Patients with bipolar disorder cycle through episodes of mania, depression and euthymia, demonstrating dramatic fluctuations in energy, social behaviour, mood and cognitive functioning. The apparent links between these associated changes suggest an important role for the study of this disorder in charting a map of the relationship between mood and cognition. To date, little is known about the nature of cognitive deficits observed in patients with bipolar disorder or about how these deficits might relate to the clinical symptoms and neurobiological substrates of the disorder. In contrast to the wealth of empirical data pertaining to neuropsychological impairment in individuals suffering from major depression, few researchers have investigated cognitive functioning in patients with manic-depressive illness.
In this review, we offer a preliminary exploration of the neuropsychology of bipolar disorder and some suggestions for its future. We begin by considering cognitive functioning in each of the three phases of this illness, thus establishing broad links between affect and cognition. We address the complex issue of general v. specific cognitive deficits in patients with bipolar disorder, focusing on the comparative study of mania, depression and schizophrenia to establish distinct neuropsychological profiles, and conclude with an examination of some of the most interesting developments in recent studies of bipolar disorder and the scope for future research in this area.
METHODOLOGICAL CONSIDERATIONS
Before discussing the nature of cognitive impairment in bipolar disorder, certain methodological issues should be addressed. Unavoidable practical considerations often interfere with ideal methodologies, clouding and weakening conclusions drawn from the results of clinical neuropsychological studies. First, researchers often neglect to indicate whether patients are in a manic, depressed or euthymic phase at the time of neuropsychological assessment. This is in part owing to difficulties with monitoring what are often rapid fluctuations in mood. Second, patients with bipolar disorder are generally receiving a combination of medications - including mood stabilisers, antidepressants, neuroleptics and benzodiazepines - that may or may not influence neuropsychological performance. Differences observed between patients and controls, or patients in different stages of bipolar illness, may be confounded by different medication regimens. Finally, in studies that compare cognitive profiles in mania and depression, differences in the patients' clinical characteristics (such as severity of illness) often make comparisons difficult.
Matching for clinical characteristics within or between patient groups presents a particularly complicated problem for research into this type of illness. Neuropsychological researchers generally attempt to minimise the effects of simpler confounds by controlling as many variables as possible; when choosing control subjects, for example, frequent attempts are made to match patients and controls for age and premorbid intelligence. Matching patients with mania and patients with depression for severity of illness, though, is more difficult, largely because assessment measures differ for each type of illness. The Young Mania Rating Scale and the Hamilton Rating Scale for Depression are often used in mania and depression, respectively, but do not allow comparison across disorders. While some investigators match patients for number of hospitalised episodes, or for some other related factor, the bases of these cross-sectional comparisons are dubious. One method of circumventing some of these problems would be to conduct longitudinal studies of patients with bipolar disorder as they enter different phases of their illness; however, as with between-subject designs, longitudinal within-subject designs cannot ensure that severity levels are equated during manic and depressed phases. In addition, potential benefits come at the price of heightened difficulty, with many researchers unable to manage the resources and lengthy time frame required by this research design.
These methodological problems, among others, make any investigation of disordered mood and cognition almost prohibitively complex, but some measures can be adopted to reduce ambiguities and confounds. For example, because knowing the stage of illness is crucial to an understanding of potential links between mood and cognitive function, this review considers only those studies that specify phase of illness. Although it is much more difficult to resolve questions posed by medication and matching for severity of illness, caution is essential, and in what follows we have attempted to be particularly sensitive to the credibility of results compromised by uncertain methodologies.
COGNITIVE FUNCTIONING IN THE AFFECTIVE DISORDERS
The first step in our reconsideration of mood and cognitive functioning is a review of the evidence relevant to neuropsychological functioning in the depressed, manic and euthymic phases of bipolar disorder. Distinguishing between unipolar and bipolar forms of depressive illness represents another contentious but essential problem in this area of research. It should be noted that the DSM-IV (American Psychiatric Association, 1994) no longer uses the terms ‘unipolar’ and ‘bipolar’ depression. Instead, the terms ‘major depressive disorder’ and ‘bipolar disorder’ are used. However, the former terms are used here for the purposes of clarity and consistency with past studies. We also consider whether differences exist between patients with major (unipolar) depressive disorder and patients in the depressed phase of bipolar illness. Finally, we address the extent to which cognitive impairment remains in patients with bipolar disorder who are euthymic at the time of neuropsychological assessment.
Cognitive impairment in depression
Until fairly recently it was thought that even severe forms of depression were associated with only minor impairments in cognitive function. An important and comprehensive review by Miller (Reference Miller1975) challenged this belief by suggesting that both mild and severe forms of depression are associated with pronounced deficits on cognitive, motor, perceptual and communication tasks. Since then, many studies have demonstrated the presence of wide-ranging neuropsychological deficits in patients with depression (Reference Weingartner, Cohen and MurphyWeingartner et al, 1981; Reference Brown, Scott and BenchBrown et al, 1994; Reference Beats, Sahakian and LevyBeats et al, 1996; Reference Elliott, Sahakian and McKayElliott et al, 1996), with current investigation focusing on the relationship of these now established deficits to clinical and neurobiological dimensions of the disorder.
Although patients with depression have been studied using a wide range of neuropsychological tests, researchers have focused on memory and executive function, as the neuroanatomical regions thought to subserve these cognitive domains are fairly well specified (see Reference ElliottElliott, 1998). Given that patients with depression frequently complain of memory difficulties, it is perhaps not surprising that these subjects demonstrate impairments on a range of memory tasks (see Reference BlaneyBlaney, 1986; Reference Johnson and MagaroJohnson & Magaro, 1987; Reference Burt, Zembar and NiedereheBurt et al, 1995, for reviews). Deficits have been reported on tests of short-term memory, verbal and visual recognition memory, spatial working memory and immediate or delayed recall (Reference Austin, Ross and MurrayAustin et al, 1992; Reference Brown, Scott and BenchBrown et al, 1994; Reference Ilsley, Moffoot and O'CanollIlsley et al, 1995; Reference Beats, Sahakian and LevyBeats et al, 1996; Reference Elliott, Sahakian and McKayElliott et al, 1996). As such a broad spectrum of findings may suggest, there has been much debate over the precise nature of memory impairment, and a number of distinct formulations have been offered to explain the observed deficits (see Reference Robbins, Joyce, Sahakian and PaykelRobbins et al, 1992, for discussion).
Executive abilities are also compromised in these patients, and it has been argued that of the neuropsychological tasks showing impairment, tests of executive function may be the most sensitive. These high-level tasks, of which the Wisconsin Card Sorting Test (WCST) (Reference Grant and BergGrant & Berg, 1948) and the Tower of London test of planning ability (Reference ShalliceShallice, 1982) are classic examples, require the coordination of cognitive processes for their successful completion, and are thought to depend on intact functioning of the prefrontal cortex. Indeed, patients with major depressive disorder have been shown to be impaired on both of these tests (Reference Martin, Oren and BooneMartin et al, 1991; Reference Franke, Maier and HardtFranke et al, 1993; Reference Elliott, Sahakian and McKayElliott et al, 1996), leading some researchers to postulate the importance of prefrontal dysfunction in the pathogenesis of clinical depression (e.g. Reference ElliottElliott, 1998).
Unipolar v. bipolar depression
Many studies are based on samples of patients with depression that includes both unipolar and bipolar disorders, presupposing the essential similarity of these conditions. Of the few studies that have directly compared the two, the general findings suggest that, at least on some neuropsychological tasks, deficits are more marked in bipolar than in unipolar depression. For example, Savard et al (Reference Savard, Rey and Post1980) administered the Halstead-Reitan Category Test to acutely depressed unipolar and bipolar groups of patients who were free of medication at the time of testing, and found that patients in the bipolar group made significantly more errors than either patients in the unipolar group or control subjects. On tests of learning and verbal fluency, Wolfe et al (Reference Wolfe, Granholm and Butters1987) similarly found more marked impairments in patients with bipolar disorder than in patients with unipolar depression matched for age and education. It should be noted that the conclusions drawn from both of these studies may be compromised by the presence of confounding variables. For example, patients in the bipolar group of Savard et al (Reference Savard, Rey and Post1980) were significantly older than those in the unipolar group, suggesting that age alone may have accounted for their findings. Additionally, Wolfe et al (Reference Wolfe, Granholm and Butters1987) cautioned that differences between their unipolar and bipolar groups might actually reflect subtle differences in severity: the rate of hospitalisation in bipolar patients was twice that noted in the unipolar patients.
Cognitive impairment in mania
In contrast to the large amount of work devoted to the cognitive changes accompanying depression, only a few studies have addressed the precise nature of impairment in patients with mania. A possible explanation for this imbalance may be the practical difficulties of using standard neuropsychological procedures to assess mania; the nature of the illness may prevent patients with mania from being reliable subjects, especially in tests of cognitive functioning. Nevertheless, it has long been recognised that mania is associated with changes in cognition as well as in affect (Reference KraepelinKraepelin, 1921; Reference Bunney and HartmannBunney & Hartmann, 1965), and more recent empirical studies confirm this view.
Patients with mania have been studied using tasks that sample aspects of learning and memory, visuospatial ability and executive function. In a study conducted by Taylor & Abrams (Reference Taylor and Abrams1986), tests of attention, visuospatial function and memory were administered to patients with mania, approximately half of whom exhibited moderate or severe global cognitive impairment. With respect to memory processes, Bunney & Hartmann (Reference Bunney and Hartmann1965) noted memory loss during manic states in a patient with regular manic-depressive cycles every 48 hours. Furthermore, Henry et al (Reference Henry, Weingartner and Murphy1971) reported impaired serial word list learning during mania, with decrements in performance directly related to increasing severity of illness. More recent findings suggest that patients with bipolar disorder in the manic phase of their illness are impaired on tests of pattern and spatial recognition memory and delayed visual recognition (Reference Murphy, Sahakian and RubinszteinMurphy et al, 1999). In an attempt to explain observed memory deficits, Henry et al (Reference Henry, Weingartner and Murphy1971) proposed that memory impairment may at least sometimes be owing to altered patterns of verbal association. Andreasen & Powers (Reference Andreasen and Powers1974) reached a similar conclusion with their finding that, relative to control subjects, the memory structures of patients with mania were loose, overinclusive and idiosyncratic, leading to difficulties in filtering environmental stimuli and a tendency to overgeneralise.
The notion that mania is associated with some form of ‘dysexecutive syndrome’ also seems reasonable, since patients typically exhibit disrupted social behaviour and decision-making reminiscent of that observed in patients with lesions to frontal regions of the cortex (Reference Bechara, Damasio and DamasioBechara et al, 1994). It is thus surprising that so little research assesses executive functioning in these patients. To date, this type of functioning has been studied using tests of attentional set-shifting (Reference MoriceMorice, 1990; Reference Clark, Iversen and GoodwinClark et al, 2000), planning ability (Reference Murphy, Sahakian and RubinszteinMurphy et al, 1999) and decision-making (Reference Clark, Iversen and GoodwinClark et al, 2000; Reference Murphy, Rubinsztein and MichaelMurphy et al, 2001). Although impairments have been observed across the full range of tasks, it is not yet clear to what extent these deficits stand over and above those observed in other non-executive domains.
Residual neuropsychological impairments in euthymia
Kraepelin (Reference Kraepelin1921) distinguished manic depression from schizophrenia on the basis of its relapsing and remitting course. Patients with affective illness, unlike those with dementia praecox, were thought to experience remission without cognitive impairment. Recent investigations of patients in the euthymic phase of bipolar disorder, however, have challenged this view. Many patients continue to experience psychological and social difficulties, and while the extent to which neuropsychological impairment remains is less clear, most studies report at least some degree of residual cognitive dysfunction in one or more tasks administered.
Asarnow & MacCrimmon (Reference Asarnow and MacCrimmon1981) used a test of attention and visual information processing to compare the performance of out-patients with manic depression or schizophrenia — both groups judged by their attending psychiatrists to be free from major symptoms — with that of healthy controls. Performance of the manic depression group was midway between that of the schizophrenia and control groups, suggesting that people with bipolar disorder demonstrate cognitive impairments that are probably not entirely due to residual psychotic symptoms. Similarly, Tham et al (Reference Tham, Engelbrektson and Mathe1997) administered an extensive range of neuropsychological tasks to patients with recurrent mood disorder (10 unipolar and 16 bipolar) who were euthymic at the time of neuropsychological assessment. Cognitive functioning was markedly impaired in a substantial number of these patients. More recently, Ferrier et al (Reference Ferrier, Stanton and Kelly1999) reported residual impairment of executive function in people with euthymic bipolar disorder after controlling for age, premorbid intelligence and depressive symptomatology. Rubinsztein et al (Reference Rubinsztein, Michael and Paykel2000) found asymptomatic patients with bipolar disorder (in remission for at least 4 months) to show deficits on tests of visuospatial recognition memory; response latency, but not accuracy, on four distinct tests of executive function, was also impaired. Other investigators have reported evidence of residual impairment as well (Reference Jones, Duncan and MirskyJones et al, 1994; Reference McKay, Tarbuck and ShapleskeMcKay et al, 1995; Reference KessingKessing, 1998 — but see Reference Kerry, McDermott and OrmeKerry et al, 1983).
While the jury is still out on the precise neuropsychological profile found in euthymic bipolar disorder, the balance of evidence from such studies supports a hypothesis of residual cognitive impairment. It is important to note that the bulk of these studies employ cross-sectional, between-subject designs that compare euthymic patients with bipolar disorder with healthy controls. As mentioned above, longitudinal, within-subject designs are more effective in assessing how cognitive performance changes with symptomatic recovery. Clearly, both types of study are necessary if we are to address whether performance of euthymic patients with bipolar disorder is inferior to that of healthy controls, and to demonstrate deterioration or improvement of cognitive functioning within a single subject group. One final note of caution is that some studies do not measure manic or depressive symptomatology during the euthymic phase under study (see Reference Rubinsztein, Michael and PaykelRubinsztein et al, 2000, for a notable exception). It is therefore possible that subclinical psychopathology may at least partially account for the residual deficits observed.
Thus, while recent experiments have established the range and depth of cognitive impairments associated with depression, mania is clearly suffering from a lack of attention. Preliminary results suggest wide-ranging deficits in patients with mania; but a comprehensive investigation of cognitive functioning across a full spectrum of tasks should still be undertaken. Comparisons of unipolar and bipolar forms of depression have revealed interesting findings; they suggest that studies presupposing the essential similarity of unipolar illness with bipolar illness may be too simplistic. Likewise, the presumption that (bipolar) mania and unipolar depression represent opposite emotional pales in a cognitive—affective continuum may also be an over-simplified model. It is also possible that the cognitive deficits observed in bipolar disorder (depressed phase) could stem from a source unrelated to that of similar impairments in unipolar depression, and that the relationship of affect to all these impairments might be more complicated.
GENERAL V. SPECIFIC DEFICITS: DISTINGUISHING MANIA FROM SCHIZOPHRENIA AND DEPRESSION
Some studies have adopted a comparative strategy for characterising possible cognitive deficits associated with mania. These studies compare mania with other neuropsychiatric disorders, such as schizophrenia and depression, to determine whether mania is associated with qualitatively different forms of cognitive impairment from those found in seemingly related illnesses. This method of establishing a specific psychological profile for mania could prove very fruitful for the more general investigation of mood and cognition, as it compares the cognitive performance of patients with mania with that of those with depression, and tests for deficits that might be identical in both illnesses. These studies determine whether the impairments observed in mania can be explained by factors specific to the manic state or whether they are, alternatively, owing to global pathology and more general problems such as psychosis or disordered thought.
Comparing mania and schizophrenia
Several studies have compared performance in mania and schizophrenia (Reference Andreasen and PowersAndreasen & Powers, 1974; Reference OltmannsOltmanns, 1978; Reference Strauss, Bohannon and StephensStrauss et al, 1984; Reference MoriceMorice, 1990; Reference Goldberg, Gold and GreenbergGoldberg et al, 1993). Findings from these studies indicate that on tests of selective attention (Reference OltmannsOltmanns, 1978), perceptual span (Reference Strauss, Bohannon and StephensStrauss et al, 1984) and shifting attentional set (measures by the WCST (Reference MoriceMorice, 1990), the deficits in patients with mania are indistinguishable from those in patients with schizophrenia. Oltmanns (Reference Oltmanns1978) found that although both sets of patients were more distractable than normal controls, they did not differ from each other. Other investigators have also demonstrated the non-specific nature of mania-related deficits. Otteson & Holzman (Reference Otteson and Holzman1976) studied patients with schizophrenia, patients with psychosis but without schizophrenia and non-psychotic patients and compared them to one another and to healthy controls on a variety of cognitive measures. While group differences emerged between psychiatric patients and control subjects, and also between patients with and without psychosis, there were no differences between the schizophrenia and mania groups. Any group differences appeared to be related to degree, rather than type, of disorganisation.
In contrast to the above, differences between patients with mania and schizophrenia have also been reported. For example, Andreasen & Powers (Reference Andreasen and Powers1974) found overinclusive thinking to be more prominent in mania than in schizophrenia. Similarly, Goldberg et al (Reference Goldberg, Gold and Greenberg1993) reported that patients with schizophrenia consistently performed at lower levels than those with affective disorder (unipolar depression, bipolar depression and bipolar mania) on tests of psychomotor speed, attention, memory and attentional set-shifting. It is perhaps noteworthy that generalised intellectual deterioration was more marked in schizophrenia than in the affective disorders, and when intelligence was controlled for, group differences emerged only on a test of memory and the WCST. Thus, the balance of evidence suggests marked similarities between the neuropsychological profiles in mania and schizophrenia.
Comparing mania and depression
Similar findings have been reported from work on comparative cognitive performance in mania and depression. Bulbena & Berrios (Reference Bulbena and Berrios1993) assessed performance of patients during acute episodes of major depression and mania using tests of attention, memory, visuospatial function and choice reaction time. Relative to controls, patients were impaired on most cognitive measures, but no differences between mania and depression were found. Moreover, Goldberg et al (Reference Goldberg, Gold and Greenberg1993) found that in bipolar disorder, patients in manic and depressed episodes did not differ on the Wechsler Adult Intelligence Scale — Revised (WAIS—R), WCST, or on neuropsychological tests of reading, line orientation and facial recognition.
While direct statistical comparison between patients with mania and depression is clearly the best approach in searching for distinct neuropsychological profiles, indirect comparison between patient groups who have been assessed using standardised neuropsychological tasks can also be informative. In a study by Murphy et al (Reference Murphy, Sahakian and Rubinsztein1999), patients in the manic phase of bipolar illness were given tests of memory and executive function taken from the Cambridge Neuropsychological Test Automated Battery (CANTAB, CeNes Plc, Cambridge, UK). These tests are reliable and valid (Robbins et al, Reference Robbins, James and Owen1994, Reference Robbins, James and Owen1998), and had been previously administered as part of a much larger test battery to a sample of patients with major depressive disorder (Reference Elliott, Sahakian and McKayElliott et al, 1996). Patients with mania demonstrated substantial impairments on tests of pattern and spatial recognition memory, and delayed visual recognition. This pattern of impairment was strikingly similar to that previously observed in patients with depression (Table 1). Executive function, as assessed by the computerised one-touch Tower of London test of planning ability, was also similarly impaired in the two patient groups (Fig. 1).
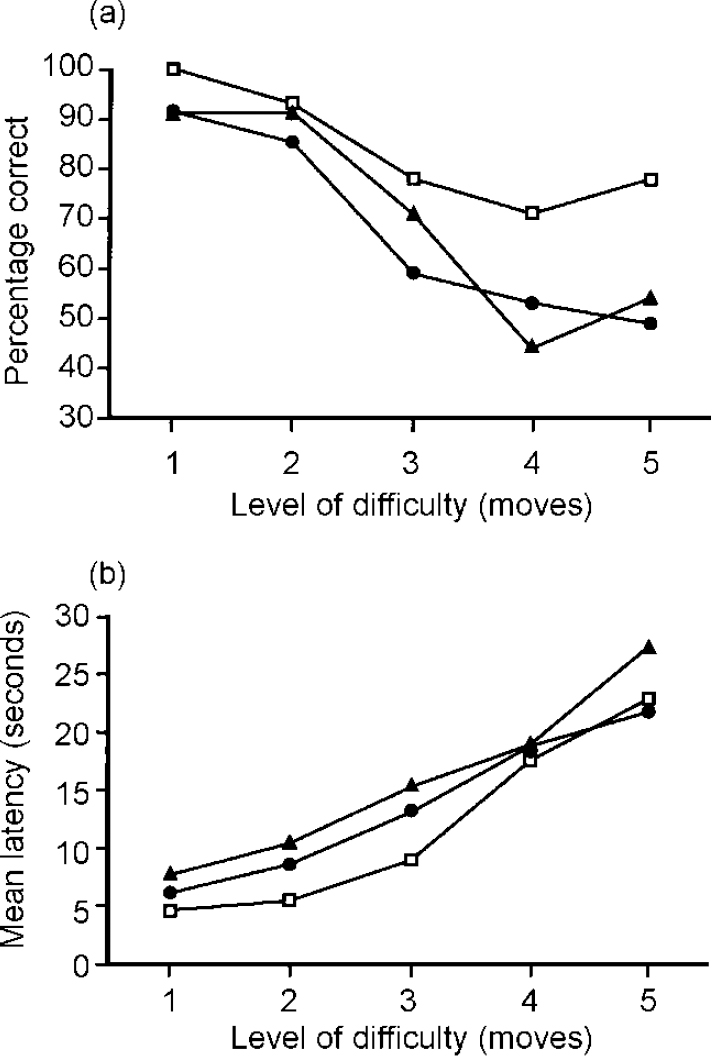
Fig. 1 Performance of patients with mania (triangles), depression (circles) and control subjects (squares) as a function of difficulty level on the one-touch Tower of London task. The dependent measures shown are (a) mean percentage of problems solved correctly by first response and (b) mean latency to first response. Data for patients with mania and depression are taken from Murphy et al (Reference Murphy, Sahakian and Rubinsztein1999) and Elliott et al (Reference Elliott, Sahakian and McKay1996), respectively.
Table 1 Neuropsychological performance of patients with major depression and bipolar disorder (manic phase) on memory tests taken from the Cambridge Neuropsychological Test Automated Battery (CANTAB)
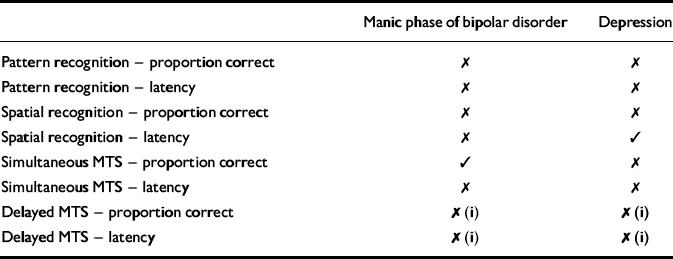
| Manic phase of bipolar disorder | Depression | |
|---|---|---|
| Pattern recognition — proportion correct | ✗ | ✗ |
| Pattern recognition — latency | ✗ | ✗ |
| Spatial recognition — proportion correct | ✗ | ✗ |
| Spatial recognition — latency | ✗ | ✓ |
| Simultaneous MTS — proportion correct | ✓ | ✗ |
| Simultaneous MTS — latency | ✗ | ✗ |
| Delayed MTS — proportion correct | ✗ (i) | ✗ (i) |
| Delayed MTS — latency | ✗ (i) | ✗ (i) |
The cognitive impairments observed in both groups of patients in these studies were interpreted as evidence for relatively global neuropsychological dysfunction (Reference Elliott, Sahakian and McKayElliott et al, 1996; Reference Murphy, Sahakian and RubinszteinMurphy et al, 1999). The deficits observed in patients with mania and depression when tested on object recognition memory were comparable to those previously reported in patients with posterior dysfunction, such as temporal lobe lesions (Reference Owen, Sahakian and SempleOwen et al, 1995a ) or mild Alzheimer's dementia (Reference Sahakian, Morris and EvendenSahakian et al, 1988). The deficits seen on tests of spatial recognition memory and planning ability, however, were similar to those in patients with frontal dysfunction (Reference Owen, Sahakian and HodgesOwen et al, 1995b ) or basal ganglia disorders such as Parkinson's disease (Reference Owen, Sahakian and HodgesOwen et al, 1995b ), in which there is disrupted functioning of frontostriatal ‘loops’ (Reference Alexander, DeLong and StrickAlexander et al, 1986). At first glance, these findings suggest that patients with mania and depression are similarly impaired on a range of cognitive tasks subserved by different neural regions, and that a single common underlying mechanism may account for the noted deficits in both groups. Investigators of depression have suggested that the pervasive deficits observed could be due to reduced motivation (Reference MillerMiller, 1975; Reference SeligmanSeligman, 1975; Reference Richards and RuffRichards & Ruff, 1989), a conservative response style (Reference Johnson and MagaroJohnson & Magaro, 1987; Reference Williams, Watts and MacLeodWilliams et al, 1997), diminished cognitive capacity and processing resources (Reference Hasher and ZacksHasher & Zacks, 1979), or a narrowing of attentional focus to depression-relevant or task-irrelevant thoughts (Reference Ellis, Ashbrook, Fiedler and ForgasEllis & Ashbrook, 1988). To date, few investigators have considered mania-related deficits within these or similar frameworks.
The bulk of research suggests that in both mania and depression, patients are impaired on a range of cognitive tasks subserved by different neural regions. In addition, although the few studies that actually compare mania and depression employ a limited range of tasks, it appears that conventional neuropsychological tests of attention, memory and executive function are unable to discriminate between patients with mania and depression. Together, these findings suggest that global pathological change, rather than factors unique to either disorder, may account for the observed deficits, and that similar processes may be involved despite markedly different clinical presentations.
New approaches to distinct profiles: biases in information processing
So far, this review has focused on the performance of cognitive and neuropsychological tasks employing neutral materials — those that are not emotionally relevant to the patient's condition, i.e. materials not seemingly positive or negative in affective or emotional tone. This exclusion of affective material effectively removes mood from the experimental dynamic; in order to assess the possible relationship between mood and cognition in the affective disorders, we must consider studies incorporating affective material in the experimental design. In patients with depression, empirical studies of mood-congruent biases in information processing are abundant, with biases reported in evaluative processes, social judgements, decision-making, attention and memory (Reference Clark and TeasdaleClark & Teasdale, 1982; Reference BlaneyBlaney, 1986; Reference Gotlib and CaneGotlib & Cane, 1987; Reference Mogg, Bradley and WilliamsMogg et al, 1995; Reference Bradley, Mogg and MillarBradley et al, 1996). One of the earliest studies examined the recall of past experiences in patients who were clinically depressed and healthy control participants (Reference Lloyd and LishmanLloyd & Lishman, 1975). The results indicated that when patients with depression were required to recall pleasant or unpleasant experiences from their past in response to various cue words (e.g. ‘house’, ‘table’), patients recalled unpleasant memories more quickly than pleasant ones as the severity of depression increased.
In light of these findings, it seemed reasonable to suppose that if differences in cognitive functioning in mania and depression do indeed exist, they will emerge on tasks involving the interaction between cognitive and affective (or emotional) processing. We attempted to address this hypothesis by administering a novel ‘affective go/no-go’ task to patients with mania and depression, and to healthy controls matched for age and premorbid intelligence (Reference Murphy, Sahakian and RubinszteinMurphy et al, 1999). This task required both attentional and affective processes for its successful completion. Specifically, subjects were required to respond to target words of either positive or negative affective tone by tapping the space bar of a computer keyboard as quickly as possible, and to inhibit this response to words of the competing affective category. As shown in Fig. 2, both groups of patients exhibited attention and response biases — in mania towards the positive stimuli and in depression towards the negative stimuli. In addition, patients with mania — but not those with depression — were impaired in their ability to inhibit behavioural responses and focus attention. These findings were particularly interesting against a background of similar impairments on conventional neuropsychological tests of memory and executive function (see above).
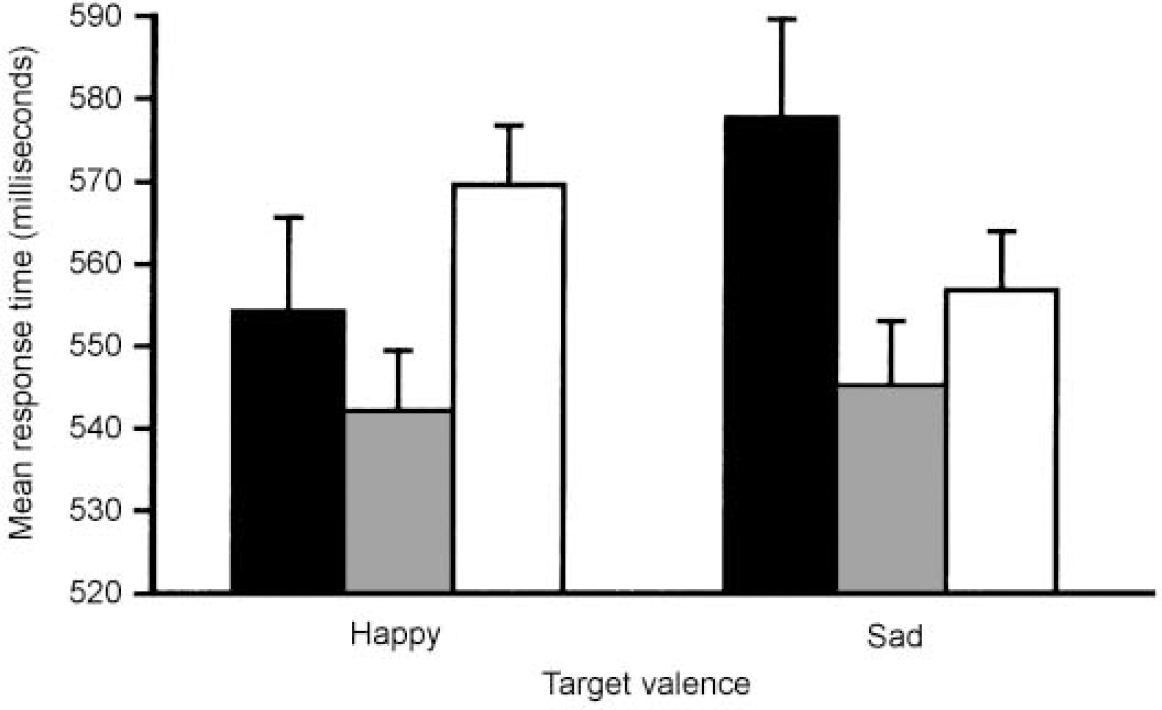
Fig. 2 Mean response times for ‘happy’ and ‘sad’ targets in the affective go/no-go task for patients with mania (black bars), depression (white bars) and control subjects (shaded bars). Bars represent one standard error of the mean (s.e.m.). Data taken from Murphy et al (Reference Murphy, Sahakian and Rubinsztein1999).
Neuroimaging studies of the neural regions that underlie cognitive processing of affective meaning suggest that medial and orbitofrontal prefrontal cortex (PFC) are particularly involved (Reference Beauregard, Chertkow and BubBeauregard et al, 1997; Reference Teasdale, Howard and CoxTeasdale et al, 1999). In line with these findings, Murphy et al (Reference Murphy, Sahakian and Rubinsztein1999) concluded that performances in mania and depression were most likely to differ on cognitive tasks subserved by functioning of the orbital/ventromedial regions of PFC. Indeed, Drevets et al (Reference Drevets, Price and Simpson1997) found that the subgenual PFC, which lies in the ventromedial PFC, is differentially activated during periods of mania and depression. The disinhibited response often observed in mania, but not in depression, provides further evidence for differential performance on tasks requiring ventromedial prefrontal functioning, as patients with medial or ventral prefrontal damage are similarly impaired on ‘go/no-go’ tasks (Reference DreweDrewe, 1975; Reference Malloy, Bihrle and DuffyMalloy et al, 1993).
At first glance it might seem puzzling that patients with mania and depression in the study by Murphy et al were differently impaired on the ‘affective go/no-go’ task but not on the Tower of London test of planning, tasks both thought to be subserved by PFC. This apparent inconsistency may be explained by the functional and anatomical distinctions between the dorsolateral and orbital/ventromedial regions of PFC that have been postulated in recent years. It is now known that tasks such as the WCST and the Tower of London test activate a neural network that includes important areas such as dorsolateral regions of PFC (Reference Berman, Randolph and GoldBerman et al, 1986; Reference Baker, Rogers and OwenBaker et al, 1996). These regions have numerous connections with cortical systems involved in information processing. In contrast, tasks that assess ability to make decisions and reverse associations between stimulus and reward are thought to be subserved by ventromedial regions (Reference Rahman, Sahakian and HodgesRahman et al, 1999; Reference Rogers, Everitt and BaldacchinoRogers et al, 1999), which are more extensively connected with limbic structures (Reference Pandya, Yeterian, Damasio, Damasio and ChristenPandya & Yeterian, 1996). As a result, it is possible that this inconsistency is related to the different neural pathways subserving cognitive function in these two tasks.
To the best of our knowledge, no other studies have compared information processing biases in mania and depression. The mood-congruent bias observed in depression is consistent with many depression studies demonstrating biases of memory and attention (see above), but this may be the first demonstration of a positive attentional bias in mania. In this context, it is worth noting that a recent study demonstrated a bias for processing negative information in bipolar mania (Reference Lyon, Startup and BentallLyon et al, 1999). While such results may seem directly contradictory to the findings reported above, the authors suggested that negative bias may be limited to implicit tests of affective orientation; the ‘go/no-go’ task used by Murphy et al and described here surely taps affective bias more explicitly.
Abnormal response to performance feedback
Another concept related to cognitive processing of emotional material and to mood-congruent bias is that of reinforcement or reward. It has been argued that the manifold signs and symptoms of manic depression may be viewed in terms of dysregulation of three major neurobiological systems: those that involve reinforcement—reward functions, central pain mechanisms and psychomotor activity (Reference CarrollCarroll, 1994). Although research has yet to demonstrate a disturbance of reinforcement—reward systems in bipolar disorder, a series of related studies has suggested that such systems may be disrupted in patients with major depression (Reference Beats, Sahakian and LevyBeats et al, 1996; Elliott et al, Reference Elliott, Sahakian and McKay1996, Reference Elliott, Sahakian and Herrod1997a ). Sahakian and colleagues have suggested that an abnormal response to negative feedback may contribute to the poor performance often observed in individuals with depression. Specifically, Elliott et al (Reference Elliott, Sahakian and McKay1996) found that on two CANTAB computerised neuropsychological tasks, which tap different cognitive functions and involve different neural substrates, failure on one problem appeared to elevate the probability of failure on the immediately subsequent problem, suggesting that negative feedback may have a detrimental effect on subsequent performance. This effect was specific to patients with depression and was not observed in any of the other clinical groups examined, i.e. those with Parkinson's disease, schizophrenia or neurosurgical legions of the frontal or temporal lobes (Reference Elliott, Sahakian and HerrodElliott et al, 1997a ). The investigators suggested that this effect may represent an important link between negative affect and the cognitive impairments associated with depression. Whether this type of effect is specific to depression or extends to patients who are manic at the time of testing, however, remains to be determined. In this regard, it is worth mentioning that in a study investigating the neural response to performance feedback, the presence of feedback increased blood flow in the ventromedial/orbitofrontal cortex for a guessing but not for a planning task (Elliott et al, Reference Elliott, Frith and Dolan1997b , Reference Elliott, Sahakian and Michael1998).
Also relevant is a study by Corwin et al (Reference Corwin, Peselow and Feenan1990) that investigated response bias (i.e. the decision rule subjects adopt when uncertain) on a task of recognition memory in patients with unipolar depression, bipolar mania and controls. An abnormally conservative response bias was associated with depression — whereas a liberal response bias was associated with mania, regardless of severity of illness. Consequently it seems that cognitive performance in depression and mania may be influenced by different emotional or affective responses to task stimuli.
CONCLUSION
In this review we have considered research on the neuropsychology of bipolar disorder with special attention to the relationship between mood and cognitive functioning. Unlike the more advanced research focusing on major (unipolar) depression, work to date on bipolar disorder has not achieved a satisfactorily comprehensive assessment of cognitive functioning. Patients suffering from depression have been shown to be cognitively impaired on a wide range of tasks, and euthymic patients have demonstrated residual impairments on some tests of attention and visual information processing. Although studies of mania indicate a wide range of possible cognitive deficits, the comprehensive review of cognition suggested by these findings has not yet been undertaken. At the same time, comparative studies of unipolar depression have brought the essential similarity of these conditions into some doubt, with complicating consequences for a perhaps oversimple understanding of the relationship between mood and cognition in affective disorders. In particular, comparative studies have sought to establish distinct neuropsychological profiles for mania, depression and schizophrenia as a way of determining whether general or specific deficits obtain in the affective disorders.
The establishment of such distinct profiles is crucial to our understanding of the neuropsychology of the affective disorders. Until recently, most comparative studies noted striking similarities between schizophrenia, mania and depression. However, these studies employed affectively neutral designs, eliminating emotional processing from the experimental dynamic and thus compromising their usefulness in the investigation of mood and cognition. More recent studies, based on the model of earlier investigations of mood-congruent bias in depression, have attempted to differentiate mania and depression by employing tasks with affective components. These studies have noted biases in informational processing and abnormal responses to feedback that appear to be consistent with other data obtained from neuroimaging work on mania and depression.
Historically, studies of mood disorders have made virtually no reference to basic research on emotion in healthy volunteers, and conventional neuropsychological testing has shied away from emphasising emotional components of cognition. A neuropsychological approach that incorporates both elements in experimental designs requiring both cognitive and emotional processing could go a long way towards a better characterisation of the deficits so far observed in depression and in mania (see, for example, Reference Murphy, Sahakian and RubinszteinMurphy et al, 1999). Such an integrated approach could benefit greatly by incorporating ideas from emotion theories that emphasise cognition—emotion interactions (e.g. Reference Barnard and TeasdaleBarnard & Teasdale, 1991; Reference Teasdale and BarnardTeasdale & Barnard, 1993; Reference Williams and RubinWilliams, 1996) and from recent advances in our understanding of the brain mechanisms that underlie emotion (e.g. Reference DamasioDamasio, 1994; Reference LeDoux and GazzanigaLeDoux, 1995). Studies focusing on the neural networks involved in such emotional processes in the neuropsychiatric affective disorders of depression and mania may provide the key to resolving these important issues.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ A comprehensive understanding of the manic, depressed and euthymic phases of bipolar disorder and their associated neuropsychological changes is important for the successful management and possible remedy of this debilitating disorder.
-
▪ Given that patients with bipolar disorder often exhibit disrupted social behaviour and sub-optimal decision-making, the study of impaired executive function could have important implications for rehabilitation.
-
▪ Congruent with their current moods, patients with mania and depression demonstrate information processing biases for positive and negative stimuli, respectively. The affective ‘go/no-go’ paradigm used to investigate these biases may be useful in monitoring fluctuations in mood and the efficacy of pharmacological or other treatments.
LIMITATIONS
-
▪ Whether different subtypes of depressive disorder (for example, unipolar and bipolar forms of depression) are associated with distinct neuropsychological profiles remains a matter of debate.
-
▪ Although preliminary results in patients with mania suggest wide-ranging neuropsychological deficits, a comprehensive investigation of cognitive functioning across a full spectrum of tasks has yet to be undertaken.
-
▪ Unavoidable methodological problems often weaken the conclusions drawn from neuropsychological studies of bipolar disorder, with group differences confounded by differences in medication regimen, severity of illness and other general illness factors.
ACKNOWLEDGEMENTS
This research was funded by a Programme Grant from the Wellcome Trust to Dr B. J. Sahakian, Professor T. W. Robbins, Professor B. J. Everitt and Dr A. C. Roberts, and was completed within the Medical Research Council Co-operative Group in Brain, Behaviour and Neuropsychiatry. Dr. F. C. Murphy is supported by the Natural Sciences and Engineering Research Council of Canada. We also thank the Searle Memorial Trust and the Charles and Elsie Sykes Trust.






eLetters
No eLetters have been published for this article.