality of life and health status. Consistent with this, prior imaging studies in schizophrenia found that smaller hippocampal volumes were associated with poorer premorbid adjustment, Reference Smith, Lang, Kopala, Lapointe, Falkai and Honer13 and with longer duration of illness. Reference Chakos, Schobel, Gu, Gerig, Bradford and Charles3,Reference Velakoulis, Pantelis, McGorry, Dudgeon, Brewer and Cook37 More severe positive symptoms have also been related to poorer hippocampal functioning. Reference Goghari, Sponheim and MacDonald38 Furthermore, lower educational levels are associated with smaller grey-matter volumes (particularly in the temporal lobes) in healthy individuals, Reference Ho, Raji, Becker, Lopez, Kuller and Hua39 which might therefore have a greater negative impact on people with mental illness such as schizophrenia, as suggested by our findings. Our results are also supported by a study of patients with first-episode psychosis, in which smaller right hippocampal volumes were associated with worse clinical outcome at 3-year follow-up. Reference De Castro-Manglano, Mechelli, Soutullo, Landecho, Gimenez-Amaya and Ortuno40
Outcomes in chronic illness
Clinical outcomes may fluctuate during the first 10–15 years of schizophrenic illness and may not consistently relate to specific structural brain differences. Reference Staal, Hulshoff Pol and Kahn41 Therefore it is extremely useful to investigate outcome measures in a cohort with chronic illness of long duration, as in our study. Prior imaging studies have explored structural markers of poor outcome in schizophrenia, differently defined based on admission rate, time spent in hospital, response to treatment, symptoms, self-care ability and general functioning. In particular, greater ventricular and lower cerebral (frontal) volumes have been reported, even with progressive changes over time in both chronic and first-episode disorder (see Bellani et al for review). Reference Bellani, Dusi and Brambilla22 Reduced volumes of the putamen and corpus callosum have also been reported by Mitelman et al in patients with very poor outcomes, identified as ‘Kraepelinian’. Reference Mitelman, Canfield, Chu, Brickman, Shihabuddin and Hazlett42,Reference Mitelman, Nikiforova, Canfield, Hazlett, Brickman and Shihabuddin43 These patients had, for the previous 5 years, been confined to hospital or needed continuous assistance, were unemployed and had no remission of symptoms. However, to the best of our knowledge, no prior report investigated specifically how hippocampal structure relates to specific psychosocial scales used to measure social functioning, as in our report. Therefore, it should be kept in mind that an impaired neuronal network, including in particular frontal cortex, putamen, corpus callosum and hippocampus, may be associated with a poor outcome in schizophrenia.
Our results suggest that illness severity and poor social outcome may reflect or promote poorer hippocampal neural plasticity in schizophrenia, with particular regional effects on the body of the hippocampal surface morphology localised predominantly on the right side, approximately including portions of the subiculum and of the cornu ammonis 1 subfields, although there was a scattered bilateral distributed impact. It should be considered that decreased environmental stimuli and chronic stress, which reduce the quality of life and the general health status of people with schizophrenia, are crucial factors that may contribute to affecting hippocampal plasticity. Reference Zhang, Zhang, Sun, Liu, Yang and Yao44,Reference Hu, Bergstrom, Brink, Ronnback and Dahlqvist45 It is interesting to note that no significant correlation was found between clinical measures and hippocampal size considering the raw total volumes obtained by region of interest tracing, Reference Baiano, Perlini, Rambaldelli, Cerini, Dusi and Bellani17 which we were able to observe using this 3D technique. This supports the strength of using advanced imaging and post-processing technique in analysing hippocampal morphology.
It is not yet fully understood whether hippocampal structural abnormalities partly reflect neurodegeneration in schizophrenia, but the hippocampus is one of the brain regions most vulnerable to neuronal loss. Diverse remodelling processes may change the morphology of hippocampus, such as retraction of dendrites, decreased neurogenesis and/or loss of glial cells, Reference Martinez-Tellez, Hernandez-Torres, Gamboa and Flores46 possibly in part due to altered glutamatergic neurotransmission. Also, oxidative DNA damage of the hippocampus has been found in elderly patients with schizophrenia and poor outcome. Reference Nishioka and Arnold47 Hippocampal neurodegeneration may thus be an index of poor social outcome in people with chronic schizophrenia, particularly in a subset with neurodegenerative features.
Limitations and future research
Some major limitations of this study should be kept in mind. First, this was a cross-sectional study. To further characterise hippocampal volume based on social outcome, patients with schizophrenia should be studied longitudinally, ideally including drug-free individuals at the first outbreak. Second, neuropsychological and genetic assessments should be performed to determine how cognition and genes influence the statistical relationship between hippocampal volume and social functioning. Poor social functioning may in part be sustained by impaired cognitive abilities such as performance memory, learning and abstraction which relate to the hippocampus. Therefore, it would be intriguing to see whether cognitive decline or particular genetic polymorphisms such as BDNF and NRG1 promote changes in hippocampal morphology and associate with social outcome. Also, from 1.5 T MRI it was not possible to reliably resolve details of the anatomical subfields of the hippocampus, so we cannot argue with certainty which specific subregion consistently correlates with different clinical or psychosocial measures. Future 3D studies should therefore obtain anatomical fine definition of hippocampal subregions, ultimately trying to delineate whether regional shrinkage specifically links, for instance, with symptoms or poor social functioning. Finally, the ventricle system was not reconstructed in this study, so we cannot completely exclude that potential enlargement of the temporal horns of the lateral ventricles might relate to distortions in surrounding anatomy, including the hippocampus. However, considering that no significant difference in hippocampal volume was found between the patient and control groups, we assume that possible effects of ventricular enlargement did not take place in this case.
In conclusion, hippocampal deflation may be a structural sign of poor clinical outcome and social functioning in schizophrenia, helping to identify a subgroup of patients who might need specific treatment. Classification of patients with hippocampal abnormalities and clinical and social impairments should, however, be based on specific criteria of illness severity and poor functioning, which might then be of clinical relevance. This may indeed assist in determining patients who would benefit from adequate therapeutic management such as psychosocial stimulation, cognitive rehabilitation or physical exercise. Such therapeutic strategies might potentially preserve or normalise hippocampal size, which could also be monitored to study the effects of clinical interventions, ultimately leading to better clinical and social outcomes.




eLetters
No eLetters have been published for this article.