Dementia is relatively uncommon before the age of 65, but the social consequences for such patients and their families are usually very serious (Reference Delany and RosenvingeDelany & Rosenvinge, 1995; Reference Newens, Forster and KayNewens et al, 1995). Alzheimer's disease of pre-senile onset (PDAT) has been reported to run a more rapid downhill course than later-onset disease (Reference Barclay, Zemcov and BlassBarclay et al, 1985), but this was not confirmed (Reference Newens, Forster and KayNewens et al, 1993). Here we report survival over a longer period than Newens et al (Reference Newens, Forster and Kay1993), in an enlarged cohort, confined to incident cases of PDAT or pre-senile vascular dementia (PVD) identified in the former Northern health region from 1985 to 1989 and followed to the end of December 1998. We compare observed with expected survival numbers, report the relation of death certification to clinical algorithms, note changes over time in place of death, and discuss the validity of diagnosis and the incidence rate of PDAT.
METHOD
Incident cases of pre-senile dementia were identified from ICD-9 codes (World Health Organization, 1977) by examining the Hospital Activity Analysis, Mental Health Enquiry or Körner information systems from general and psychiatric hospitals in the former Northern health region (less South Cumbria) during the 5 years 1985 to 1989 (mean population at risk aged 45-64, 652 000). In addition, enquiries about cases of dementia were addressed to all day hospitals, social services departments, community psychiatric nurses, private nursing homes and clinical psychologists, and the records of the two neuro-radiology centres serving the region during the period were searched for suspected cases of dementia using DSM-III-R guidelines, as described in detail by Newens et al (Reference Newens, Forster and Kay1993). All cases, therefore, had contact with specialist National Health Service (NHS) hospital-based services. A diagnostic algorithm was applied to the in-patient or out-patient case notes. This algorithm first separated out cases of dementia which had been diagnosed by a hospital consultant as secondary to a specific clinical diagnosis other than PDAT or PVD: for example, neurological conditions such as hydrocephalus or multiple sclerosis, and those associated with alcohol misuse (n=18). The remaining 233 cases either met National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria for the clinical diagnosis of Alzheimer's disease (Reference McKhann, Drachman and FolsteinMcKhann et al, 1984) or showed one of the following: sudden onset, classical stepwise progression, specific focal abnormality on computed tomography (CT) scan, or Hachinski Ischaemic Score (Reference Hachinski, Iliff and ZilkhaHachinski et al, 1975) of 7 or above, suggestive of a diagnosis of PVD. If a patient was readmitted and the diagnosis then changed to a non-dementia disorder, the case was excluded. For calculation of incidence, an estimate was made of the number of patients whose records were missing, who probably fulfilled the criteria, as described by Newens et al (Reference Newens, Forster and Kay1993), but since they could not be identified by name they were excluded from the survival analysis. The study was approved by ethical committees throughout the Northern region.
The diagnosis of PDAT was further validated by interview, in 1990-92, of 74 (41%) of the 179 algorithm-defined PDAT patients as part of a case-control study of PDAT using the Mini-Mental State Examination (MMSE) (Reference Folstein, Folstein and McHughFolstein et al, 1975) and the History and Aetiology Schedule (HAS) (Reference Copeland and DeweyCopeland & Dewey, 1991). Finally, 80% of the main carers of patients in this subgroup were followed up by post in 1995, when all the surviving patients were reported to have considerable impairment in Activities in Daily Living (ADL) (e.g. all required help in dress or bathing and fewer than 10% could feed themselves unaided). In PVD, cases lacking an ICD-9 coding for dementia would have been missed and its incidence rate was not calculated. Deaths were ascertained through the National Health Service Central Registry (NHSCR), which supplied a copy of the death draft entry, giving the certified causes of death. When the entry gave an ICD code, this was entered into our database; when it did not, we supplied the appropriate code. We identified 233 incident cases of pre-senile dementia, of whom six (2.3%) could not be found by the NHSCR and were excluded from the survival analysis, which was based on 227 cases, including the 85 cases of PDAT described by Newens et al (Reference Newens, Forster and Kay1993) and 142 new cases of pre-senile dementia.
Statistical methods
Non-parametric statistics were used to compare the markedly skewed age distributions, and the frequencies of nominal variables. The relationship between diagnoses made from case notes and from algorithms was examined by McNemar's test. The t-test for independent samples was used to compare the means of continuous variables with approximately normal distributions. Survival analysis was performed using the SPSS/PC version 5.0.1 program; subgroups were compared with the Wilcoxon (Gehan) test. The expected length of survival of persons of age and gender equivalent to those of the whole cohort was estimated using English life tables for 1990-92 (Office of Population Censuses and Surveys, 1997). A longevity quotient, equal to the proportion of expected lifetime survived, was calculated by dividing the observed by the expected survival and multiplying by 100. The independent contributions of age, gender, Hachinski score and clinical diagnosis of PDAT or PVD to the prediction of death or survival and length of survival were further examined by logistic or multiple regression.
RESULTS
Clinical diagnosis
The clinical algorithm defined 179 cases as PDAT and 48 as PVD; 89.9% had had CT scans (PDAT 83.8%, PVD 93.8%). There were 117 (51.5%) men, 84 (47%) with PDAT and 33 (69%) with PVD; the gender difference (relatively more women with PDAT and more men with PVD) was significant (χ2 with continuity correction P=0.012). The number of cases entering the study in the five 5-year age groups 40-44, 45-49, 50-54, 55-59, 60-64 were 0, 3, 18, 72 and 134, respectively; the median age was 60.2 years, range 45-64. The age distributions in PDAT and PVD were similar. The case note diagnoses were made by neurologists for 60%, psychiatrists for 26% and physicians for 13% of cases (unknown for 1%). When the case note diagnoses were compared with the algorithmic (NINCDS) diagnoses, we found 94.3% agreement for Alzheimer's disease and 75.6% for vascular dementia; most of the case note diagnoses of unspecified dementia were probable Alzheimer's disease, according to the algorithm (Table 1). After combining unspecified dementia and Alzheimer's disease, the difference between case note and algorithmic diagnoses was not significant (McNemar's test, P=0.69). The κ coefficient of agreement was 0.66.
Table 1 Comparison of case note diagnosis with algorithm diagnosis

| Case note diagnosis | Clinical algorithm diagnosis n (%) | ||
|---|---|---|---|
| Probable Alzheimer's dementia | Vascular dementia | Total | |
| Alzheimer's disease | 132 (94.3) | 8 (5.7) | 140 (100.0) |
| Dementia unspecified | 36 (85.7) | 6 (14.3) | 42 (100.0) |
| Vascular dementia | 11 (24.4) | 34 (75.6) | 45 (100.0) |
| Total | 179 (78.9) | 48 (21.1) | 227 (100.0) |
Incidence
The mean annual incidence of PDAT, allowing for missing case notes, was 6.2 per 100 000 of population aged 45-64. The rate for 1989 of 4.3 was significantly lower than that for 1985-1988 (mean 6.7 per 100 000).
Duration before diagnosis
The median age of onset of symptoms was 59.2 years (range 44-64). The duration of symptoms of the illness before diagnosis was 3-6 months in 18%, 6-12 months in 37%, 1-2 years in 25%, and over 2 years in 20%. It was significantly longer (χ2=13.08, d.f.=4, P=0.011) in women than in men, and significantly longer (χ2=27.02, d.f.=4, P<0.0001) in PDAT than PVD, for which a duration of over 1 year was unusual. It was not significantly related to the period survived or to dead/alive status at the end of observation.
Certificated causes of death
Of the 192 death certificates, 139 specifically mentioned dementia; two other cases, one with Binswanger's encephalopathy and hypertension, and one with cerebral atrophy (coroner's post-mortem), in whom the presence of dementia seemed implicit, were classified with vascular dementia and unspecified dementia, respectively, making 141 cases with dementia (73.4%). Table 2 shows that Alzheimer's disease was specifically mentioned in 61 cases (31.8%) and vascular dementia in 12 (6.3%), of which 11 were specified as multi-infarct dementia. Dementia not specified as Alzheimer or vascular was entered in 52 cases (27.1%) (9 senile, 25 pre-senile, 17 unspecified dementia, 1 cerebral atrophy). In 14 cases (7.3%) both Alzheimer's disease or unspecified dementia and cerebrovascular disease were recorded. In addition, there were 20 (10.4%) cases of cerebrovascular disease without mention of dementia (CVD). In the following, ‘CVD’ refers only to these cases. Included with dementia or CVD were two cases (0.9%) of Parkinson's disease (one with dementia and one with cerebral atherosclerosis) and five cases (2.6%) with epilepsy (two with Alzheimer's disease, one with senile and one with pre-senile dementia and one with dementia of unspecified type). Dementia was not recorded on 53 (27.6%) certificates and neither dementia nor CVD on 33 (17.2%) certificates.
Table 2 NINCDS clinical algorithm diagnosis1 and certificated causes of death
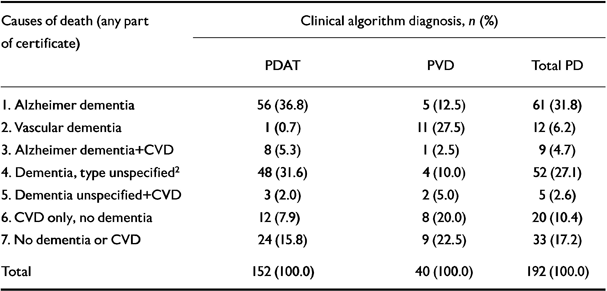
| Causes of death (any part of certificate) | Clinical algorithm diagnosis, n (%) | ||
|---|---|---|---|
| PDAT | PVD | Total PD | |
| 1. Alzheimer dementia | 56 (36.8) | 5 (12.5) | 61 (31.8) |
| 2. Vascular dementia | 1 (0.7) | 11 (27.5) | 12 (6.2) |
| 3. Alzheimer dementia+CVD | 8 (5.3) | 1 (2.5) | 9 (4.7) |
| 4. Dementia, type unspecified2 | 48 (31.6) | 4 (10.0) | 52 (27.1) |
| 5. Dementia unspecified+CVD | 3 (2.0) | 2 (5.0) | 5 (2.6) |
| 6. CVD only, no dementia | 12 (7.9) | 8 (20.0) | 20 (10.4) |
| 7. No dementia or CVD | 24 (15.8) | 9 (22.5) | 33 (17.2) |
| Total | 152 (100.0) | 40 (100.0) | 192 (100.0) |
As Table 2 shows, there was substantial agreement between the cause of death and the diagnosis made by clinical algorithm. As for the 52 certificated cases of dementia of unspecified type, 48 (92.3%) were clinically PDAT. Of the 14 cases whose death certificates recorded both Alzheimer's disease or unspecified dementia and cerebrovascular disease, 11 were PDAT. Dementia was recorded on the death certificate significantly less frequently in cases of PVD than of PDAT (χ2 with continuity correction=4.71, d.f.=1, P=0.03). Cerebrovascular disease with or without dementia was recorded in 15.1% of cases of PDAT and 27.5% of PVD, and CVD alone in 7.9% and 20%, respectively (Table 2).
Heart disease and other conditions
Heart disease such as myocardial infarction, coronary atherosclerosis, ischaemic or hypertensive heart disease, or congestive failure, was mentioned on 36 certificates (18.7%), in 27 (75%) of which it was given as the immediate cause of death. Heart disease was significantly more common (χ2=26.1, d.f.=1, P<0.001) among deaths without recorded dementia (43.4%) than in those with dementia (9.4%), and among deaths without recorded cerebral disease of any kind (63.6%) than in those with cerebral disease (9.4%) (χ2=49.5, d.f.=1, P<0.001). Heart disease was not related to the clinical NINCDS diagnosis of PDAT or PVD. Respiratory diseases (nearly all bronchopneumonia) were the immediate cause in 73 cases (38%), which was similar in PDAT and PVD. In seven cases, adult onset diabetes mellitus was the direct or a contributory cause of death; six were cases of PDAT, but the death certificates showed only two with Alzheimer's disease, and the others with CVD or heart disease. Renal failure was mentioned in three PDAT cases.
Gender differences
Alzheimer's disease or unspecified dementia were significantly more frequent on the death certificates of women, and vascular dementia or CVD on those of men (χ2 with continuity correction=4.10, d.f.=1, P=0.043). Dementia was absent from the death certificates significantly more often in men than women (χ2 with continuity correction=6.67, d.f.=1, P=0.010).
Survival
‘Survival’ refers to survival after diagnosis unless otherwise stated. Figure 1 shows the cumulative percentage surviving in PDAT and PVD and the expected survival in the general population. Table 3 shows the number dying, the cumulative deaths and the number surviving in successive years in the whole cohort. The difference in median survival between PDAT (6.09 years) and PVD (6.00 years) was not significant (P>0.05), and the pattern is so similar (Fig. 1) that the following data apply to the whole cohort unless otherwise stated. The median survival was 6.08 years: 5.97 years in men and 6.22 years in women, an insignificant difference. For each year of entry, the cohort was reasonably homogeneous in respect of survival: median survival (years) was 6.0, 6.0, 5.9, 7.0 and 7.0 for the 5 years from 1985 to 1989, and the differences between adjacent years, and between 1985-87 and 1988-89, were not significant. Of the patients entering the study in 1985-86, 8.6% were still alive at the end, as were 22.5% of those entering later. The difference between younger and older patients (below and above median age at diagnosis) was not significant. Mean age at death was 65.4 years (s.d. 4.99) (median 66.3, range 49-76), with no significant differences by gender or diagnosis. Considering only those who had died, median survival was 5.47 years (mean 5.53, s.d. 2.78). Calculated from the onset of symptoms, the median survival time of the whole cohort was 7.50 years, and in those who died 6.77 years. Cumulative survival was 64% after 5 years and 20% after 10 years, compared with age/gender-matched survival in the general population of 93% and 83%, respectively. The proportions of expected lifetime for which the cases actually survived (the longevity quotient) were 68.5% and 24.2% at the end of 5 and 10 years, respectively.
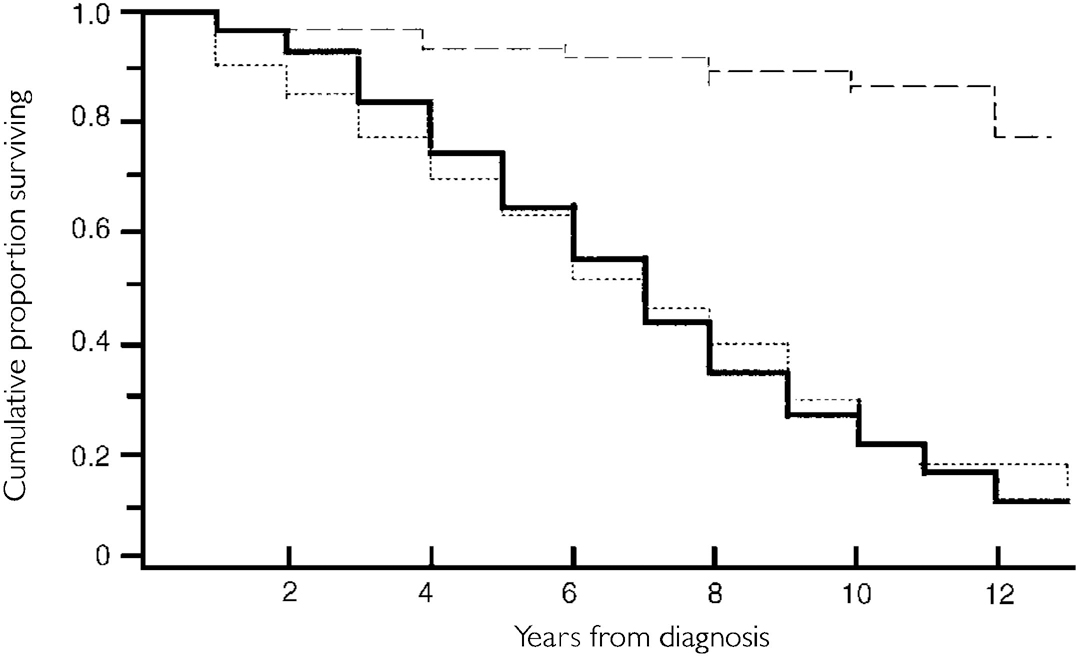
Fig. 1 Survival analysis of 179 incident cases of clinically diagnosed pre-senile dementia of Alzheimer type (PDAT) and 48 incident cases of pre-senile vascular dementia (PVD), with life expectancy of controls matched for age and gender from English Life Tables for 1990-92 (Office of Population Censuses and Surveys, 1997). General population, - - -; PDAT, —; PVD, ···· .
Table 3 Survival analysis of 179 incident cases of clinically diagnosed pre-senile dementia of Alzheimer type (PDAT) and 48 incident cases of pre-senile vascular dementia (PVD): number dying, cumulative deaths and number surviving in successive years

| Number of years since start | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-1 | 1-2 | 2-3 | 3-4 | 4-5 | 5-6 | 6-7 | 7-8 | 8-9 | 9-10 | 10-11 | 11-12 | 12-13 |
| Total cumulative deaths (PDAT+PVD) | ||||||||||||
| 9 | 21 | 40 | 60 | 82 | 112 | 131 | 149 | 170 | 180 | 186 | 191 | 192 |
| Patients dying in interval | ||||||||||||
| 9 | 12 | 19 | 20 | 22 | 30 | 19 | 18 | 21 | 10 | 6 | 5 | 1 |
| Number surviving | ||||||||||||
| 1 | 8 | 11 | 6 | 9 | ||||||||
Survival of 2 years or less from the onset of disease was associated with clinical diagnosis of PVD (χ2 with continuity correction=7.07, d.f.=1, P=0.008) and death from CVD (χ2 with continuity correction=6.71, d.f.=1, P=0.009). Patients who died with no mention of cerebral disease on the death certificate had survived for a significantly shorter time (mean 4.6 years) than those with cerebral disease (mean 5.7 years) (t=2.13, d.f.=190, P=0.034), and these groups also differed in mean survival from symptom onset (t=2.77, d.f.=188, P=0.006). Cases for whom heart disease was given on the death certificate were also associated with significantly shorter survival from the onset of symptoms (t=2.39, d.f.=188, P=0.018), but not from the time of diagnosis (mainly owing to shorter duration of symptoms before diagnosis). Of the 10 patients (six with PVD) who died within 2 years of onset, death was due to heart disease or CVD in eight.
When the cohort of non-survivors was divided into two by median length of survival from symptom onset, cerebral disease of any kind was found to have been recorded more frequently in those who survived longer (90.5% v. 76.8%) (P=0.01). Of the 28 patients who had died 10 years or more after the onset of their symptoms, cerebral disease was recorded in 86% (dementia in 64%), and the distribution of causes of death (Table 2) did not differ significantly from that for the remaining 162 cases (P=0.64), though CVD without dementia was a somewhat more common cause of death in the longer-term survivors (21%) than in the remainder (9%).
Prediction
Although neither death nor length of survival were predicted by age, gender or clinical diagnosis, clinical diagnosis of PVD and higher Hachinski score were associated with death with CVD or multiinfarct dementia within the study period. Clinical diagnosis of PDAT and lower Hachinski score were associated with death with Alzheimer's disease recorded, and female gender was associated with any mention of dementia. Multivariate analyses gave similar results, except that the Hachinski score did not add to prediction in the presence of clinical diagnosis, and age contributed to the prediction of death with CVD.
Place of death
Among the 192 deaths, 37 (19.3%) died at home, 108 (56.2%) in hospital and 47 (24.5%) in a residential or nursing home. Between the periods 1985-91 and 1992-98 the proportion of deaths at home and in hospital fell and that in residential and nursing homes increased (χ2=9.14, d.f.=2, P=0.01) (Table 4). More of those patients who had survived longer died in residential or nursing homes, whereas those whose illnesses had been shorter died at home or in hospital.
Table 4 Place of death by year of death in pre-senile dementia

| Year of death | Place of death, n (%) | |||
|---|---|---|---|---|
| Home | Hospital | Residential or nursing home | Total | |
| 1985-91 | 20 (25.3) | 48 (60.8) | 11 (13.9) | 79 (100.0) |
| 1992-98 | 17 (15.0) | 60 (53.1) | 36 (31.9) | 113 (100.0) |
| Total | 37 (19.3) | 108 (56.2) | 47 (24.5) | 192 (100.0) |
DISCUSSION
Diagnosis
Onset was measured both from time of diagnosis and from time of symptom onset (as elicited by our enquiries); the latter was probably less reliable, but added one or more years to the total survival time in over a third of cases. Delay in diagnosis, which was over 1 year in nearly half the cases, was found in another cohort to be more often due to lack of insight in the patient and reluctance to consult a doctor; the shorter delay in men may be due to evidence of serious effects of the disease in the workplace (Reference Newens, Forster and KayNewens et al, 1994). A home visit is justified when requested by the carer if a patient will not attend. The longer duration before diagnosis in PDAT could be a consequence of the insidious onset, whereas cerebrovascular features in PVD would draw attention to the illness sooner.
With neuropathological diagnosis as the standard, the sensitivity and specificity of a clinical diagnosis of Alzheimer's disease and multi-infarct dementia have exceeded 70% (Reference Mölsä, Paljarvi and RinneMölsä et al, 1985). In the present study a clinical algorithm, modified from NINCDS criteria and based on information in case notes, was used to standardise diagnosis and to reduce the number of categories from three to two (PDAT and PVD); dementia of unspecified type was usually classifiable as PDAT. In other respects, disagreements between case note and algorithmic diagnoses were few.
However, the clinical diagnosis of presenile dementia has been reported to be frequently erroneous (Reference Ron, Toone and GarraldaRon et al, 1979). We had several opportunities to review the illness and discard doubtful or misdiagnosed cases before the survival analysis was undertaken. In the first place, all case note diagnoses had been carefully screened by applying the algorithm, which included memory impairment accompanied by personality change or at least one other cognitive deficit such as dysphasia; interference with social functioning; and illness duration of at least 3 months (Reference Newens, Forster and KayNewens et al, 1993). Patients diagnosed by neurologists were younger than those seen by physicians or psychiatrists, but did not differ in other respects. Second, the case notes of readmitted patients were discarded if the final discharge diagnosis was a nondementia disorder. Third, dementia was confirmed clinically for some of the patients with PDAT when an interview with patient and relative was possible. Finally, the carers of these patients later reported a deteriorating course with considerable impairments in activities of daily living, and in no case had the initial clinical diagnosis of dementia been changed (Reference Newens, Forster and KayNewens et al, 1995).
On the other hand, there were fewer opportunities to review the diagnosis of PVD, and this group was probably incomplete and possibly unrepresentative, since the clinical algorithm could not be applied to stroke patients with cognitive impairment unless a diagnosis of dementia had also been coded, and it is likely that this was not done in several possible cases.
Incidence
For this reason, no estimate was made of the incidence of PVD. The mean annual incidence rate of PDAT of 6.2 per 100 000 population aged 45-64 does not differ significantly from the rate of 7.2 previously reported by Newens et al (Reference Newens, Forster and Kay1993), nor from the rate of 11.1 in Finland (Reference Mölsä, Marttila and RinneMölsä et al, 1982). The drop in rate was mainly due to a particularly low rate in 1989, which may have been due to less retrospective information being available in the final year of the study than in earlier years. An annual rate of about 7 per 100 000 may be the best estimate from our data overall.
Death certification
The validity of the clinical diagnosis of presenile dementia is supported by the death certificates, 70% of which mentioned dementia. While this is strong evidence that these patients were suffering from dementia, failure to record dementia does not necessarily mean that dementia was absent. The 24% of PDAT cases in which there was no mention of dementia is less than the 30% without mention of dementia in a cohort of patients with a well-established diagnosis of senile dementia, Alzheimer type (Reference Burns, Jacoby and LuthertBurns et al, 1990). The distinction between PDAT and PVD is also supported (Table 2). The more frequent mention of dementia in PDAT than PVD is in agreement with the Scottish study of Thomas et al (Reference Thomas, Starr and Whalley1997). We did not find significant differences in other causes of death between PDAT and PVD, whereas Thomas et al (Reference Thomas, Starr and Whalley1997) found small increases in cardiac disease, vascular disease (other than cerebrovascular disease) and other disorders as underlying or contributory causes of death in PVD compared with PDAT. In the present study, the finding that heart disease as a cause of death was more frequent when neither dementia nor CVD was present could be due to dementia being considered unimportant when there was an obvious cause of death, although in the five patients who died from heart disease within 2 years, confusional states could possibly have been mistaken for dementia. But it is also possible that cardiovascular factors are indeed present, not only in PVD but in some cases of PDAT; the Hachinski score does not distinguish between multi-infarct dementia and mixed dementia (e.g. Reference Mölsä, Paljarvi and RinneMölsä et al, 1985).
Cerebrovascular disease
Cerebrovascular disease, with or without dementia, was mentioned on the certificates in 16% of cases of PDAT. In these cases the direct causes of death were: vascular dementia (1), cerebral haemorrhage (1), and cerebral thrombosis (2); and among direct (12) or antecedent (9) causes were unspecified types of acute stroke or cerebral atherosclerosis. This association supports a role for vascular factors in Alzheimer's disease (Reference StewartStewart, 1998) but requires confirmation. Neuropathological studies are needed to resolve this question and to uncover the range of conditions that may present clinically as pre-senile dementia. Meanwhile, deaths in this cohort will continue to be flagged by the NHSCR until all the participants in the study have died.
Survival
The median (50%) survival time of 6.08 years from diagnosis in patients with PDAT, with a maximum follow-up period of 12.5 years, is close to the 6.04 years survived after a maximum follow-up of 7 years in the earlier incidence cohort (Reference Newens, Forster and KayNewens et al, 1993); as already noted there is a 37% overlap between the two cohorts. Contrary to the findings of some earlier studies (e.g. Reference Seltzer and SherwinSeltzer & Sherwin, 1983), patients with PDAT usually survived some time before dying. The median survival of 7.6 years after symptom onset in PDAT is similar to the median survival of 8.1 years reported in an Israeli population (Reference Treves, Korczyn and ZilberTreves et al, 1986). Nor have previous studies of senile dementia of the Alzheimer type (SDAT) found age of onset to be related to duration of survival or rate of progression (e.g. Reference Bracco, Gallato and GrigolettoBracco et al, 1994). However, Barclay et al (Reference Barclay, Zemcov and Blass1985), studying incident cases of Alzheimer's disease, found that the longevity quotient 5 years after diagnosis was considerably lower (46%) in those diagnosed before the age of 65 than in those diagnosed at the age of 65 or later, indicating that, compared with the normal, younger patients have a decreased life expectancy. In our study the longevity quotient of 68% after 5 years is more in accord with the results of Mölsä et al (Reference Mölsä, Marttila and Rinne1986), suggesting that survival in Alzheimer's disease is related to a constant effect of disease progression, relatively independent of age.
Uniformity or diversity?
Our sample was remarkably homogeneous in respect of survival time and causes of death. Predictive features were few. We found no difference in median or mean survival between PDAT and PVD. Hier et al (Reference Hier, Warach and Gorelick1989) also failed to find a significant difference in survival time between patients aged 65 and over with multi-infarct dementia and those with senile dementia, but Mölsä et al (Reference Mölsä, Marttila and Rinne1986) attributed higher mortality in vascular dementia to the cerebrovascular disease and stroke. Specific clinical features, such as stroke or hypertension, which contribute to the Hachinski scale, were not individually identifiable in our study, but we found an increase in the number of deaths from cerebrovascular disease and stroke, although not from cardiovascular morbidity, in PVD compared with PDAT. In a study of deaths in Scottish mental hospitals, survival after symptom onset was shorter in multi-infarct dementia (5.8 years) than in PDAT (7.4 years), mainly because of a longer period between onset and entering hospital in PDAT (Reference McGonigal, McQuade and ThomasMcGonigal et al, 1992). In the present study, also, duration before diagnosis was longer in PDAT and, as in Scotland, age at onset or gender did not affect survival in either condition.
Since the absence of post-mortem data must raise questions about diagnosis, survivors and non-survivors were studied further. First, fewer of the patients entering the study in earlier years had survived than of those entering later, suggesting (if the patients in the two time periods are comparable, as they appear to be) that further deaths associated with pre-senile dementia will occur in the more recently recruited patients. Second, there were no significant differences among survivors between those who had survived longer or shorter than the median (n=17 and 18, respectively) in respect of age at diagnosis, gender, type of consultant, head scan, clinical algorithm or duration before diagnosis. Nor were there significant differences in any of these respects between survivors and non-survivors. Third, 31 long-term survivors were compared with 28 patients who had died 10 years or more after onset. The only significant difference was that those who had died were older at the time of diagnosis (mean age 60.8 v. 58.2 years, t=2.75, d.f.=57, P=0.008).
Very short survival (i.e. death within 2 years from symptom onset) was associated with clinical diagnosis of PVD and death from CVD or heart disease. Apart from these cases, cerebral disease was recorded more often in patients who had survived beyond the median; and even in those who had died 10 years or more after onset, cerebral disease of some kind was recorded in 86% (dementia in 64%), and the distribution of causes of death (Table 2) did not differ significantly from that in the remaining cases (P=0.637). The high proportion of deaths in which cerebral disease was mentioned in persons surviving over 10 years from onset suggests that long survival is compatible with a diagnosis of pre-senile dementia. In the cohort with PDAT reported by McGonigal et al (Reference McGonigal, McQuade and Thomas1992), survival ranged from 1.2 to 16 years. Although cases with purely functional psychoses have probably been excluded by our rigorous selection procedures, the failure to specify the type of dementia in 30% of deaths raises the possibility that the sample was heterogeneous as regards the type of brain disease. Yet, except for two cases of Parkinson's disease, no other specific neurodegenerative diseases have been entered on the death certificates (one death from cerebellar degeneration has occurred since the closing date).
Service aspects
Thirty-five (19.3%) patients died at home, but this proportion fell during the period studied, as did the proportion dying in hospital, whereas the proportion dying in residential or nursing homes increased. This provides a minimal estimate of the number of patients cared for at home, since we were unable to distinguish between patients who were admitted to a general ward with an acute illness and those who were receiving long-term hospital care. The changes in the place of death during the period 1985-91 compared to 1992-97 presumably reflect the reduction in the number of NHS beds for the elderly and the implementation of the National Health Service and Community Care Act 1990.
Our findings have a number of implications for services. First, they are important for the counselling of relatives, both on an emotional plane and for planning future financial provision and support in individual families (Reference Luscombe, Brodaty and FreethLuscombe et al, 1998). Second, in conjunction with the studies of incidence and prevalence, disabilities and service use, they have an important role in the planning of hospital and community care for populations. Because pre-senile dementia is relatively uncommon, it is difficult for health authorities to commission totally separate services for physically active younger patients (Reference Delany and RosenvingeDelany & Rosenvinge, 1995), and a recent survey confirmed that only 12 out of 304 (3.9%) relevant hospital trusts provided a dedicated specialist service for younger people with dementia (Reference BarberBarber, 1997). Some have suggested that the investigation and diagnosis of pre-senile dementia are the domain of neurologists, whereas the caring skills and facilities of psychiatry of old age should be extended to younger patients for long-term management (Reference Allen and BaldwinAllen & Baldwin, 1995). Our study did not include cases of dementia due to specific neurological diseases or alcohol misuse, though these conditions are important components of pre-senile dementia in a wider sense (Reference Ferran, Wilson and DoranFerran et al, 1996). An alternative model, which recognises that the old age psychiatry system may not always be the most appropriate for younger age groups, is to integrate pre-senile dementia within the context of diagnostic and rehabilitative services for all types of neurological and neurodegenerative disease in middle and late-middle age. Our findings suggest that this question merits careful attention.
CLINICAL IMPLICATIONS AND LIMITATIONS
CLINICAL IMPLICATIONS
-
▪ Counselling of relatives and planning of services need to take account of the chronicity and progression of pre-senile dementia.
-
▪ The value of death certification would be enhanced by the routine recording of chronic conditions.
-
▪ Vascular disease in pre-senile dementia of Alzheimer type appears to warrant further study.
LIMITATIONS
-
▪ Cases were identified through contact with National Health Service hospital services and did not constitute a true population-based sample.
-
▪ The method of ascertaining cases would have missed cases of pre-senile vascular dementia when the ICD-9 diagnostic code was not accompanied by a code for dementia.
-
▪ Diagnosis was not confirmed neuropathologically.
ACKNOWLEDGEMENTS
We are grateful to the Northern Region Locally Organised Research Scheme and to the Medical Research Council for research grants, and to the National Health Service Central Registry for providing death drafts. We thank patients, relatives and general practitioners for their help and cooperation.








eLetters
No eLetters have been published for this article.