Submicroscopic, rare chromosomal deletions and duplications known as copy number variants (CNVs) have been found to contribute to increased risks of different neurodevelopmental disorders, notably autism, schizophrenia and intellectual disability, and most recently attention-deficit hyperactivity disorder (ADHD). Reference Abrahams and Geschwind1–Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 However, the types of CNVs most consistently associated with ADHD and other neurodevelopmental disorders are individually rare (<1% frequency). This raises the question of whether such mutations are associated with clinically and developmentally typical disorder. We have previously found that large, rare CNVs are significantly more common in children with ADHD than those without ADHD. Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 In the present paper, based on an extension of our previously reported sample, we address a different question. Specifically, we set out to identify whether ADHD accompanied by the presence of at least one large, rare CNV is associated with distinctive clinical, cognitive and developmental features, differential family loading of psychiatric disorder and specific pre-/perinatal markers.
Method
Sample
Our sample consisted of 567 children aged 5–17 years (mean = 10.6, s.d. = 2.82) with a DSM-IV 10 or DSM-III-R 11 diagnosis of ADHD or ICD-10 12 diagnosis of hyperkinetic disorder. Participants were referred to the study by clinicians from child and adolescent psychiatry or paediatric out-patient clinics across the UK. All were of White British origin. Children with schizophrenia, known autism-spectrum disorder, bipolar disorder, Tourette syndrome, epilepsy, brain damage or any other known neurological or genetic disorder were excluded from the study prior to analysis (n = 18). All children were also screened for autism-spectrum disorder using the Autism Screening Questionnaire (ASQ). Reference Berument, Rutter, Lord, Pickles and Bailey13 All those who scored above the cut-off point on the ASQ were reassessed using the same items and reviewed by a clinical child and adolescent psychiatrist. Those who still showed high scores or were judged by a clinician to have possible autism-spectrum disorder were excluded.
This sample is an extension of the 366 children included in a previous genome-wide study of CNV in ADHD. Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 As such, all methods are identical to those previously described. Ethical approval for the study was obtained from the North West England and Wales Multicentre Research Ethics Committees. Written informed consent from parents and assent from children (and consent for those aged 16 years and older) were obtained for all individuals.
Measures
Clinical
Attention-deficit hyperactivity disorder symptoms and diagnoses in the child were confirmed using the Child and Adolescent Psychiatry Assessment (CAPA), Reference Angold, Prendergast, Cox, Harrington, Simonoff and Rutter14 a research diagnostic interview undertaken with the parent. The presence of impairment and onset of symptoms/impairment before the age of 7 years that are needed to confirm diagnoses were also assessed using the CAPA. All interviewers were trained to a high level of reliability (kappa = 1.00 for agreement on ADHD diagnosis). Information on the pervasiveness of ADHD symptoms and impairment in a school setting was obtained using the Child ADHD Teacher Telephone Interview (CHATTI) Reference Holmes, Lawson, Langley, Fitzpatrick, Trumper and Pay15 or the Conners Teacher Rating Scale. Reference Conners16
The CAPA Reference Angold, Prendergast, Cox, Harrington, Simonoff and Rutter14 was also used to assess current symptoms and diagnoses of comorbid oppositional defiant disorder, conduct disorder, anxiety disorders (generalised anxiety, social anxiety, separation anxiety), depression, mania and tic disorders. Symptoms of these comorbid conditions were also assessed using the child version of the CAPA Reference Angold and Costello17 for children aged 12 years and over. Comorbid symptoms were endorsed if reported by either parent or child. Reference Angold and Costello17 DSM-IV diagnoses and total current symptom counts for all of the assessed disorders were generated from the CAPA. Total scores from the ASQ were also computed.
Cognitive measures
Cognitive ability was assessed using the Wechsler Intelligence Scale for Children, Reference Weschler18,Reference Weschler19 which obtains an estimate of full scale IQ. For those aged under 12 years, reading ability was measured using the Wechsler Objective Reading Dimensions (WORD) assessment package. Reference Wechsler20
Pre- and perinatal factors and early development
Data on pre- and perinatal factors were obtained using a well-validated parent-rated retrospective questionnaire, Reference Lewis and Murray21 supplemented with additional questions that have been included in previous investigations. Reference Rice, Lewis, Harold, van den Bree, Boivin and Hay22–Reference Langley, Holmans, van den Bree and Thapar24 We focused on factors previously found to be associated with ADHD or other neurodevelopmental problems. Parents reported whether the child had spent any time in a special care baby unit, the child’s birth weight and the mother’s and father’s age at the birth of the child. The presence of smoking during pregnancy (yes/no) was assessed using the question ‘Did you smoke at all during your pregnancy?’ Parents were also asked to report on the child’s early development Reference Thapar, Harrington, Ross and McGuffin25 using measures found to discriminate those with ADHD from those without. Specifically, parents were asked about a delay in speech (not talking by age 2 years), as well as a delay in walking (not walking by age 18 months). Parents were also asked whether their child had suffered from any serious medical conditions. Medical conditions reported by the parent were coded as congenital in origin by two independent physicians (e.g. cardiac murmur coded as congenital, but not asthma).
Family factors
Parents reported on the family’s total annual income and their educational attainment. Low income was defined as annual income <£20 000 and low level of education if one or both parents had left school without qualifications (GCSEs or O-levels at 16 years).
Family history was assessed by asking parents to report on the presence of any mental health problem (ADHD, depression, anxiety, schizophrenia, mania/bipolar disorder and autism) for all first-degree relatives of the index child. This information was used to define the presence of psychiatric disorder in any first-degree relative. Mothers and fathers (where available) also provided data on their own current (in the past 6 months) and childhood ADHD symptoms. 10,Reference Barkley and Murphy26 Symptoms that were present at both time points (childhood onset symptoms that continued until adult life) were summed to give a total number of maternal and paternal ADHD symptoms Reference Kessler, Adler, Barkley, Biederman, Conners and Demler27 and these scores were adjusted for parental age using linear regression analysis.
CNV identification
The ADHD sample used in this study is an extension of the n = 366 previously analysed for large, rare CNVs and therefore all quality control and CNV detection protocols were identical to those previously described. Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 Briefly, single nucleotide polymorphisms were genotyped using the Human660W-Quad BeadChip (Illumina, San Diego, California, USA) and BeadStudio was used to call genotypes, normalise the signal intensity data and determine the log R ratio and B allele frequency at each single nucleotide polymorphism according to the standard Illumina protocols. Copy number variants were defined by PennCNV 25 with loci spanning at least 15 consecutive informative single nucleotide polymorphisms, those having copy number calls <2 and >2 being classed as deletions and duplications respectively. All samples with a single nucleotide polymorphism call rate <0.95, a high standard deviation in their genome-wide log R ratio (>0.30), carrying more than 30 apparent CNVs over 100 kb or any duplicate or related samples (identity by descent >0.3) were excluded prior to analysis (n = 40). Finally, the application of a number of CNV validation and quality control procedures (full details previously published) Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 allowed the definition of the set of large (classified as those >500 kb), rare (<1% frequency) CNVs that were used in this analysis.
Statistical analyses
The ADHD sample was divided into those with (n = 77) and without (n = 490) at least one large, rare CNV, greater than 500 kb in size. The two groups were compared on each of the clinical, cognitive, pre- and perinatal, developmental and family factors. All analyses are presented for raw scores/data for ease of interpretation. To avoid unnecessary multiple testing, the primary analyses were based on quantitative measures where possible and categorical definitions of the same constructs were subsequently tested only if initial association had been found. Where a variable was not normally distributed, the scores were natural logarithmically transformed (ln x) and analyses were run on transformed scores. Continuous measures were compared across groups using t-tests and categorical variables were examined using Pearson chi-squared tests.
As ADHD (like autism and schizophrenia) is associated with lower IQ and its prevalence is higher in individuals with intellectual disability (full-scale IQ <70), Reference Simonoff, Pickles, Wood, Gringras and Chadwick29 we did not exclude children with intellectual disability from this study. Results are, however, separately presented for individuals without intellectual disability (n = 473, of whom 54 have large, rare CNVs) as well as for the full sample. IQ test scores were not available for 42 children. All analyses were undertaken using SPSS, version 16 for Windows. To take into account multiple testing, using Bonferroni correction for the number of variables tested, alpha was set at P = 0.002 (0.05/27).
Results
Sample description
Of the total sample of 567 children, 492 (86.8%) were boys. At the time of assessment, 71.3% (n = 404) currently met criteria for DSM-IV ADHD combined type, 8.3% (n = 48) for inattentive type, 11.8% (n = 67) for hyperactive-impulsive type and 92.9% (n = 527) for current DSM-III-R ADHD. The rates of comorbid disorder were 43% (n = 244) for current DSM-IV oppositional defiant disorder, 15% (n = 85) for conduct disorder, 5.1% (n = 29) for any anxiety disorder and 0.7% (n = 4) for any depressive disorder. Of the total sample, 52 were defined as having intellectual disability (IQ <70), and 473 had IQ test scores of 70 or above (42 had scores missing for the Weschler Intelligence Scale for Children). Reference Weschler18,Reference Weschler19 Overall, 490 (86.4%) children had no large, rare CNV, 69 (12.2%) had one CNV and 8 (1.4%) had two CNVs (see online Table DS1 for a full list of CNVs). The proportion of CNV carriers was similar in this extended sample (13.6%) to our previously reported rate (14% cases v. 7% controls). Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9
Clinical and cognitive characteristics of children with and without large, rare CNVs
Table 1 shows the clinical and cognitive characteristics of children with and without CNVs. As age and gender were not associated with CNV presence, these variables were not included as covariates in subsequent analyses. As previously observed, Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 CNVs were significantly more common in children with intellectual disability (IQ <70) but were not restricted to this group. The majority of CNV carriers in our sample (n = 54, 70.1%) were still in the group of children without intellectual disability. There were associations with lower IQ and reading ability test scores but these did not reach statistical significance following Bonferroni correction. The IQ distributions in the total sample of children with ADHD and the subgroup of CNV carriers are shown in Figs 1 and 2. There were no other clinical phenotypic differences in children with and without CNVs.
When children with intellectual disability were excluded, CNV carriers were still not distinguishable on any clinical characteristic, although CNV carriers showed lower reading ability but not lower IQ scores. The number of children with CNVs in loci previously implicated in autism and schizophrenia was small (n = 20) and thus results need to be interpreted with caution. When this group (without intellectual disability) was clinically compared with non-CNV carriers, the pattern of results was similar. No significant differences (after correction for multiple testing) were detected, although CNV carriers showed lower reading ability and, interestingly, ASQ scores (none had autism) were higher (online Table DS2).
Pre-/perinatal, developmental and family factors
Table 2 shows that children with CNVs could not be distinguished on any pre-or perinatal factor, by an atypical early developmental history or by familial loading for psychiatric disorder.
Discussion
Children with ADHD who carry large, rare CNVs were found to be indistinguishable from children without CNVs in terms of the pattern and severity of ADHD symptoms and psychiatric comorbidity. Nor were there any pre-/perinatal, developmental or familial markers of large, rare CNVs. This suggests that large, rare CNVs do not appear to be associated with an atypical form of disorder.
The IQ scores for this sample were normally distributed and, in keeping with previous studies of ADHD, Reference Biederman, Faraone, Mick, Williamson, Wilens and Spencer30,Reference Kuntsi, Eley, Taylor, Hughes, Asherson and Caspi31 the IQ distribution was comparable to the normal population but with a lower mean. Carriers of large, rare CNVs had a significantly higher rate of intellectual disability (as previously reported in a subsample of the present study) and there were associations with lower IQ scores and reading ability, although the latter did not survive correction for multiple testing. However, there was no relationship between possession of CNVs and IQ in those without intellectual disability. The higher rate of CNVs in children with ADHD and intellectual disability might be explained by several factors. First, as shared genetic and familial risk factors contribute to the association between ADHD and lower IQ in the general population, Reference Kuntsi, Eley, Taylor, Hughes, Asherson and Caspi31,Reference Wood, Rijsdijk, Johnson, Andreou, Albrecht and Arias-Vasquez32 CNVs might have a common risk effect on these two different but related neurodevelopmental problems. Given that we have previously observed an overlap of CNVs found in ADHD with those found in schizophrenia and autism, Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 the hypothesis that CNVs influence multiple neurodevelopmental phenotype outcomes is plausible. Reference O'Donovan, Kirov and Owen33 A second possibility is that CNVs might be increasing ADHD risk through effects on cognition and learning. Reference O'Donovan, Kirov and Owen33 This would explain our observed association between CNVs, intellectual disability and reading problems. However, the lack of association between the presence of CNVs and IQ in children without intellectual disability is inconsistent with this view. Overall, regardless of explanation, the results suggest that large, rare CNVs in ADHD, although more common in individuals with intellectual disability, are not confined to this group and are relevant across the IQ spectrum. Nevertheless, given the high rate of large, rare CNVs in children with intellectual disability, as we have previously highlighted, Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 this group of children (with both ADHD and intellectual disability) might benefit from a clinical genetics opinion.
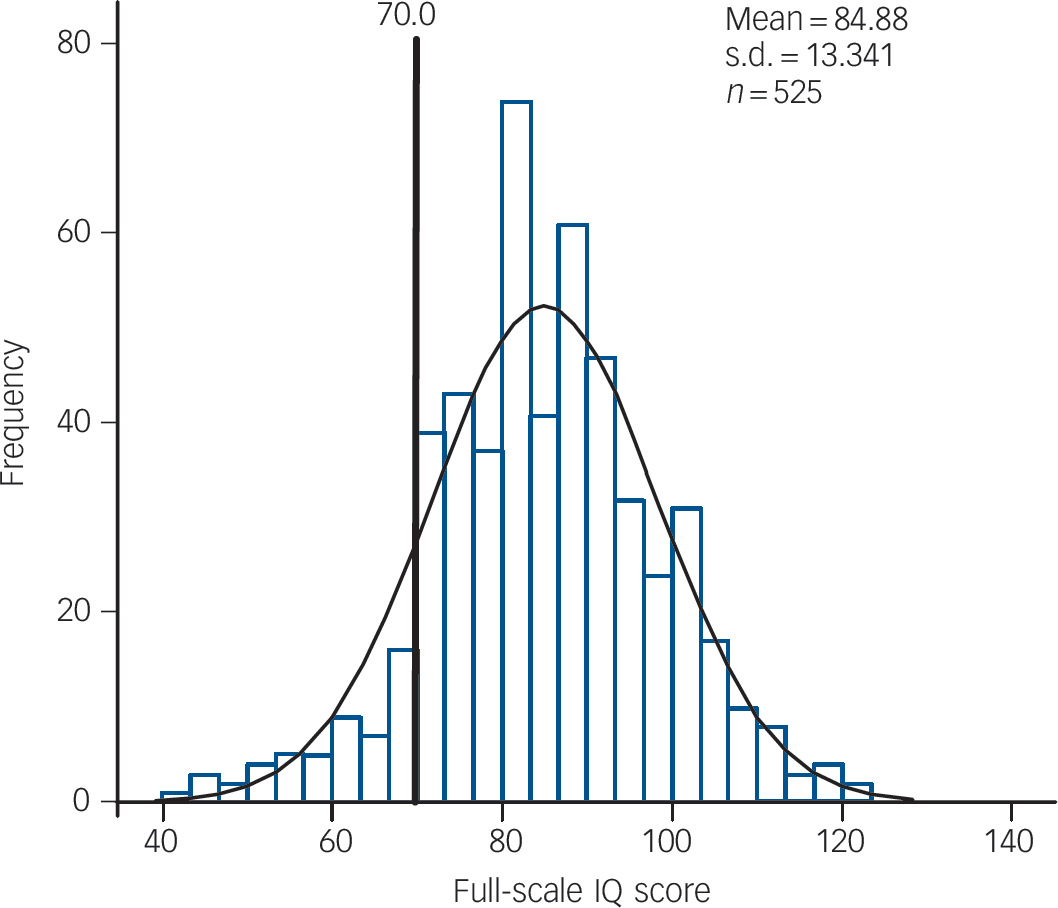
Fig. 1 Distribution of IQ scores of the full sample.
As already highlighted, structural variants including large, rare CNVs are increasingly being considered to contribute to the risk of ADHD, autism and schizophrenia. Reference Abrahams and Geschwind1–Reference Williams, Zaharieva, Martin, Langley, Mantripragada and Fossdal9 It had been well recognised, prior to the recent CNV studies, that there are specific ‘syndromic’ types of ADHD, autism and schizophrenia that appear to be caused by structural chromosomal abnormalities. For example, chromosome 22q11 microdeletion syndrome (velocardiofacial syndrome) is associated with increased risks of ADHD and schizophrenia. Reference Vorstman, Morcus, Duijff, Klaassen, Heineman-de Boer and Beemer34 There is some work, however, to
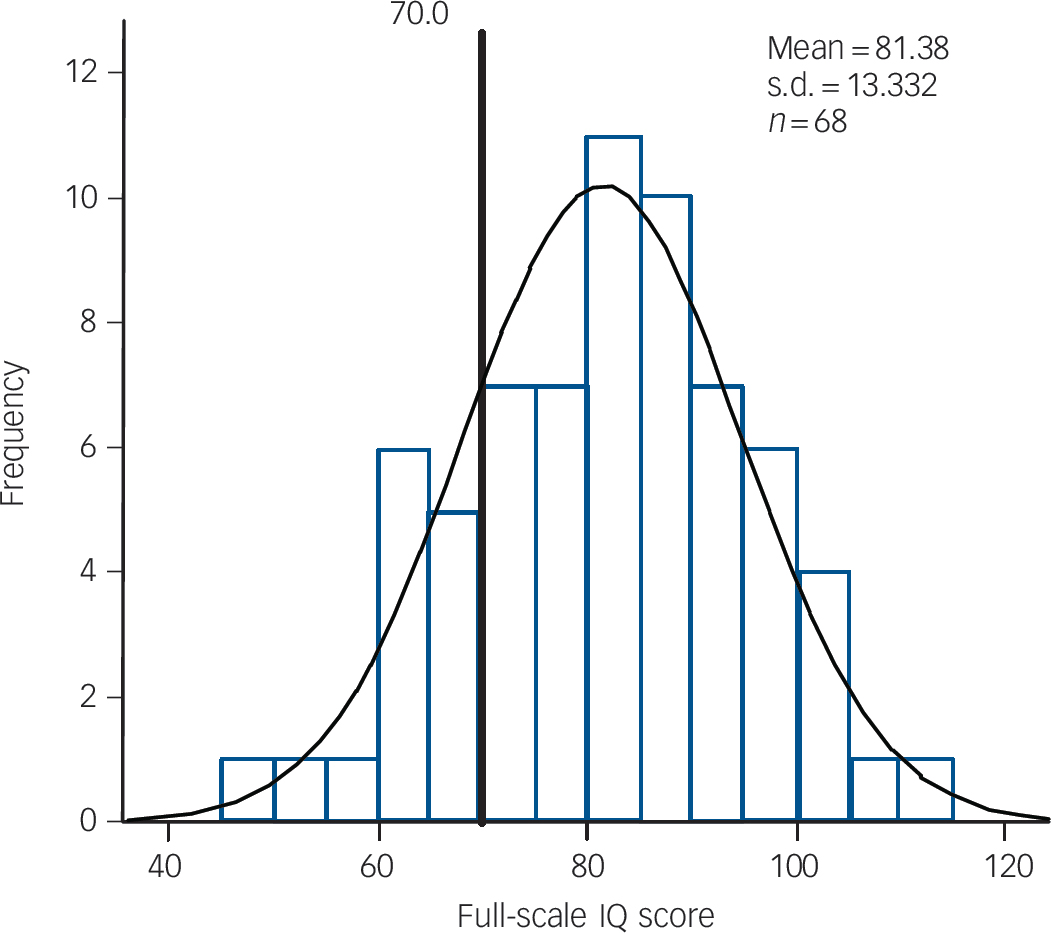
Fig. 2 Distribution of IQ scores of all the children with copy number variants.
Table 1 Clinical and cognitive characteristics of children with and without copy number variants (CNVs)
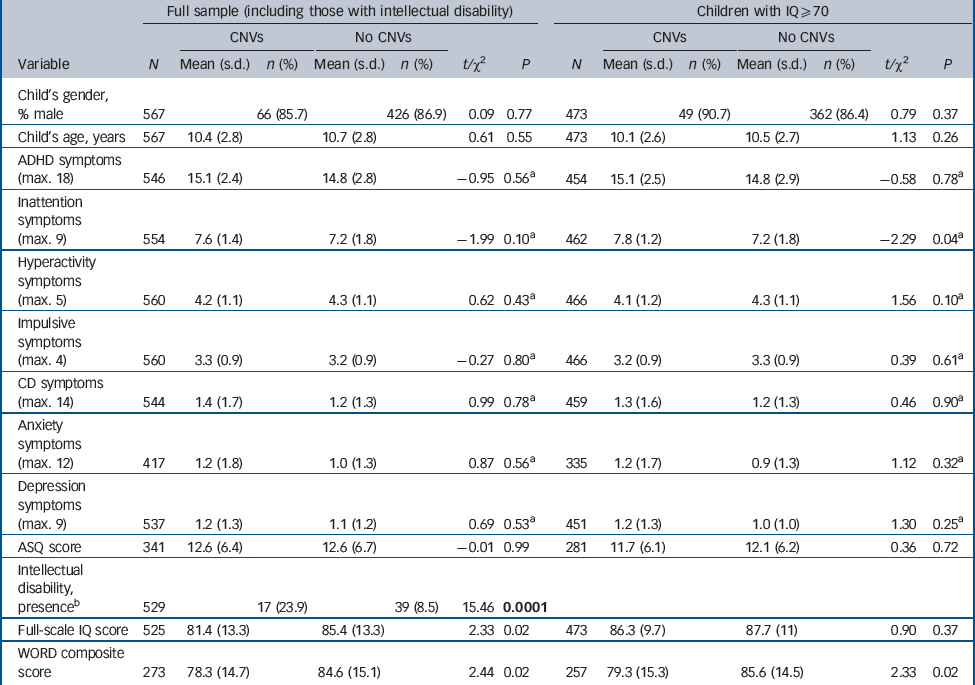
| Full sample (including those with intellectual disability) | Children with IQ ≥ 70 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNVs | No CNVs | CNVs | No CNVs | |||||||||||
| Variable | N | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | t/χ2 | P | N | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | t/χ2 | P |
| Child's gender, % male | 567 | 66 (85.7) | 426 (86.9) | 0.09 | 0.77 | 473 | 49 (90.7) | 362 (86.4) | 0.79 | 0.37 | ||||
| Child's age, years | 567 | 10.4 (2.8) | 10.7 (2.8) | 0.61 | 0.55 | 473 | 10.1 (2.6) | 10.5 (2.7) | 1.13 | 0.26 | ||||
| ADHD symptoms (max. 18) | 546 | 15.1 (2.4) | 14.8 (2.8) | –0.95 | 0.56 a | 454 | 15.1 (2.5) | 14.8 (2.9) | –0.58 | 0.78 a | ||||
| Inattention symptoms (max. 9) | 554 | 7.6 (1.4) | 7.2 (1.8) | –1.99 | 0.10 a | 462 | 7.8 (1.2) | 7.2 (1.8) | –2.29 | 0.04 a | ||||
| Hyperactivity symptoms (max. 5) | 560 | 4.2 (1.1) | 4.3 (1.1) | 0.62 | 0.43 a | 466 | 4.1 (1.2) | 4.3 (1.1) | 1.56 | 0.10 a | ||||
| Impulsive symptoms (max. 4) | 560 | 3.3 (0.9) | 3.2 (0.9) | –0.27 | 0.80 a | 466 | 3.2 (0.9) | 3.3 (0.9) | 0.39 | 0.61 a | ||||
| CD symptoms (max. 14) | 544 | 1.4 (1.7) | 1.2 (1.3) | 0.99 | 0.78 a | 459 | 1.3 (1.6) | 1.2 (1.3) | 0.46 | 0.90 a | ||||
| Anxiety symptoms (max. 12) | 417 | 1.2 (1.8) | 1.0 (1.3) | 0.87 | 0.56 a | 335 | 1.2 (1.7) | 0.9 (1.3) | 1.12 | 0.32 a | ||||
| Depression symptoms (max. 9) | 537 | 1.2 (1.3) | 1.1 (1.2) | 0.69 | 0.53 a | 451 | 1.2 (1.3) | 1.0 (1.0) | 1.30 | 0.25 a | ||||
| ASQ score | 341 | 12.6 (6.4) | 12.6 (6.7) | –0.01 | 0.99 | 281 | 11.7 (6.1) | 12.1 (6.2) | 0.36 | 0.72 | ||||
| Intellectual disability, presence b | 529 | 17 (23.9) | 39 (8.5) | 15.46 | 0.0001 | |||||||||
| Full-scale IQ score | 525 | 81.4 (13.3) | 85.4 (13.3) | 2.33 | 0.02 | 473 | 86.3 (9.7) | 87.7 (11) | 0.90 | 0.37 | ||||
| WORD composite score | 273 | 78.3 (14.7) | 84.6 (15.1) | 2.44 | 0.02 | 257 | 79.3 (15.3) | 85.6 (14.5) | 2.33 | 0.02 | ||||
ADHD, attention-deficit hyperactivity disorder; ASQ, Autism Screening Questionnaire; CD, conduct disorder; max., maximum; WORD, Wechsler Objective Reading Dimensions scale.
a Analysis on transformed data.
b Four individuals known to have intellectual disability based on testing by the clinic (e.g. British Ability Scale) but with no study IQ test scores available.
suggest that the pattern of clinical features of ADHD might be different in this group from those with ‘idiopathic ADHD’. One study found that children with ADHD and velocardiofacial syndrome, when compared with idiopathic ADHD, showed higher rates of ADHD inattentive type and comorbidity. Reference Antshel, Faraone, Fremont, Monuteaux, Kates and Doyle35 In terms of symptom presentation, we found that children with ADHD who possess large, rare CNVs when ascertained from non-specialist clinics were clinically indistinguishable from those who do not show such mutations. However, the mean level of inattention symptoms was higher in the CNV carriers and it is possible that our sample size was not large enough to detect a significant difference. They also did not differ in terms of familial loading for ADHD and other psychiatric disorders. These results are in keeping with the view that these individuals do not have atypical, rare, syndromic forms of ADHD.
There are only a few studies investigating the phenotypic manifestations of CNVs in other disorders. One study was based on 100 individuals with autism-spectrum disorder. Reference Qiao, Riendeau, Koochek, Liu, Harvard and Hildebrand36 The sample was ascertained for developmental complexity and CNVs were rated as present if considered pathogenic (de novo or absent in controls) but psychiatric phenotype data were not available. Those with pathogenic CNVs were most strongly distinguished by a higher rate of intellectual disability but not by pre-/perinatal factors. Other studies of autism-spectrum disorder have been mixed. Reference Lintas and Persico37 Interestingly, some studies have found associations between specific CNVs and larger and smaller head sizes in intellectual disability and schizophrenia. Reference McCarthy, Makarov, Kirov, Addington, McClellan and Yoon38 We did not have measures of head circumference. Others have also suggested that higher paternal age is associated with autism Reference Reichenberg, Gross, Weiser, Bresnahan, Silverman and Harlap39 and schizophrenia, Reference Malaspina, Harlap, Fennig, Heiman, Nahon and Feldman40 but not ADHD, Reference Gabis, Raz and Kesner-Baruch41 and it is this that might result in increased rates of mutations in sperm and offspring genomic alterations. Reference Hultman, Sandin, Levine, Lichtenstein and Reichenberg42 In our study, there was no relationship between those carrying a CNV and older parental age.
Although our sample is the largest CNV study of ADHD published to date, there are a number of limitations. First, the sample size may have been too small to distinguish phenotypic differences of small effect size. Second, we did not have data on whether the CNVs in this full sample were de novo or inherited to test these separately. A challenge to examining parent of origin effects in ADHD in UK community clinic samples is that many fathers are not available. We also had no formal assessments of dysmorphic features or detailed assessments of subtle congenital anomalies. Another issue is that in most CNV studies, including our own, large, rare CNVs are located at different chromosomal loci and it is likely that all CNVs are not equally predisposing. Finally, although an increased burden of CNVs has been reported in ADHD and other neurodevelopmental disorders, it is difficult to assess whether they play a causal role. We cannot determine that by observing association alone.
In summary, rare structural variants have increasingly been implicated as contributing to ADHD and other neurodevelopmental disorders. However, it is not known whether the form of ADHD that occurs in carriers of large, rare CNVs is typical of the disorder as a whole. Our results suggest that, apart
Table 2 Pre-/perinatal, developmental and family characteristics of children with and without copy number variants (CNVs)
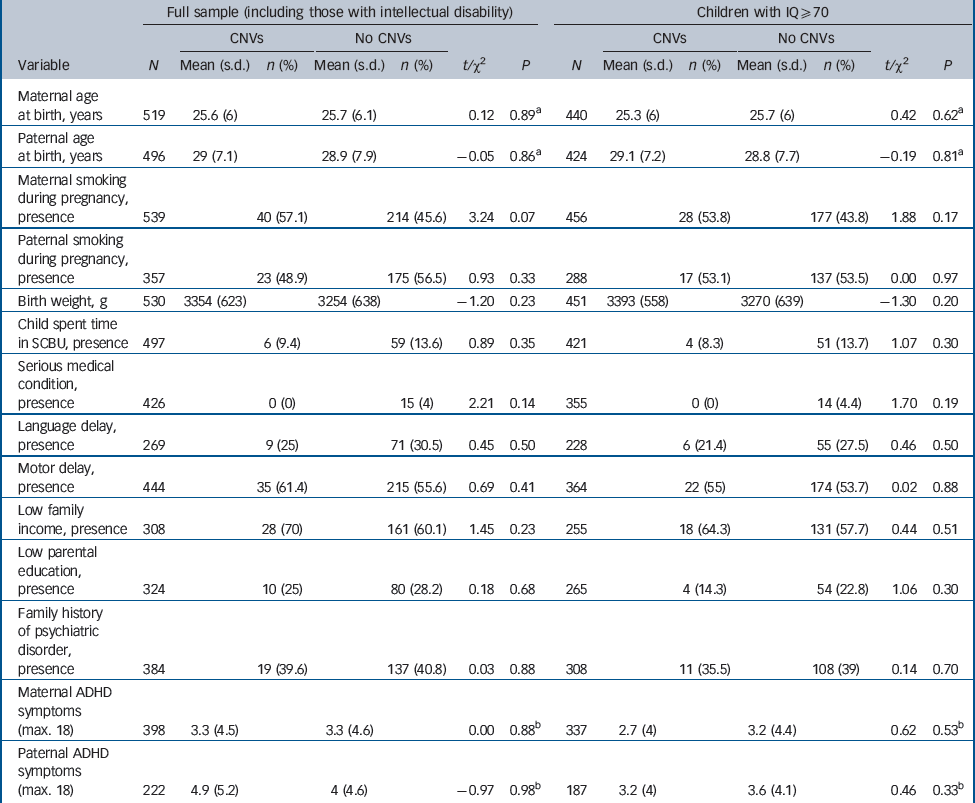
| Full sample (including those with intellectual disability) | Children with IQ ≥ 70 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNVs | No CNVs | CNVs | No CNVs | |||||||||||
| Variable | N | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | t/χ2 | P | N | Mean (s.d.) | n (%) | Mean (s.d.) | n (%) | t/χ2 | P |
| Maternal age at birth, years | 519 | 25.6 (6) | 25.7 (6.1) | 0.12 | 0.89 a | 440 | 25.3 (6) | 25.7 (6) | 0.42 | 0.62 a | ||||
| Paternal age at birth, years | 496 | 29 (7.1) | 28.9 (7.9) | –0.05 | 0.86 a | 424 | 29.1 (7.2) | 28.8 (7.7) | –0.19 | 0.81 a | ||||
| Maternal smoking during pregnancy, presence | 539 | 40 (57.1) | 214 (45.6) | 3.24 | 0.07 | 456 | 28 (53.8) | 177 (43.8) | 1.88 | 0.17 | ||||
| Paternal smoking during pregnancy, presence | 357 | 23 (48.9) | 175 (56.5) | 0.93 | 0.33 | 288 | 17 (53.1) | 137 (53.5) | 0.00 | 0.97 | ||||
| Birth weight, g | 530 | 3354 (623) | 3254 (638) | –1.20 | 0.23 | 451 | 3393 (558) | 3270 (639) | –1.30 | 0.20 | ||||
| Child spent time in SCBU, presence | 497 | 6 (9.4) | 59 (13.6) | 0.89 | 0.35 | 421 | 4 (8.3) | 51 (13.7) | 1.07 | 0.30 | ||||
| Serious medical condition, presence | 426 | 0 (0) | 15 (4) | 2.21 | 0.14 | 355 | 0 (0) | 14 (4.4) | 1.70 | 0.19 | ||||
| Language delay, presence | 269 | 9 (25) | 71 (30.5) | 0.45 | 0.50 | 228 | 6 (21.4) | 55 (27.5) | 0.46 | 0.50 | ||||
| Motor delay, presence | 444 | 35 (61.4) | 215 (55.6) | 0.69 | 0.41 | 364 | 22 (55) | 174 (53.7) | 0.02 | 0.88 | ||||
| Low family income, presence | 308 | 28 (70) | 161 (60.1) | 1.45 | 0.23 | 255 | 18 (64.3) | 131 (57.7) | 0.44 | 0.51 | ||||
| Low parental education, presence | 324 | 10 (25) | 80 (28.2) | 0.18 | 0.68 | 265 | 4 (14.3) | 54 (22.8) | 1.06 | 0.30 | ||||
| Family history of psychiatric disorder, presence | 384 | 19 (39.6) | 137 (40.8) | 0.03 | 0.88 | 308 | 11 (35.5) | 108 (39) | 0.14 | 0.70 | ||||
| Maternal ADHD symptoms (max. 18) | 398 | 3.3 (4.5) | 3.3 (4.6) | 0.00 | 0.88 b | 337 | 2.7 (4) | 3.2 (4.4) | 0.62 | 0.53 b | ||||
| Paternal ADHD symptoms (max. 18) | 222 | 4.9 (5.2) | 4 (4.6) | –0.97 | 0.98 b | 187 | 3.2 (4) | 3.6 (4.1) | 0.46 | 0.33 b | ||||
ADHD, attention-deficit hyperactivity disorder; max., maximum; SCBU, special care baby unit.
a Analysis on transformed data.
b Analysis on transformed data corrected for parental age.
from an increased risk of CNVs in children with comorbid intellectual disability, large, rare CNVs do not appear to be restricted to an atypical subgroup of children with ADHD. These findings have implications for selecting populations for CNV screening and suggest that other classes of rare mutations might also be found in typical clinical cases.
Funding
This work has been funded by the Wellcome Trust, Action Research and Baily Thomas Charitable Trust.
Acknowledgements
We are very grateful to all the families who participated in this project and the clinicians who supported the work. We thank Dr Irina Zaharieva for genotyping the samples, Ms Emma Evans for initial assistance with the data and Dr Ajay Thapar for rating the medical disorders.







eLetters
No eLetters have been published for this article.