Dementia is one of the greatest health and social care challenges we face, with costs set to increase substantially as the numbers of people with dementia rise. The estimated global costs of dementia, US$604 billion (£386 billion) in 2010, are projected to increase by 85% by 2030. Reference Wimo and Prince1 In England alone, the cost of long-term care of older people with dementia is set to increase from £5.4 billion in 2002 to £16.7 billion in 2031. Reference Comas-Herrera, Wittenberg, Pickard and Knapp2
Depression is a common and important comorbidity in dementia. Prevalence estimates vary from 5 to 40% depending on sampling and definition. Reference Devanand, Jacobs, Tang, Del Castillo-Castaneda, Sano and Marder3–Reference Steffens, Fisher, Langa, Potter and Plassman5 Depression in dementia is a risk factor for increased carer distress, disability, suicide and mortality, and is also associated with a high level of use of medical in-patient beds. Reference Kales, Blow, Copeland, Bingham, Kammerer and Mellow6 Treating depression in dementia is therefore a clinical priority with the potential to improve the well-being, quality of life and level of function of people with dementia; it might also have an effect on costs.
However, there is only weak evidence on the effectiveness of antidepressants for depression in dementia, Reference Bains, Birks and Dening7 and no cost-effectiveness evidence on the treatment of depression in dementia. Reference Barrett, Byford and Knapp8 Despite this, antidepressants are frequently used for treating depression in dementia. The Health Technology Assessment Study of the Use of Antidepressants for Depression in Dementia (HTA-SADD) trial was designed to investigate the clinical effectiveness and cost-effectiveness of the two most commonly used classes of antidepressants compared with placebo. We have published our analyses of clinical effectiveness Reference Banerjee, Hellier, Dewey, Romeo, Ballard and Baldwin9 and reported that those randomised to sertraline or mirtazapine did no better than those receiving placebo. Here we report on the co-primary aim: to examine the cost-effectiveness of sertraline and mirtazapine compared with placebo over 13 weeks and 39 weeks in people with depression and dementia.
Method
Research setting
We have published the details of the HTA-SADD trial method and results elsewhere. Reference Banerjee, Hellier, Dewey, Romeo, Ballard and Baldwin9 To summarise, the trial involved people with probable or possible dementia of the Alzheimer's type according to NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association) criteria Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan10 and depression (defined as a Cornell Scale for Depression in Dementia (CSDD) Reference Alexopoulos, Abrams, Young and Shamoian11 score of over 7) referred to old age psychiatric services in nine English sites: Birmingham, Cambridge, Leicester, Liverpool, Manchester, Newcastle, North London, Southampton and South London. Ethical approval was obtained from the North West 7 (Greater Manchester) Ethics Committee.
Sample size
A sample size of 507 was calculated to provide 90% power to detect a two-point CSDD difference (s.d. = 5; standardised effect-size (SES) = 0.4) for 13-week sertraline/placebo and mirtazapine/placebo comparisons, and 86% power at 39 weeks. This was revised during the trial because of slower than forecasted recruitment to 339. Reference Banerjee, Hellier, Dewey, Romeo, Ballard and Baldwin9
Randomisation
After baseline assessment and consent, participants were randomised to three groups: sertraline, mirtazapine and placebo (trial registration: ISRCTN88882979 and EudraCT 2006-000105-38). All participants also received normal clinical care. The target doses were 150 mg sertraline or 45 mg mirtazapine daily with titration over 8 weeks. Thereafter it was open to clinicians to adjust the dose. The Clinical Trials Unit at the Institute of Psychiatry independently undertook treatment allocation.
Economic evaluation
The primary economic evaluation was a cost-effectiveness analysis comparing differences in treatment costs for patients receiving sertraline, mirtazapine or placebo with differences in effectiveness as measured by the primary outcome, total CSDD score Reference Alexopoulos, Abrams, Young and Shamoian11 over two time periods: 0–13 weeks and 0–39 weeks. The secondary analysis was a cost-utility analysis using quality-adjusted life years (QALYs) computed from the Euro-Qual (EQ-5D) 12 and societal weights (obtained from data from a sample of the general public to aggregate items into an overall EQ-5D score) Reference Dolan, Gudex, Kind and Williams13 over those same periods. Both the primary and secondary economic evaluations were undertaken from two perspectives: (a) health and social care agencies; and (b) health and social care agencies and unpaid carers.
Resource use
Resource-use data for each person were collected over a retrospective period of 6 months before randomisation. At 13 weeks, follow-up data were collected retrospectively for a 3-month period and at 39 weeks for a retrospective period of 6 months. Services and support received by the study participants were recorded on a resource-use questionnaire adapted from the Client Service Receipt Inventory (CSRI), Reference Beecham, Knapp and Thornicroft14 including in-patient stays, out-patient attendances, day hospital treatment, visits to social clubs, meals at lunch clubs, day care visits and hours spent in contact with community-based professionals. The study also collected data on volunteer support, befriending and telephone care-line support, and also on unpaid support provided by friends and relatives. Contacts made with voluntary workers and support provided by friends and relatives were also measured in hours of care support time. The prescribed daily doses for the medications were calculated from the trial medication log, and prescribing periods were weighted to the changing dose regime.
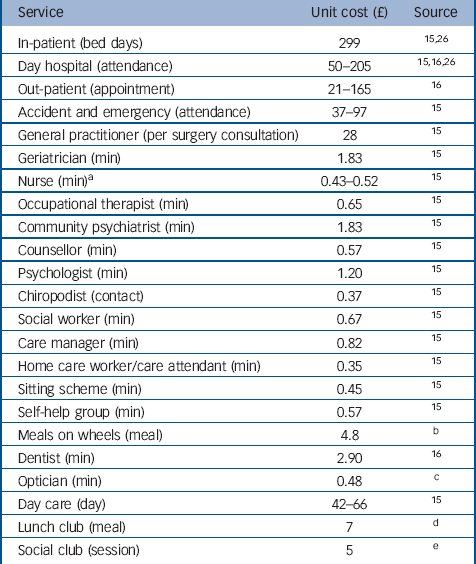
Unit costs
All unit costs were estimated at 2009/2010 prices and were collected from sources in the public domain (Table 1). Costs per unit of measurement for each type of service (such as per in-patient day, per appointment, per attendance, per visit or per contact with health and community-based professionals including voluntary services) were taken from a widely used compendium. Reference Curtis15 The National Health Service Schedule of Reference Costs was used to estimate the cost of out-patient attendances. 16 The unit cost of medication was obtained from the British National Formulary. 17
We collected information from each study participant's main carer on time they spent providing unpaid care and support, and time spent by friends or relatives regularly providing help for the trial participant. We asked respondents to estimate the hours of unpaid care and support from all such sources in an average or typical week. Opportunity costs were attached to these hours using, first, an estimate of replacement cost (the unit cost of a paid local authority home care worker Reference Curtis15 ) and, second, the cost of lost employment (gross hourly wage of a carer in paid employment; zero for a carer not in paid employment).
Cost estimation
Data on resource use from the CSRI and medication prescribed from the medication logs were combined with unit cost data to estimate total costs for each participant in the trial. Three main categories of costs were analysed: medication costs, aggregated health and social care costs (primary care and hospital-based visits and community-based contacts) and (opportunity) cost of time spent caregiving by relatives and friends. Costs were categorised in this way to facilitate comparison of costs alongside measures of effectiveness from the various study perspectives. The costs of services and support used by patients were derived by combining medication, health and social care resource utilisation data with unit costs. Costs were calculated for the periods 0–13 weeks and 0–39 weeks.
Table 1 Unit cost for 2009–2010
| Service | Unit cost (£) | Source |
|---|---|---|
| In-patient (bed days) | 299 | Reference Curtis15 , Reference Banerjee, Murray, Foley, Atkins L Schneider and Mann26 |
| Day hospital (attendance) | 50–205 | Reference Curtis 15 , 16 , Reference Banerjee, Murray, Foley, Atkins L Schneider and Mann 26 |
| Out-patient (appointment) | 21–165 | 16 |
| Accident and emergency (attendance) | 37–97 | Reference Curtis15 |
| General practitioner (per surgery consultation) | 28 | Reference Curtis15 |
| Geriatrician (min) | 1.83 | Reference Curtis15 |
| Nurse (min) a | 0.43–0.52 | Reference Curtis15 |
| Occupational therapist (min) | 0.65 | Reference Curtis15 |
| Community psychiatrist (min) | 1.83 | Reference Curtis15 |
| Counsellor (min) | 0.57 | Reference Curtis15 |
| Psychologist (min) | 1.20 | Reference Curtis15 |
| Chiropodist (contact) | 0.37 | Reference Curtis15 |
| Social worker (min) | 0.67 | Reference Curtis15 |
| Care manager (min) | 0.82 | Reference Curtis15 |
| Home care worker/care attendant (min) | 0.35 | Reference Curtis15 |
| Sitting scheme (min) | 0.45 | Reference Curtis15 |
| Self-help group (min) | 0.57 | Reference Curtis15 |
| Meals on wheels (meal) | 4.8 | b |
| Dentist (min) | 2.90 | 16 |
| Optician (min) | 0.48 | c |
| Day care (day) | 42–66 | Reference Curtis15 |
| Lunch club (meal) | 7 | d |
| Social club (session) | 5 | e |
a Practice nurse, district nurse health visitor, community psychiatric nurse, cardiac nurse, incontinence nurse.
b http://www.ic.nhs.uk/webfiles/publications/009_Social_Care/pss0910expfinal/pss0910updateOct2011/Personal_Social_Services_Expenditure_Report_2009_10.pdf
c New calculation: there is a recommended fee payable to ophthalmic medical practitioners who administer sight tests, although optometrists undertake most tests. Optometrist salaries vary depending on practice setting (private or hospital or combination of the two). Typical salaries in private practice based on salary data collected June 2009 (http://www.prospects.ac.uk/optometrist_salary.htm) ranged from £19 500 to £28 000. In hospital settings, optometrist salaries are usually covered by the Agenda for Change pay scale. Average salary for private practice was used. Cost per hour was estimated based on 41 weeks per annum, 38 hours per week.
d http://cash-online.org.uk/content/1/6/3/; uprated using the Consumer Price Index (CPI). Reference Curtis15
e Cost of adult social club at 2004/05 uprated using the pay and prices inflator. Reference Curtis15
Statistical analysis
An ‘intention-to-treat’ analysis was carried out to preserve the unbiased distribution of factors in the groups produced by randomisation. Missing resource-use data were singly imputed. If there was no report on the use of a particular resource, we assumed that it was not used. If participants reported on a resource but not the quantity used, we imputed this amount from within treatment-group means for participants with data for that item at the same assessment point.
Health and social care costs for 0–13 weeks and 0–39 weeks (and health/social care and costs of unpaid carer costs for the parallel analysis from the broader perspective for the same time periods) were regressed in turn on treatment allocation, baseline cost, baseline CSDD and centre. To mitigate the effects of skewness, non-parametric bootstrapping methods were used to estimate 95% confidence intervals (CIs) for mean costs. Where the bias-corrected 95% CIs of between-group change scores excluded zero, they could be judged to be significant at P = 0.05 or lower.
Estimates of bootstrapped mean cost and effectiveness were used to estimate an incremental cost-effectiveness ratio (ICER) for each analysis. The ICER for each replication was calculated as:
which summarises the cost difference between two treatments per incremental difference in the outcome (CSDD and EQ-5D in turn). The EQ-5D was measured directly from patients – as recommended by National Institute for Health and Clinical Excellence (NICE) guidelines 18 – and weighted by a valuation of changes in quality of life reported from UK population data. Reference Dolan, Gudex, Kind and Williams13 Health effects were then expressed in terms of QALYs. The ratio statistic compared the treatments in terms of observed differences in costs and effects, regardless of whether those differences were statistically significant.
Uncertainty about the cost and effectiveness estimates was addressed by plotting cost-effectiveness acceptability curves (CEAC). A CEAC was used to assess trade-offs between costs and outcomes, showing the likelihood of each of the two medications in turn being seen as cost-effective relative to the other or relative to placebo, given different (implicit monetary) values placed on incremental outcome improvements. In this net-benefit approach, monetary values of incremental effects and incremental costs for each case are combined, and the net benefit derived as:
where: a is the comparator, b is drug treatment, NB is net benefit, and λ is willingness to pay for a unit improvement in CSDD-depression severity score (primary evaluation) or an additional QALY (secondary evaluation). The impact on costs given uncertainty around the value attached to informal care inputs was assessed in one-dimensional sensitivity analysis. All analyses were completed in Stata (version 11) and SPSS 17 on Windows.
Results
Baseline comparisons
Overall, 339 participants were recruited to the trial. At baseline, full service-use data were available for 326 participants (111 placebo, 107 sertraline, 108 mirtazapine). At 13 weeks, economic data were available for 97 (87%) participants in the placebo group, 78 (73%) in the sertraline group and 88 (81%) in the mirtazapine group. By 39 weeks there were economic data on 84 (76%) participants in the placebo group, 69 (64%) in the sertraline group and 78 (72%) in the mirtazapine group. This drop-out level is comparable with other trials in the area.
Service use and support
Contacts made by study participants with services and support over weeks 0–13 and 0–39 are shown in Tables 2 and 3 respectively. In the 0–13-week period (Table 2), there were no differences in service use between the treatment groups reaching statistical significance at the 5% level. However, taking the whole 0–39 week period (Table 3), it was striking that the mean number of hours per week spent by unpaid carers caring for patients in the placebo-treated group and the sertraline group were almost twice that for patients in the mirtazapine-treated group. This difference in unpaid carer time between the placebo and mirtazapine-treated group was statistically significant at the 5% level.
Outcomes
At 39 weeks, the difference in mean CSDD score between placebo and sertraline was 0.05 (95% CI −1.83 to 1.67); between placebo and mirtazapine was −0.80 (95% CI −2.55 to 1.21); and between mirtazapine and sertraline was −0.9 (95% CI −1.10 to 2.73). The secondary measure of outcome was QALY gain at 39 weeks; the mean difference between placebo and sertraline was 0.03 (95% CI −0.09 to 0.03); between placebo and mirtazapine was 0.05 (95% CI −0.10 to 0.01); and between mirtazapine and sertraline was 0.02 (95% CI −0.03 to 0.07). There were no statistically significant differences in either the primary or secondary measure of outcome between groups at 39 weeks (or at 13 weeks) (Table 4).
Costs
Daily medication costs for sertraline 50 mg of £0.05 and mirtazapine 15 mg of £0.23 were applied (mean cost of medication per person: £7 (95% CI 6–8) and £37 (95% CI 32–41)). Mean total costs over 0–13 weeks and 0–39 weeks are detailed in Table 4. Pair-wise comparisons were made between the two antidepressants and placebo using regression analysis and bootstrapping. There were no statistically significant differences between the groups in either of the time periods, either when health and social care service costs only were considered, or when health and social care services and unpaid carer costs were summed. After adjustment for baseline costs, CSDD score at baseline and site, there were no statistically significant differences in health and social care costs – or in health/social care and unpaid carer costs – in any pair-wise comparison in either time period.
Unpaid carer costs exceeded health and social care costs by a factor of 1.2 to 1.7. Including these unpaid carer costs results in a change in the ranking of total costs, with mirtazapine being the least expensive of all treatments in both periods.
Cost-effectiveness
As noted earlier, the primary economic evaluation focused on CSDD as the outcome over, first, the period 0–13 weeks after randomisation, and, second, the period 0–39 weeks after randomisation. The secondary evaluation focused on QALYs computed from the EQ-5D and societal weights over the same periods. Data used in the estimation of the ICERs are shown in Table 5. As noted earlier, there were no statistically significant differences in CSDD scores or QALYs in any of the pair-wise comparisons. There were also no significant pair-wise differences in costs from either perspective between the treatment groups.
Table 2 Service use, week 0–13

| Placebo (n = 97) | Sertraline (n = 78) | Mirtazapine (n = 88) | ||||
|---|---|---|---|---|---|---|
| n | Mean a (s.d.) | n | Mean a (s.d.) | n | Mean a (s.d.) | |
| Hospital-based care | ||||||
| In-patient (bed day) b | 8 | 1.65 (7.98) | 5 | 1.58 (6.82) | 5 | 0.49 (2.19) |
| Out-patient (attendance) | 33 | 0.53 (1.08) | 25 | 0.60 (1.10) | 26 | 0.53 (1.10) |
| Accident and emergency (attendance) | 8 | 0.12 (0.48) | 5 | 0.08 (0.27) | 4 | 0.57 (0.28) |
| Day hospital (contact) | 3 | 0.23 (1.44) | 6 | 0.83 (4.11) | 4 | 0.32 (1.66) |
| Community-based care | ||||||
| General practitioner (contact) | 57 | 1.36 (2.36) | 44 | 1.09 (1.44) | 49 | 1.22 (2.84) |
| Geriatrician (contact) | 3 | 0.03 (0.17) | 0 | 0 | 88 | 0.03 (0.18) |
| Nurse c (contact) | 41 | 0.87 (1.49) | 29 | 2.50 (10.83) | 43 | 1.56 (3.71) |
| Occupational therapist (contact) | 11 | 0.21 (0.66) | 7 | 0.35 (1.70) | 5 | 0.08 (0.38) |
| Community psychiatrist (contact) | 21 | 0.26 (0.54) | 14 | 0.24 (0.63) | 19 | 0.27 (0.58) |
| Psychologist (contact) | 2 | 0.82 (0.64) | 3 | 0.06 (0.37) | 2 | 0.09 (0.62) |
| Counsellor (contact) | 1 | 0.01 (0.10) | 3 | 0.36 (2.94) | 2 | 0.17 (1.32) |
| Care manager (contact) | 7 | 0.10 (0.42) | 1 | 0.01 (0.11) | 4 | 0.05 (0.21) |
| Social worker (contact) | 15 | 0.21 (0.69) | 10 | 0.19 (0.58) | 12 | 0.28 (0.87) |
| Home care worker/care attendant (contact) | 19 | 18.57 (60.57) | 17 | 21.92 (72.77) | 22 | 28.33 (72.19) |
| Chiropodist (contact) | 33 | 0.43 (0.71) | 16 | 0.26 (0.57) | 23 | 0.40 (0.88) |
| Sitting scheme (contact) | 5 | 1.21 (6.71) | 5 | 0.68 (4.29) | 3 | 0.59 (3.75) |
| Self-help group (contact) | 0 | 0 | 0 | 0 | 1 | 0.03 (0.32) |
| Meals on wheels (contact) | 3 | 0.30 (1.77) | 3 | 5.82 (33.95) | 4 | 2.32 (12.74) |
| Dentist (contact) | 10 | 0.13 (0.49) | 10 | 0.15 (0.43) | 15 | 0.23 (0.58) |
| Optician (contact) | 10 | 0.12 (0.39) | 13 | 0.19 (0.46) | 12 | 0.15 (0.39) |
| Day services | ||||||
| Day services (day) | 15 | 4.15 (11.95) | 17 | 6.50 (15.64) | 16 | 5.47 (13.33) |
| Lunch club (visit) | 3 | 1.88 (15.92) | 0 | 0 | 3 | 1.18 (8.51) |
| Social club (visit) | 2 | 0.27 (1.86) | 4 | 0.67 (2.89) | 2 | 0.44 (3.08) |
| Informal care | ||||||
| Care giving (hours/week) | 45 | 10.05 (17.65) | 37 | 11.63 (21.59) | 42 | 9.84 (23.85) |
a Across full sample.
b Psychiatric and non-psychiatric in-patient bed days.
c Practice nurse, district nurse health visitor, community psychiatric nurse, cardiac nurse, incontinence nurse.
Table 3 Service use, week 0–39

| Placebo (n = 84) | Sertraline (n = 69) | Mirtazapine (n = 78) | ||||
|---|---|---|---|---|---|---|
| n | Mean a (s.d.) | n | Mean a (s.d.) | n | Mean a (s.d.) | |
| Hospital-based care | ||||||
| In-patient (bed day) b | 9 | 3.05 (10.48) | 11 | 2.55 (9.26) | 14 | 4.54 (15.08) |
| Out-patient (attendance) | 44 | 0.83 (1.15) | 33 | 0.90 (1.41) | 29 | 0.69 (1.15) |
| Accident and emergency (attendance) | 13 | 0.17 (0.41) | 8 | 0.25 (0.86) | 7 | 0.10 (0.35) |
| Day hospital (contact) | 1 | 0.01 (0.11) | 8 | 2.61 (9.42) | 3 | 0.56 (3.30) |
| Community-based care | ||||||
| General practitioner (contact) | 57 | 1.51 (1.83) | 40 | 1.52 (2.15) | 55 | 1.88 (2.40) |
| Geriatrician (contact) | 4 | 0.05 (0.21) | 0 | 0 | 2 | 0.03 (0.16) |
| Nurse c (contact) | 37 | 1.24 (2.34) | 33 | 5.84 (29.57) | 34 | 1.46 (3.53) |
| Occupational therapist (contact) | 9 | 0.17 (0.53) | 8 | 0.45 (2.23) | 5 | 0.10 (0.44) |
| Community psychiatrist (contact) | 22 | 0.33 (0.67) | 15 | 0.26 (0.53) | 29 | 0.60 (1.48) |
| Psychologist (contact) | 5 | 0.21 (1.34) | 2 | 0.03 (0.17) | 1 | 0.01 (0.11) |
| Care manager (contact) | 6 | 0.52 (2.87) | 3 | 0.04 (0.21) | 5 | 0.10 (0.44) |
| Social worker (contact) | 12 | 0.58 (2.98) | 15 | 0.42 (0.98) | 17 | 0.44 (1.47) |
| Home care worker/care attendant (contact) | 16 | 33.56 (107.73) | 19 | 38.07 (95.60) | 17 | 38.95 (110.10) |
| Chiropodist (contact) | 35 | 0.88 (1.37) | 20 | 0.53 (1.52) | 32 | 1.11 (1.89) |
| Sitting scheme (contact) | 0 | 0 | 5 | 1.23 (5.49) | 4 | 0.76 (3.69) |
| Meals on wheels (contact) | 2 | 0.63 (5.67) | 2 | 3.77 (21.70) | 2 | 3.14 (19.49) |
| Dentist (contact) | 18 | 0.33 (0.96) | 18 | 0.47 (1.25) | 19 | 0.34 (0.81) |
| Dietician (contact) | 0 | 0 | 0 | 0 | 1 | 0.01 (0.11) |
| Day services | ||||||
| Day services (day) | 16 | 5.57 (14.31) | 18 | 7.26 (15.13) | 16 | 5.17 (12.63) |
| Lunch club (visit) | 1 | 0.31 (2.84) | 1 | 0.38 (3.15) | 3 | 0.83 (4.84) |
| Social club (visit) | 2 | 0.62 (4.47) | 3 | 0.57 (2.69) | 1 | 0.33 (2.94) |
| Informal care | ||||||
| Care giving (hours per week) | 40 | 12.27 (21.24) | 34 | 12.32 (24.07) | 33 | 6.74 (11.82) |
a Across full sample.
b Psychiatric and non-psychiatric in-patient bed days.
c Practice nurse, district nurse health visitor, community psychiatric nurse, cardiac nurse, incontinence nurse.
Table 4 Health and social care and informal care costs and outcome

| Placebo | Sertraline | Mirtazapine | Bootstrapped mean difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (s.d.), £ | n | Mean (s.d.), £ | n | Mean (s.d.), £ | Sertraline – placebo | Mirtazapine – placebo | Mirtazapine – sertraline | |
| (a) Medication costs | |||||||||
| 0–13 weeks | 97 | 0 | 78 | 7 (5) | 88 | 37 (22) | 7 (6 to 8) | 37 (32 to 41) | 30 (25 to 34) |
| 0–39 weeks | 84 | 0 | 69 | 7 (5) | 78 | 37 (22) | 7 (6 to 8) | 37 (32 to 41) | 30 (25 to 34) |
| (b) Health and social care costs | |||||||||
| 0–13 weeks | 97 | 1438 (3339) | 78 | 1434 (2326) | 88 | 1094 (1871) | –4 (–900 to 798) | –344 (–1207 to 322) | –340 (–1049 to 283) |
| 0–39 weeks | 84 | 2146 (4402) | 69 | 2832 (4111) | 78 | 2513 (4290) | 686 (–630 to 1973) | 367 (–977 to 1596) | –319 (–1643 to 1023) |
| (c) Informal care cost | |||||||||
| 0–13 weeks | 97 | 2744 (4819) | 78 | 3175 (5897) | 88 | 2687 (6511) | 431 (–1000 to 2242) | –57 (–1686 to 1537) | –488 (–2380 to 1470) |
| 0–39 weeks | 84 | 3351 (5799) | 69 | 3363 (6573) | 78 | 1841 (3228) | 12 (–1940 to 2256) | –1510 (–3088 to −136) | –1522 (–3398 to −72) |
| Total costs excluding informal care inputs (a+b) | |||||||||
| 0–13 weeks | 97 | 1438 (3339) | 78 | 1441 (2327) | 88 | 1131 (1869) | 3 (–893 to 806) | –307 (–1172 to 358) | –310 (–910 to 299) |
| 0–39 weeks | 84 | 2146 (4402) | 69 | 2839 (4112) | 78 | 2550 (4289) | 693 (–622 to 1980) | 404 (–972 to 1626) | –289 (–1545 to 1151) |
| Total costs including informal care inputs (a+b+c) | |||||||||
| 0–13 weeks | 97 | 4182 (5821) | 78 | 4616 (6488) | 88 | 3818 (7060) | 434 (–1340 to 2356) | –365 (–2212 to 1560) | –798 (–2754 to 1498) |
| 0–39 weeks | 84 | 5497 (7922) | 69 | 6202 (8241) | 78 | 4391 (5285) | 705 (–1855 to 3234) | –1106 (–3137 to 970) | –1811 (–4048 to 543) |
| Depression score (CSDD) | |||||||||
| 13 weeks | 95 | 7.8 (4.1) | 78 | 8.6 (4.9) | 85 | 7.9 (5.0) | 0.84 (–0.60 to 2.14) | 0.16 (–1.53 to 1.11) | –0.7 (–0.57 to 2.52) |
| 39 weeks | 82 | 8.5 (5.5) | 68 | 8.6 (5.5) | 76 | 7.7 (6.2) | 0.05 (–1.83 to 1.67) | –0.80 (–2.55 to 1.21) | –0.9 (–1.10 to 2.73) |
| QALY 39 weeks (EQ-5D) | 57 | 0.55 (0.17) | 53 | 0.57 (0.14) | 52 | 0.60 (0.13) | 0.03 (–0.09 to 0.03) | 0.05 (–0.10 to 0.01) | 0.02 (–0.03 to 0.07) |
CSDD, Cornell Scale for Depression in Dementia; QALY, quality-adjusted life years; EQ-5D, Euro-Qual.
Probability estimates were plotted for a range of implicit monetary values attached to improvements in depression score and QALY gain. We are not aware of any studies that have attached monetary values to incremental changes in CSDD to provide any guidance on the appropriate range of willingness-to-pay values.
In Fig. 1, we see that mirtazapine had a low likelihood (around 30%) of being more cost-effective than placebo if society were not willing to pay anything for a unit improvement in the CSDD depression score. The likelihood of cost-effectiveness rose to 80% if society were willing to pay £5000 for a unit improvement in CSDD score, and stayed at 80% over values of willingness to pay for an improvement in CSDD score up to £30 000.
Figure 2 shows the CEACs from the secondary economic evaluation, where health and social care costs including unpaid carer inputs and health and social care costs excluding unpaid carer inputs were considered alongside QALYs in turn. It suggests that from a health and social care perspective mirtazapine was 14% likely to be more cost-effective than placebo even if society were willing to pay nothing for a QALY gain. This probability increased to over 20% for a willingness to pay £30 000 for a QALY gain. However, when health and social care costs including unpaid carer inputs were considered alongside QALYs, mirtazapine was 89% likely to be more cost-effective than placebo even if society were willing to pay nothing for a QALY gain. The £30 000 value is important as it is the threshold often associated with positive decisions on health technologies taken by NICE.

Fig. 1 Probability that mirtazapine is cost-effective compared with placebo: health and social care costs and Cornell Scale for Depression in Dementia score (CSDD) over 39 weeks.

Fig. 2 Probability that mirtazapine is cost-effective relative to placebo: health and social care costs and quality-adjusted life years (QALYs) over 39 weeks.
Table 5 Differences in incremental cost, effect, and cost-effectiveness

| Sertraline – placebo | Mirtazapine – placebo | Mirtazapine – sertraline | ||||
|---|---|---|---|---|---|---|
| 0–13 weeks | 0–39 weeks | 0–13 weeks | 0–39 weeks | 0–13 weeks | 0–39 weeks | |
| Incremental cost, £ (2009/2010) | ||||||
| Health and social care, mean (95% CI) | 3 (–893 to 806) | 693 (–622 to 1980) | –307 (–1172 to 358) | 404 (–972 to 1626) | –310 (–910 to 299) | –289 (–1545 to 1151) |
| Health and social care and informal care, mean (95% CI) | 434 (–1340 to 2356) | 705 (–1855 to 3234) | –365 (–2212 to 970) | –1106 (–3137 to 970) | –798 (–2754 to 1498) | –1811 (–4048 to 543) |
| Incremental effect | ||||||
| CSDD score, mean (95% CI) a | 0.84 (–0.60 to 2.14) | 0.05 (–1.83 to 1.67) | 0.16 (–1.53 to 1.11) | –0.80 (–2.55 to 1.21) | –0.7 (–0.57 to 2.52) | –0.9 (–1.10 to 2.73) |
| QALY (EQ-5D), mean (95% CI) b | – | 0.03 (–0.09 to 0.03) | – | 0.05 (–0.10 to 0.01) | – | 0.02 (–03 to 0.07) |
| Incremental cost-effectiveness, £: health and social care and: | ||||||
| (a) CSDD score | 4 (dominated) | 13 860 (dominated) | 1919 (lower costs; worse outcome) | –505 (higher costs; better outcome) | 443 (mirtazapine dominant) | 321 (mirtazapine dominant) |
| (b) QALY (EQ-5D) | – | 23 100 (higher costs; better outcome) | – | 8080 (higher costs; better outcome) | – | –14 450 (mirtazapine dominant) |
| Incremental cost-effectiveness, £: health and social care and informal care costs and: | ||||||
| (a) CSDD score | 517 (dominated) | 14 100 (dominated) | 2281 (lower costs; worse outcome) | 1382 (mirtazapine dominant) | 1140 (mirtazapine dominant) | 2012 (mirtazapine dominant) |
| (b) QALY (EQ-5D) b | – | 23 500 (higher costs; better outcome) | – | –22 120 (mirtazapine dominant) | – | –90 550 (mirtazapine dominant) |
QALY, quality-adjusted life year; EQ-5D, Euro-Qual; dominated, active treatment has higher costs and worse outcome; dominant, active treatment has lower costs and better outcome.
a On Cornell Scale for Depression in Dementia (CSDD) higher scores are related to a worse outcome; therefore negative incremental CSDD scores indicate better outcome for active treatment. In case of the comparison between mirtazapine and sertraline this is mirtazapine.
b Patient rated.
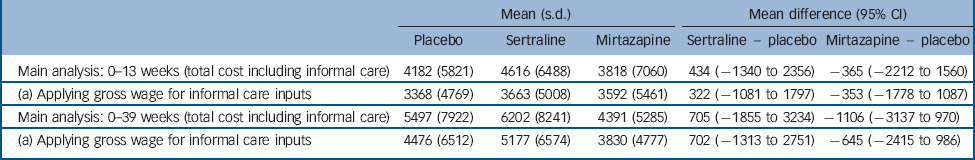
We assessed uncertainty around parameter estimates included in the cost analysis. For the main analyses, unpaid carer costs were based on the hourly cost of a home care worker. This hourly value for the caregiving inputs by friends and family was replaced in sensitivity analysis by the gross hourly wage of a carer in paid employment and zero for a carer not in paid employment. Using these alternative values of caregiver time inputs did not alter the findings (Table 6).
Discussion
As far as we are aware this is the first study to explore the cost-effectiveness of mirtazapine and sertraline in treating depression in dementia. Our results show that mirtazapine and sertraline are not cost-effective compared with placebo as a treatment for depression in dementia when looking at the primary outcome of change in depressive symptoms (i.e. neither of the antidepressants reduced CSDD score more than placebo).
However, mirtazapine did halve unpaid carer time and therefore carer costs. So, when costs were considered alongside QALY gains, a different picture emerged. Mirtazapine had the highest likelihood of cost-effectiveness compared with sertraline and placebo. Some previous studies have similarly reported a cost-effectiveness advantage when using the QALY as the outcome measure even though there was no discernible cost-effectiveness difference on the primary symptom measure, including studies of dementia Reference Knapp, Thorgrimsen, Patel, Spector, Hallam and Woods19 and depression. Reference Kendrick, Chatwin, Dowrick, Tylee, Morriss and Peveler20 The probabilities of cost-effectiveness at a willingness-to-pay value of £30 000 per QALY and under are relevant given that NICE uses this value as a threshold to guide decisions about whether or not to recommend health technologies. Reference Appleby, Devlin and Parkin21
We considered possible reasons for the finding that mirtazapine treatment had a good chance of being cost-effective compared with placebo or sertraline when the outcome under consideration is QALY. The trend towards lower incremental costs for mirtazapine was driven by the statistically significantly lower unpaid carer inputs. The small improvements in quality of life for mirtazapine relative to the other treatments also contributed to the cost-effectiveness result, and can perhaps be mediated plausibly via the putative ability of mirtazapine to ameliorate sleep disturbances and anxiety. Reference Schittecatte, Dumont, Machowski, Cornil, Lavergne and Wilmotte22,Reference Muhlbacher, Konstantinidis, Kasper, Eichberger, Hinterhuber and Hofmann23 Improvements in sleep could potentially improve life quality and therefore patient-reported EQ-5D scores; they could also release carer time directly and so ameliorate an important source of carer distress. Reference Naglie, Tomlinson, Tansey, Irvine, Ritvo and Black24 In this way mirtazapine might have a general effect, beneficial for both the patient and the carer, without exerting a specific antidepressant effect. The extent to which this is generalisable to other antidepressants is not clear from our study. The potential positive effects of mirtazapine act more in the realm of general behavioural and psychological symptoms in dementia than depression per se. It is possible that a positive effect on sleep, anxiety or agitation in the person with dementia might result in relief, not only for the person with dementia but also the carer, in terms of hours of care needed. However, it is also important to note that complex ethical issues are raised potentially when treatment is given to patients for the benefit of their carers.
Table 6 Sensitivity analysis
| Mean (s.d.) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|
| Placebo | Sertraline | Mirtazapine | Sertraline – placebo | Mirtazapine – placebo | |
| Main analysis: 0–13 weeks (total cost including informal care) | 4182 (5821) | 4616 (6488) | 3818 (7060) | 434 (–1340 to 2356) | –365 (–2212 to 1560) |
| (a) Applying gross wage for informal care inputs | 3368 (4769) | 3663 (5008) | 3592 (5461) | 322 (–1081 to 1797) | –353 (–1778 to 1087) |
| Main analysis: 0–39 weeks (total cost including informal care) | 5497 (7922) | 6202 (8241) | 4391 (5285) | 705 (–1855 to 3234) | –1106 (–3137 to 970) |
| (a) Applying gross wage for informal care inputs | 4476 (6512) | 5177 (6574) | 3830 (4777) | 702 (–1313 to 2751) | –645 (–2415 to 986) |
Strengths and limitations of the study
This is the first randomised controlled trial with an economic evaluation of pharmacotherapy for people with dementia and depression, based on individual patient data, and so fills an important evidence gap. Further, its findings are likely to have a wide application because of the broad nature of the study group, in terms of both the range of depressive symptoms and the severity of dementia. The study included individuals with probable and possible Alzheimer's. This group is close to the population found in clinical practice where there is often a vascular component to dementia. However, we would limit generalisability of the study's findings to those with Alzheimer's disease and mixed dementia only, and not to other subtypes such as vascular dementia, dementia with Lewy bodies or frontotemporal dementia.
There were some incomplete data from the CSRI, which was to be expected given the size and spread of the sample and the comprehensive nature of the service-use data-collection exercise. It would not have been feasible to collect these data from alternative sources. Missing responses were therefore assigned a value by imputation to make efficient use of the data provided.
The computation of unpaid carer costs is always difficult and we built estimates in this study on two different assumptions about unit cost, and did not reach different conclusions about cost-effectiveness. However, we were still reliant on carers' self-reported numbers of hours spent providing support to trial participants, and this is an aspect of evaluation that needs more attention to ensure sufficient accuracy.
Implications
Following the current policy-making stance of focusing on health and social care costs, the findings reported here suggest that using these drugs (relative to placebo) for treating depression in dementia is unlikely to be cost-effective if a narrow focus on depression score is adopted. However, treatment with mirtazapine appears likely to be cost-effective if a broad cost-perspective is used (to include the impact on health and social care services and the impact on unpaid carers) and if a broader approach is taken to outcome measurement to look at health-related quality of life. Family carers are a vital resource in dementia care and their support is an explicit policy priority in England. Reference Banerjee25 Given the priority and the potential value of supporting family carers of people with dementia, further research is warranted to investigate the potential of mirtazapine to help with behavioural and psychological symptoms in dementia and in supporting carers.
Acknowledgements
We would like to thank all the participants and carers that gave their time to be part of this study. We thank Pfizer for their kind donation of the sertraline and sertraline placebo for this trial. Thanks are due to the members of the HTA-SADD Data Monitoring and Ethics Committee and the HTA-SADD Trial Steering Committee – Peter Connelly (chair of the Data Monitoring and Ethics Committee), Robin Jacoby (chair of the Trial Steering Committee), Rowan Harwood, Cornelius Kelly, Angela Clayton-Turner, Craig Ritchie and Ed Juszczak. We thank the Alzheimer's Society for providing patient and public involvement support into the study. We are grateful for the practical help provided by the NIHR Mental Health Research Network (MHRN) and Dementia and Neurodegenerative Disease Research Network (DeNDRoN).











eLetters
No eLetters have been published for this article.