Executive functions are mental abilities that allow the individual to respond appropriately to unfamiliar and complex situations and support several cognitive, emotional and social capacities. Impairment of executive functions occurs in the majority of stroke patients (Reference Lesser, Boone and MehringerLesser et al, 1996), and may slow rehabilitation and return to premorbid levels of psychosocial adjustment.
Antidepressant treatment improves outcome following stroke, independently of depression. Nortriptyline and fluoxetine improve activities of daily living (Reference Narushima and RobinsonNarushima & Robinson, 2003) and mortality (Reference Jorge, Robinson and ArndtJorge et al, 2003), and sertraline improves morbidity (Reference Rasmussen, Lunde and PoulsenRasmussen et al, 2003). Antidepressants may exert outcome-improving effects on the brain in at least two ways: one is through modulation of cortical-striato-pallido-thalamo-cortical pathways subsequent to action on the raphe nuclei, locus ceruleus and ventral tegmental area (Reference Alexopoulos, Meyers and YoungAlexopoulos et al, 2000); another is through reorganisation of neural circuitry favoured by their activity on brain-derived neurotrophic factor (BDNF) (Reference Saarelainen, Hendolin and LucasSaarelainen et al, 2003).
This evidence prompted our study hypothesis that antidepressants improve frontal executive function following stroke. This hypothesis has not been tested before. The positive effect of treatment with antidepressants on the cognitive abilities of older adults (Reference Allard, Artero and RitchieAllard et al, 2003) and the identification of similar mechanisms of executive deficit in late life and stroke (Coffey et al, Reference Coffey, Figiel and Djang1988a ,Reference Coffey, Figiel and Weiner b ) further supported the rationale behind this study. We predicted that antidepressant treatment would improve executive dysfunction independently of depression.
METHOD
Patient selection
Data allowing examination of the study hypothesis were collected during a double-blind placebo-controlled treatment study of post-stroke depression (Reference Robinson, Schultz and CastilloRobinson et al, 2000). Participants were patients consecutively admitted to the Younkers Rehabilitation Center at the Iowa Methodist Medical Center in Des Moines, Iowa. Patients were included if they had experienced a stroke within the preceding 6 months. Exclusion criteria were:
-
(a) medical illness threatening the patient's life or recovery;
-
(b) severe comprehension deficit, i.e. failing part 1 of the Token Test (Reference De Renzi, Faglioni and PrevidiDe Renzi et al, 1978);
-
(c) a history of head injury or other brain disease.
Patients taking antidepressants discontinued them for a 2-week wash-out period before the study. Written consent was obtained in accordance with institutional review board requirements. Ninety-two patients entered the double-blind placebo-controlled phase and 69 completed it (Fig. 1).
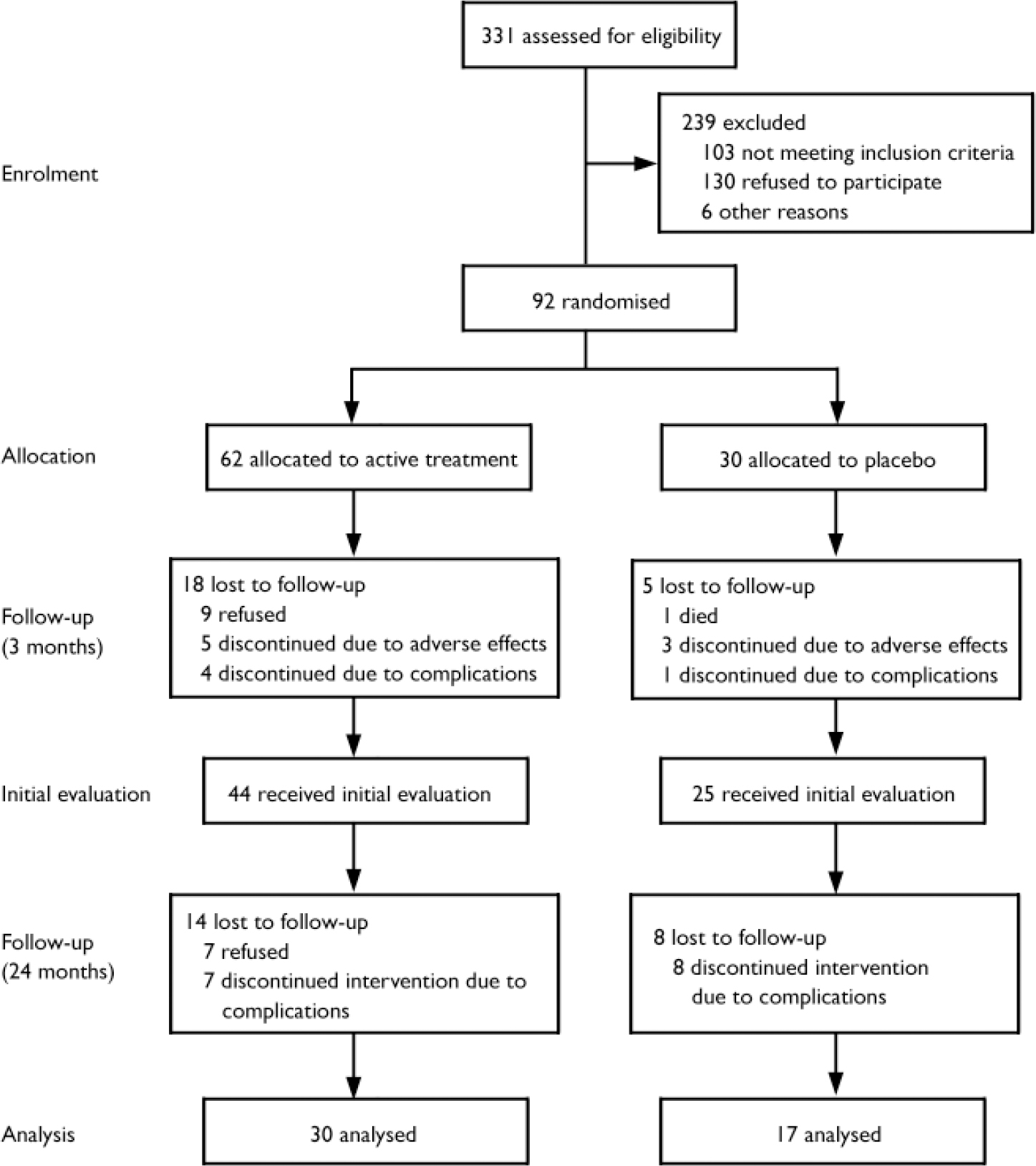
Fig. 1 Trial CONSORT diagram
After completing the treatment phase, seven patients refused to repeat the assessment. Fifteen patients developed complications and were excluded. In the active treatment group, one patient developed Parkinson's disease, one had a transient cerebrovascular ischaemic event just before the evaluation and five experienced seizures. In the placebo group, one patient developed psychosis, two had a stroke, and five experienced seizures. All patients who developed seizures took anticonvulsants. There was no significant group difference in the frequency of complications (χ2=3.04, P=0.08). Clinical and background variables of patients not included in the analysis were not significantly different from those of participants included in the analyses. Data from 47 patients were analysed, 30 of these patients were treated with either nortriptyline (n=11) or fluoxetine (n=19), and 17 received placebo.
Treatment protocol
Patients were randomly assigned to 12 weeks of either fluoxetine, nortriptyline or placebo, unless there was a definite contra-indication. Nortriptyline was chosen because it is reported to be effective in post-stroke depression (Reference Lipsey, Robinson and PearlsonLipsey et al, 1984); fluoxetine was chosen because it is a commonly used selective serotonin reuptake inhibitor with the advantage of a long half-life. Nortriptyline was not given to patients with cardiac abnormalities, and fluoxetine was contraindicated in patients with intracerebral haemorrhage. Eight patients had a contraindication to nortriptyline and nine to fluoxetine: these patients were randomly reassigned to the alternative active medication or to placebo. Thus, 85% of the patients were randomly assigned to nortriptyline, fluoxetine or placebo. All patients were randomly assigned to either active or placebo medication.
The dosages of nortriptyline were 25 mg per day for the first week, 50 mg per day for weeks 2 and 3, then 75 mg per day for weeks 4–6 and 100 mg per day for the final 6 weeks. Dosages of fluoxetine were 10 mg per day for the first 3 weeks, 20 mg per day for weeks 4–6, then 30 mg per day for weeks 7–9 and 40 mg per day for the final 3 weeks. In nine patients the dosages had to be decreased owing to severe side-effects; five were being treated with nortriptyline and four with fluoxetine. Reduction of dosage was achieved in double-blind fashion. All the patients were fully compliant with their medication regimen throughout the treatment period. After completion of the treatment phase of the study, all the medications were discontinued.
Assessment
The initial neuropsychological examination took place after the completion of the 12-week double-blind phase. This was followed by a second evaluation 21 months after the end of active treatment.
The neurological examination was conducted by a neurologist using the National Institutes of Health (NIH) Stroke Scale (Reference Brott, Adams and OlingerBrott et al, 1989). Severity of depression was assessed using the 17-item version of the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960). Overall cognitive functioning was assessed with the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975). All assessments were conducted by examiners masked to the participants' background conditions.
The following tests of executive function constituted the primary outcome variables: the Controlled Oral Word Association (COWA; Reference BentonBenton, 1967) test measured initiation and psychomotor speed; the Wisconsin Card Sorting Test Perseverative Errors (WCST–PE; Reference Grant and BergGrant & Berg, 1948) and the Wechsler Adult Intelligence Scale–Revised (WAIS–R; Reference WechslerWechsler, 1981) Similarities sub-tests measured conceptualisation and problem-solving; and the WAIS–R Arithmetic and Digit Span sub-tests measured attention and working memory.
Neuroimaging
Computerised tomography or magnetic resonance scans were obtained and evaluated for anatomical location and lesion volume by a neuroradiologist or a neurologist unaware of the psychiatric findings. Lesion volume was estimated using the ratio of the largest cross-sectional area of the lesion to the area of the brain slice that included the body of the lateral ventricles (Reference Robinson, Bolla-Wilson and KaplanRobinson et al, 1986).
Statistical analysis
Between-group comparisons were made using means, standard deviations and repeated-measures analysis of variance (ANOVA). Frequency distributions were compared using the chi-squared test. Individual executive function scores were transformed into standardised z scores which were combined into an ‘executive index’. The follow-up executive index scores were based on z scores calculated using the baseline means and standard deviations. Changes between the initial and follow-up measures were evaluated by change scores, where positive scores showed improvement and negative scores showed deterioration. Multiple linear regression was used to examine the potential contribution of clinical factors to executive improvement. All tests were two-tailed and significance was set at P<0.05.
RESULTS
Patient characteristics, neurological and radiological findings
There was no statistically significant group difference in background characteristics and demographic data (Table 1). During the 21-month post-treatment period, one patient in the active treatment group refused to participate, two complained of side-effects, and four died; in the placebo group three patients refused follow-up evaluations and one died. Thus, a total of 36 patients completed all evaluations. There was no significant group difference in rates of withdrawal. The background variables of patients who completed the follow-up neuropsychological evaluations were not significantly different from those of patients who did not complete them.
Table 1 Demographic characteristics of the active and placebo treatment groups

| Characteristic | Nortriptyline or fluoxetine group (n=30) | Placebo group (n=17) | P | ||||
|---|---|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 65.5 | (12.5) | 71.7 | 5.6 | 0.09 | ||
| Education, years: mean (s.d.) | 12.4 | (1.9) | 11.4 | 3.5 | 0.21 | ||
| Initial HRSD score: mean (s.d.) | 9.4 | (6.5) | 6.4 | (4.8) | 0.11 | ||
| Time since stroke, days: mean (s.d.) | 68.0 | (85.9) | 37.1 | (23.3) | 0.17 | ||
| Duration of antidepressant treatment after 12-week treatment, months: mean (s.d.) | 4.7 | (6.3) | 2.8 | (5.8) | 0.34 | ||
| Gender: male, n (%) | 20 | (67) | 8 | (47) | 0.19 | ||
| Ethnicity: White, n (%) | 28 | (93) | 17 | (100) | 0.40 | ||
| Married, n (%) | 23 | (77) | 9 | (53) | 0.10 | ||
| Socio-economic status: Hollingshead class IV or V, n (%) | 13 | (44) | 6 | (35) | 0.59 | ||
| Right-handedness, n (%) | 28 | (93) | 17 | (100) | 0.17 | ||
| Prior psychiatric history, n (%) | 4 | (13) | 2 | (12) | 0.88 | ||
| Family psychiatric history, n (%) | 14 | (47) | 5 | (29) | 0.24 | ||
| DSM–IV major or minor depression (initial) | 12 | (40) | 4 | (24) | 0.24 | ||
| DSM–IV major or minor depression (follow-up) | 5 | (17) | 3 | (18) | 0.93 | ||
| Receiving antidepressant treatment after the 12-week treatment | 13 | (43) | 4 | (24) | 0.17 | ||
HRSD, Hamilton Rating Scale for Depression
In the period between the initial and follow-up evaluations, some patients were given antidepressants by their treating physicians. Type, dosage and duration of all prescribed medications were recorded. The frequency and the duration of antidepressant treatment in the two groups were not significantly different (Table 1). Additionally, there was no significant group difference in MMSE scores at the initial evaluation (active treatment, mean=27.5, s.d.=0.71; placebo group, mean=27.0, s.d.=0.95; P=0.66) and at follow-up (active treatment, mean=27.8, s.d.=0.85; placebo group, mean=25.6, s.d.=0.95; P=0.12). There was no significant group difference in neurological and radiological variables (Table 2).
Table 2 Stroke characteristics and neurological findings
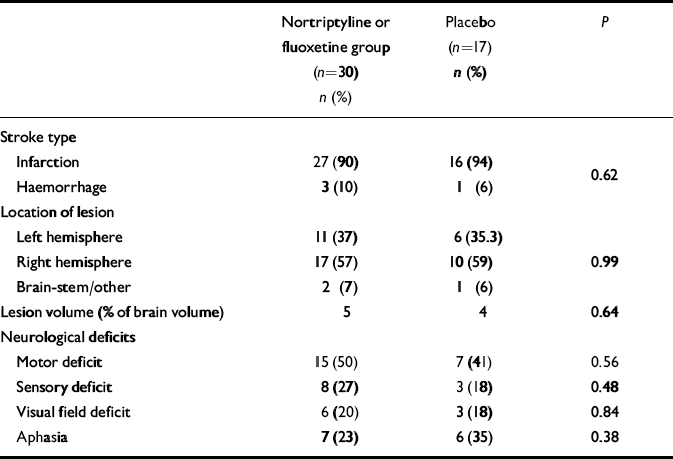
| Nortriptyline or fluoxetine group (n=30) n (%) | Placebo (n=17) n (%) | P | |
|---|---|---|---|
| Stroke type | |||
| Infarction | 27 (90) | 16 (94) | 0.62 |
| Haemorrhage | 3 (10) | 1 (6) | |
| Location of lesion | |||
| Left hemisphere | 11 (37) | 6 (35.3) | |
| Right hemisphere | 17 (57) | 10 (59) | 0.99 |
| Brain-stem/other | 2 (7) | 1 (6) | |
| Lesion volume (% of brain volume) | 5 | 4 | 0.64 |
| Neurological deficits | |||
| Motor deficit | 15 (50) | 7 (41) | 0.56 |
| Sensory deficit | 8 (27) | 3 (18) | 0.48 |
| Visual field deficit | 6 (20) | 3 (18) | 0.84 |
| Aphasia | 7 (23) | 6 (35) | 0.38 |
Executive index
At the completion of the treatment phase, executive performance showed no statistically significant between-group effect (F=0.01, d.f.=1,45, P=0.91).
A significant time-by-group interaction was found between the initial and follow-up evaluation. This was true for both efficacy analysis (i.e. analysing only patients who completed the 24-month follow-up: F=13.1, d.f.=1,34, P=0.001) and intention-to-treat (ITT) analysis (i.e. analysing all patients who enrolled in the treatment study, with missing data interpolated based on actual observations using the last observation carried forward method: F=12.1, d.f.=1,45, P=0.001). Patients given active treatment showed an improvement in executive performance after 21 months, whereas patients given placebo showed a decline (Fig. 2).
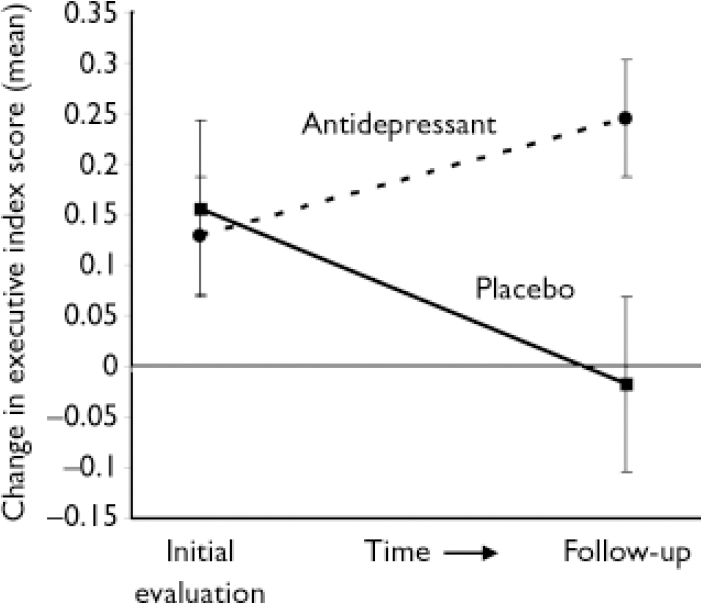
Fig. 2 Executive index change between initial and follow-up evaluations (mean and standard errors).
There was no significant difference between patients treated with nortriptyline and fluoxetine. Paired t-test analysis among actively treated patients showed significant improvement (efficacy analysis: t=–2.39, d.f.=22, P=0.03; ITT analysis: t=–2.33, d.f.=29, P=0.03), whereas placebo-treated patients showed significant deterioration (efficacy analysis: t=2.75, d.f.=12, P=0.02, ITT analysis: t=2.59, d.f.=16, P=0.02). All but one patient who received placebo showed deterioration of executive function 2 years after the initiation of the study.
Individual tests of executive function
Participants' performance on the individual tests composing the executive index was also examined (Fig. 3). The COWA and WCST–PE tests showed significant treatment effects compared with placebo in change scores (COWA test, efficacy analysis: F=6.46, d.f.=1,34, P=0.02; COWA test, ITT analysis: F=6.22, d.f.=1,45, P=0.02; WCST–PE, efficacy analysis: F=7.19, d.f.=1,34, P=0.01, WCST–PE ITT analysis: F=6.87, d.f.=1,45, P=0.01). The WAIS–R Similarities, Digit Span and Arithmetic sub-tests showed the same direction of change, but failed to reach statistical significance (Fig. 3).
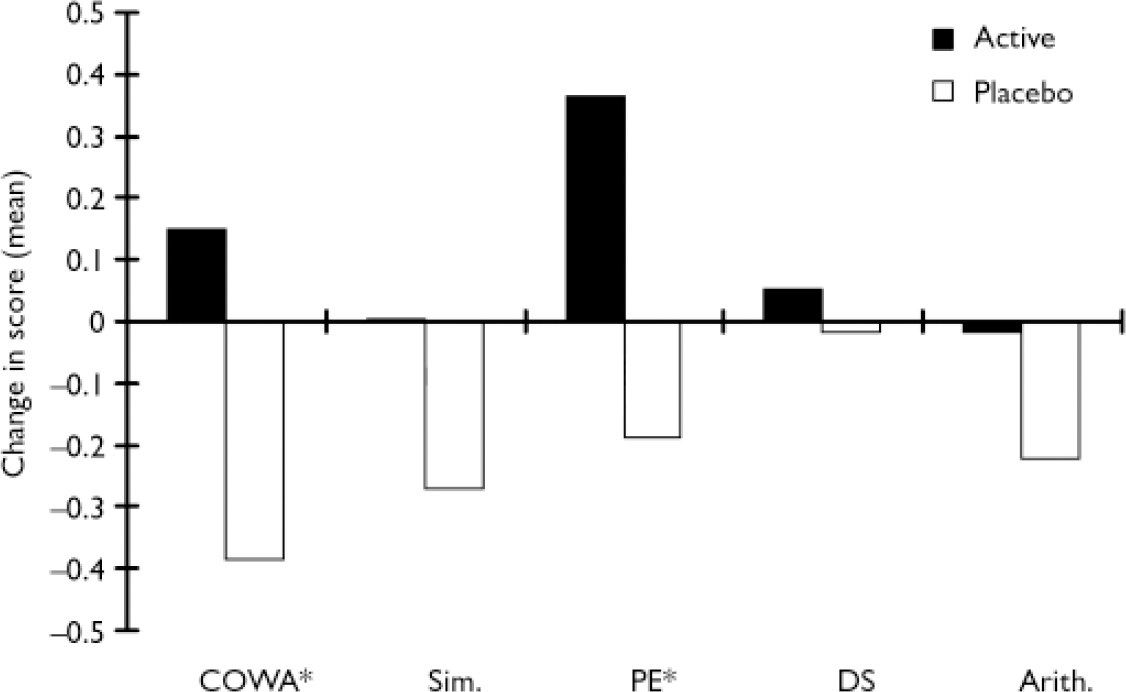
Fig. 3 Individual executive function test scores change between initial and follow-up evaluations (Arith., Arithmetic sub-test; COWA, Controlled Oral Word Association test; DS, Digit Span; PE, Perseverative Errors; Sim., Similarities sub-test). Significant group differences are shown for the COWA and PE tests (*); the other tests showed either improvement or lack of deterioration in the active group but the difference between active and placebo treatment failed to reach statistical significance. The PE score is inverted to be consistent with the other tests in the figure.
Multiple linear regression analysis
Many factors may influence executive function recovery in addition to antidepressant treatment. In order to examine the contribution of these potentially confounding factors, an exploratory stepwise regression analysis was conducted including the following variables: active treatment or placebo; severity of depressive symptoms at baseline, on completion of the 3-month placebo-controlled treatment phase and after 2 years; age; gender; past and family psychiatric history; antidepressant treatment during the 21-month follow-up period; presence of neurological impairment (motor, sensory or visual impairments, or aphasia); lesion location (left hemisphere, right hemisphere or brainstem); lesion's proximity to the frontal pole (i.e. relative distance between the frontal pole and the most rostral margin of the stroke lesion compared with the distance between the frontal and occipital poles); type (infarction or haemorrhage) and volume of lesion.
The final model predicting executive function consisted of active treatment or placebo, age, past psychiatric history, neurological impairment and total lesion volume (F(5,25)=3.91, P<0.05). The only factor that showed a significant independent effect was active treatment or placebo (F(1,25)=14.9, P<0.01) (Table 3).
Table 3 Multiple linear regression analysis of change in executive function during 21 months of follow-up
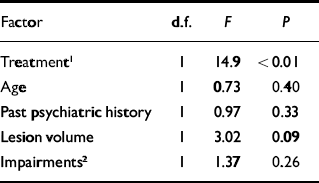
| Factor | d.f. | F | P |
|---|---|---|---|
| Treatment 1 | 1 | 14.9 | <0.01 |
| Age | 1 | 0.73 | 0.40 |
| Past psychiatric history | 1 | 0.97 | 0.33 |
| Lesion volume | 1 | 3.02 | 0.09 |
| Impairments 2 | 1 | 1.37 | 0.26 |
1. Active v. placebo treatment
2. Motor/sensory/visual deficits or aphasia
DISCUSSION
To our knowledge this is the first study to examine the effect of antidepressant treatment on executive function following stroke. We found that 12 weeks of treatment, started within 6 months of the stroke, exerted a positive effect on executive function 21 months after treatment. The effect on cognitive function was independent of improvement in depression, was delayed and was independent of intervening antidepressant treatment. The effect of antidepressants on cognition did not extend to overall cognitive status as measured by the MMSE.
Caveats
Before discussing these findings, some factors that might have influenced our results should be acknowledged. The majority of patients were high-school or college-educated, White, married and belonged to Hollingshead social class I to III (I is the highest, V is the lowest). Therefore, the results may not be applicable to all stroke patients. As expected in studies examining elderly people, follow-up evaluations could not be obtained for all patients. This may limit generalisation of our findings to people willing or able to receive follow-up evaluations. Some patients were prescribed antidepressants during the naturalistic follow-up, and a small number of patients were reassigned to the alternative active treatment or placebo for medical reasons; these factors might have introduced bias (although naturalistic antidepressant treatment was not an independent predictor of recovery). Finally, there was no neuropsychological assessment to ensure the two groups had equivalent levels of executive function prior to treatment; hence, despite randomisation, this might have influenced the results.
Clinical implications
The findings in this study have important implications for the neuropsychiatry of stroke and rehabilitation medicine. Antidepressants administered within 6 months of stroke appear to improve long-term executive function outcome. This effect is two-fold. As can be observed in Fig. 1, antidepressants both improve and prevent decline of executive function. How can this interesting and clinically important phenomenon be explained?
Mechanisms
Frontal cortical–subcortical circuits
Five frontal cortical–subcortical circuits – motor, oculomotor, dorsolateral prefrontal, lateral orbital frontal, and anterior cingulate – subserve distinct motor and cognitive abilities (Reference Alexander, DeLong and StrickAlexander et al, 1986). All circuits originate in the prefrontal cortex, project to the striatum, synapse at the level of the globus pallidus, substantia nigra and thalamus, and finally return to the prefrontal cortex, forming closed loops – the cortical-striato-pallido-thalamo-cortical (CSPTC) pathway. The dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortical circuits subserve both executive (Reference DreweDrewe, 1974; Reference Baker, Rogers and OwenBaker et al, 1996) and affective functions (Baxter et al, Reference Baxter, Phelps and Mazziotta1985, Reference Baxter, Schwartz and Phelps1989; Reference Drevets, Videen and PriceDrevets et al, 1992; Reference Baker, Rogers and OwenBaker et al, 1996; Reference Elliott, Baker and RogersElliott et al, 1997). They can be modulated by the activity of monoaminergic nuclei, including the raphe nuclei, the locus ceruleus and the ventral tegmental area (Reference Alexopoulos, Meyers and YoungAlexopoulos et al, 2000), which are sites of action of antidepressant medications. Thus, the mechanism of executive function recovery may be the modulation of monoaminergic nuclei exerting effects on CSPTC circuits.
Neurogenesis
Another possible mechanism stems from the association of chronic antidepressant administration and neurogenesis. Neurogenesis in the adult brain is generally thought to be restricted to germinal centres in the subventricular zone and the hippocampal/dentate gyrus (Reference PetersonPeterson, 2002). Chronic administration of antidepressants enhances the development of immature neurons and promotes the survival and function of adult neurons by enhancing BDNF and its receptor trkB, resulting in functional and anatomical changes. Activation of BDNF and trkB receptor has been shown to be required for antidepressants to induce behavioural effects (Reference Saarelainen, Hendolin and LucasSaarelainen et al, 2003), and has been posited as an explanation for the delayed treatment effect of antidepressants (Reference Nibuya, Morinobu and DumanNibuya et al, 1995). Although there has been no demonstration of neurogenesis in the prefrontal cortex, chronic antidepressant treatment induces activation of trkB receptor in the prefrontal cortex and is responsible for the sensitisation to the effects of BDNF (Reference Saarelainen, Hendolin and LucasSaarelainen et al, 2003). Neurotrophins, particularly BDNF, have been shown to regulate neurite outgrowth (membrane-enclosed protrusions of neuronal cell cytoplasm), synaptic plasticity and the selection of functional connections in the central nervous system in general (Reference Katz and ShatzKatz & Shatz, 1996; Reference McAllister, Katz and LoMcAllister et al, 1999). Consistent with these findings is the recent notion that chronic administration of antidepressants prevents stress-induced reduction of BDNF (Reference Manji and DumanManji & Duman, 2001; Reference McEwen and LasleyMcEwen & Lasley, 2003; Reference Brown, Varghese and McEwenBrown et al, 2004). The finding in our study may make the study of neurotrophin-mediated mechanisms of improved executive function worth pursuing in greater detail.
Future studies
Our study has shown that early treatment with antidepressants following stroke has a remote positive effect on recovery and prevention of decline in executive function. Monoaminergic modulation of frontal executive functions and/or enhancement of neuronal plasticity and reorganisation of limbic and frontal structures may underlie this phenomenon. Our findings require confirmation in further studies, which might also explore whether any particular antidepressant is to be preferred, and the optimal time, duration and dosage of treatment.
Acknowledgements
We thank Stephan Arndt, PhD, Teresa Kopel, MA, Stephanie Rosazza, BS, and J. Todd Kosier, BS, for their help in this research project. Thanks to Lauren Wagner, MD, for help in editing the manuscript. We are indebted to the patients of the Younkers Rehabilitation Center of the Iowa Methodist Medical Center in Des Moines, Iowa. The study was supported in part by National Institute of Mental Health R01 grants MH-40355, MH-52879, MH-53592, MH63405 (R.G.R.), MH-52879 (S.P.) and Research Scientist Award MH-00163 (R.G.R.). S.P. is supported by the Edward J. Mallinckrodt foundation and by a National Institutes of Health sponsored Institutional Career Development Award (K12).









eLetters
No eLetters have been published for this article.