Polycyclic aromatic hydrocarbons (PAH), a group of over 100 different chemicals formed during incomplete combustion of organic matter, have potential toxicity, mutagenic and carcinogenic properties, and are widely distributed in the environment, including the food chain(1). Among these compounds, benzo(a)pyrene (BaP) is best known and has been widely used as a marker of exposure and to analyse the health effects of carcinogenic PAH in food(2).
Several studies have shown that both active and passive tobacco smoke are major sources of exposure to PAH(Reference Lodovici, Akpan and Evangelisti3, Reference Suwan-ampai, Navas-Acien and Strickland4). Other than tobacco smoke, diet is the main source of human exposure to PAH in the non-occupationally exposed populations(1, Reference Phillips5, Reference Suzuki and Yoshinaga6). Foods can be contaminated by PAH present in polluted air, soil or water. PAH are also formed in foods as a consequence of processing and cooking methods such as drying, smoking, grilling, roasting or frying. The amount of PAH produced during cooking rises with increased fat content, longer exposure to flames and proximity to the heat source(7).
The carcinogenic effects of exposure to PAH have been exhaustively studied and evaluated(1, 2). However, there is evidence, although limited, on other possible child health effects of prenatal exposure to PAH. Both laboratory and recent epidemiological studies have found associations between prenatal exposure to PAH and adverse reproductive or child health outcomes, including low birth weight and length, preterm birth, reduced head circumference at birth and lower scores on childhood tests of neurodevelopment(Reference Choi, Jedrychowski and Spengler8–Reference Perera, Rauh and Whyatt10). These associations have been observed for airborne PAH as well as for DNA adducts, the latter serving as a marker of total PAH exposure, including both airborne and dietary sources. It is uncertain whether there may be adverse health effects of prenatal exposure arising principally from dietary sources. Moreover, it remains uncertain to what extent intake of micronutrients involved in antioxidant pathways may help to reduce adverse health effects of PAH exposure. Supplementation with vitamin C or higher levels of plasma vitamin A have been associated with lower levels of BaP–DNA adducts(Reference Mooney, Madsen and Tang11, Reference Sram, Farmer and Singh12).
Dietary intake of BaP and total PAH in adults has been estimated in several studies(Reference Phillips5, Reference Ibanez, Agudo and Berenguer13–Reference Falco, Domingo and Llobet17). However, no study has estimated the dietary intake of these compounds among pregnant women. The present study aimed to estimate the dietary intake of BaP and total PAH in a population of pregnancy women, identifying the main dietary sources of these compounds and characterising factors associated with higher intake among women with different tobacco smoke exposure. We also examined the dietary intake of antioxidants in women with different exposure to tobacco. Finally, in order to determine factors that explain variability in intake of PAH, associations between PAH intake and food consumption were assessed.
Materials and methods
Population and study design
Data come from a population-based birth cohort established in Sabadell (Catalonia, Spain) as part of the INMA – INfancia y Medio Ambiente (Environment and Childhood) Project. Methods have been described previously(Reference Ribas-Fito, Ramon and Ballester18). Briefly, between July 2004 and July 2006, 657 women were recruited during their first trimester of pregnancy while attending their first ultrasound visit. Questionnaires on dietary habits, socio-economic characteristics, health status, reproductive history, smoking habits during pregnancy and other factors were administered by trained interviewers during the first and third trimesters of pregnancy. Data on tobacco smoke exposure were available for 612 women (93·2 %). Women who smoked throughout pregnancy were defined as current active smokers. The present study was approved by the Clinical Research Ethical Committee of the Municipal Institute of Health Care, and informed consent was signed by all participants.
Dietary assessment
Individual dietary consumption was assessed during recruitment using a 101-item semi-quantitative FFQ that was previously validated in Spanish adults(Reference Vioque19). Items were classified into seventeen food groups: meats, seafood, dairy products, cereals/potatoes, fruit, vegetables, eggs, legumes, dairy desserts, nuts, fats/oils, beverages, alcoholic beverages, sugar, sauces, sweet and salty snacks. Since there were important differences in PAH concentrations among food items classified within the same broad group (e.g. processed/cured meats v. other meats), subcategories were created for the following food groups: meats, seafood, dairy products, cereals/potatoes and fruit. Frequencies of intake were reported using nine categories ranging from ‘never or fewer than once a month’ to ‘6 times or more per day’. Women were asked for their usual intake frequency of each food item since the start of pregnancy. Daily intake (g/d) was estimated using reference portion sizes typical in Spanish adults(20). In addition, daily intakes of energy, fat (as PAH are lipophilic)(1), folate and several antioxidant micronutrients (β-carotene and vitamin C) were estimated. Folate may be involved in enzymes responsible for detoxification of contaminants(Reference Fenech21).
Estimation of total polycyclic aromatic hydrocarbons and benzo(a)pyrene intake
First, a compilation of all available data on food concentrations of the sum of up to sixteen PAH (benz(a)anthracene, benzo(b)fluoranthene, benzo(j)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene, benzo(a)pyrene, chrysene, cyclopenta(c,d)pyrene, dibenz(a,h)anthracene, dibenzo(a,e)pyrene, dibenzo(a,h)pyrene, dibenzo(a,i)pyrene, dibenzo(a,l)pyrene, indeno(1,2,3-cd)pyrene, 5-methylchrysene, 7H-benzo(c)fluorene) and BaP individually were undertaken to construct a food composition table with estimated levels of PAH in the 101 food items in the FFQ. Exclusion criteria for the selection of eligible values included (i) values published before 1990 and (ii) values from possibly highly polluted settings (e.g. foods locally produced in oil-producing countries such as Kuwait)(Reference Husain, Naeemi and Dashti22). In addition, several extreme outliers (e.g. three fold higher than all other published data in foods with elevated concentrations) were also excluded to avoid unduly influencing the mean values. When published values were below detection limits, or not detectable, half the detection limit was assigned (see Appendix 1 for sources of published data). Average concentrations for the sum of PAH (hereafter ‘total PAH’) and BaP in each food were calculated using (i) all available eligible data and (ii) all available data excluding the highest and the lowest value – a trimmed mean (TM). When no data were available for a food item in the FFQ, concentrations of PAH were imputed from similar items (e.g. values for skimmed milk were imputed from semi-skimmed milk).
Finally, daily PAH intake was estimated by multiplying food item concentrations of BaP and total PAH by intake in grams for each woman. For example, in women who report consuming a standard serving of cured ham (50 g, about two to three slices) once a day, given a concentration of 0·077 μg/100 g, the mean daily intake of BaP from this food would be 0·038 μg. The total dietary intake of these compounds was assessed by summing the intake of all food items. The mean concentrations of BaP and total PAH in each food group were also calculated and expressed as μg/100 g.
Other variables
Pre-pregnancy BMI (self-reported weight (kg)/height (m2)), age, social class based on occupation, educational level (university, secondary, primary or below), region of origin (Europe, Latin America) and parity were collected by questionnaire in the first trimester of pregnancy. Social class was classified based on the Spanish Society of Epidemiology guidelines(23). Tobacco smoke exposure was collected during the third trimester and categorised into three groups: (i) active smokers (women who reported any smoking during pregnancy); (ii) passive smokers (women residing with an active smoker or exposed to tobacco smoke at their place of work); and (iii) non-smokers unexposed to passive smoke.
Statistical analysis
The intake of BaP and total PAH, food groups, micronutrients (folate, β-carotene, vitamin C), total fat and energy was estimated for non-smokers, passive and active smokers and expressed as means and standard deviations. One-way ANOVA was used to identify significant differences in intake among smoking groups. To identify the main food sources of BaP and total PAH, the mean intake of these compounds (μg/d) and the proportion contributed to total intake were calculated for each food group stratified by tobacco smoke exposure. Multiple linear regression models were used to assess whether tobacco smoke exposure was associated with higher intake of BaP and total PAH after adjusting for education, region of origin and age. Several potential covariates that were not significantly related to PAH intake (social class, BMI, marital status and parity) were excluded from the final model for parsimony. In separate models, associations between food group intake and intake of BaP and total PAH were estimated in order to determine which food groups were most predictive of high dietary PAH intake among pregnant women. We also used multivariate linear models to analyse the association between dietary PAH and birth weight, adjusting for tobacco use and the covariates listed above; preterm births were excluded from the present analysis. Data were analysed using the STATA Statistical software package version 10·1 (Stata Corp., College Station, TX, USA).
Results
Figure 1 shows the mean concentrations of BaP and total PAH estimated for each food group (see Appendix 2 for items included in each food group). The food group with the highest levels of BaP was shellfish (0·110 μg/100 g), followed by alcoholic beverages (0·077 μg/100 g) and processed fish (0·043 μg/100 g). For total PAH, the highest levels were found in processed fish (2·358 μg/100 g), processed/cured meats (2·124 μg/100 g) and nuts (1·528 μg/100 g). Using the more conservative estimation of BaP concentrations (the TM, which excluded the highest and the lowest published values) did not influence the ranking of the top three contaminated food groups. For total PAH, use of the TM levels changed this ranking slightly, with nuts, processed fish and bread becoming the most contaminated food groups.
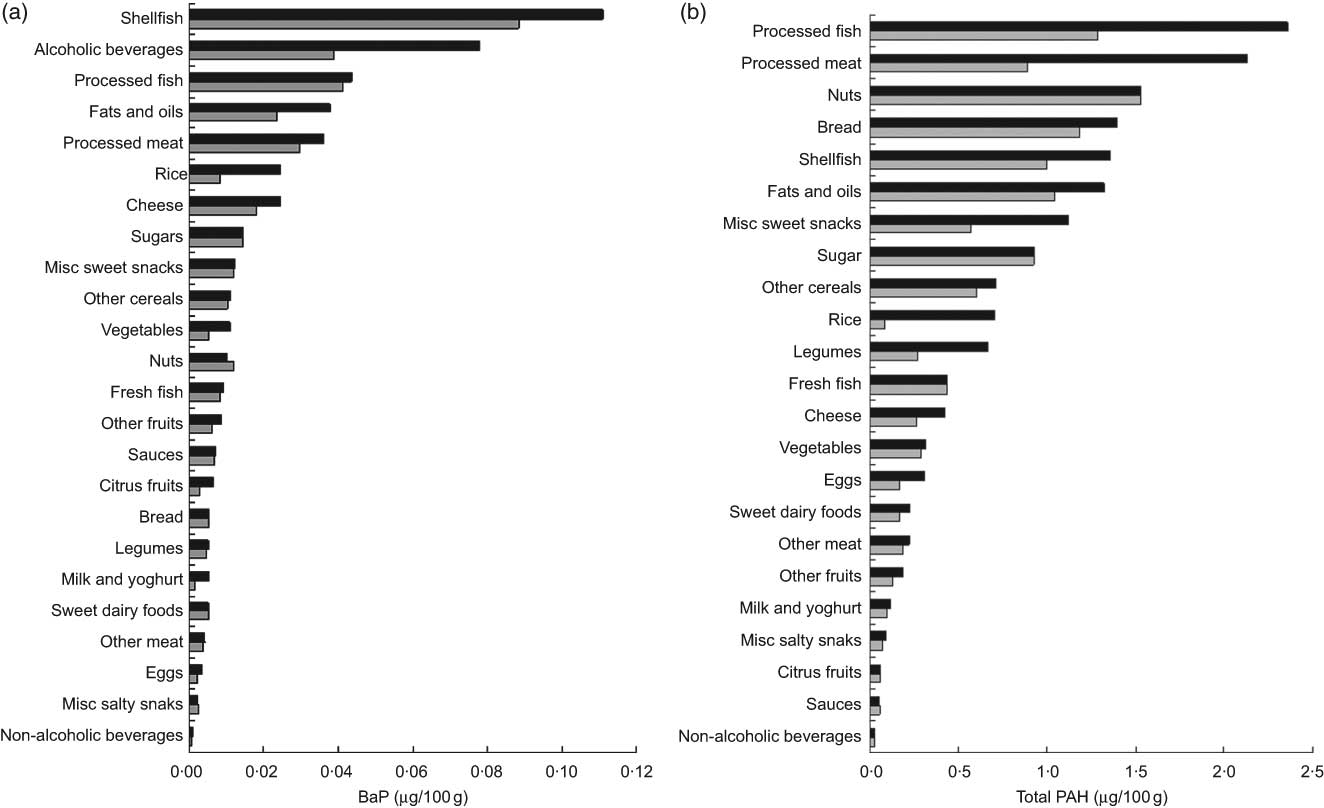
Fig. 1 Mean concentration (μg/100 g) of (a) benzo(a)pyrene (BaP) (![]() , BaP;
, BaP; ![]() , BaP trimmed mean) and (b) total polycyclic aromatic hydrocarbons (total PAH) (
, BaP trimmed mean) and (b) total polycyclic aromatic hydrocarbons (total PAH) (![]() , total PAH;
, total PAH; ![]() , total PAH trimmed mean) for food groups used to estimate intake of these compounds among pregnant women from the INMA–Sabadell cohort
, total PAH trimmed mean) for food groups used to estimate intake of these compounds among pregnant women from the INMA–Sabadell cohort
As shown in Table 1, the mean dietary intake of BaP and total PAH was significantly higher in active smokers (0·199 and 10·207 μg/d, respectively) and passive smokers (0·197 and 9·427 μg/d) than in non-smokers (0·182 and 8·850 μg/d; P value 0·005 and < 0·001). During the third trimester, the dietary intake of BaP and total PAH was similar, remaining significantly higher among active smokers (0·196 and 11·425 μg/d, respectively) and passive smokers (0·187 and 10·167 μg/d) than in non-smokers (0·181 and 9·900 μg/d; P value 0·040 and < 0·001). For each smoking group, intake in the first v. third trimester did not differ significantly (t test, P values > 0·05). Higher PAH intake in active and passive smokers was attributable to a significantly higher consumption of processed/cured meats, shellfish, milk/yoghurt, bread, sweet dairy foods, alcoholic beverages and sugar (Table 1). In addition, both active and passive smokers had a lower intake of fruit than non-smokers (P value 0·002). Active smokers also had a lower intake of β-carotene and vitamin C than passive and non-smokers (P value < 0·05). After multivariate adjustment, both active and passive smoking during pregnancy were associated with a higher dietary intake of BaP and total PAH (Table 2). Older age, lower educational level and Latin American origin were also associated with dietary exposure to BaP.
Table 1 Mean estimated intake of BaP, total PAH, food groups and key nutrients among pregnant women with different smoking habits from the INMA–Sabadell cohort
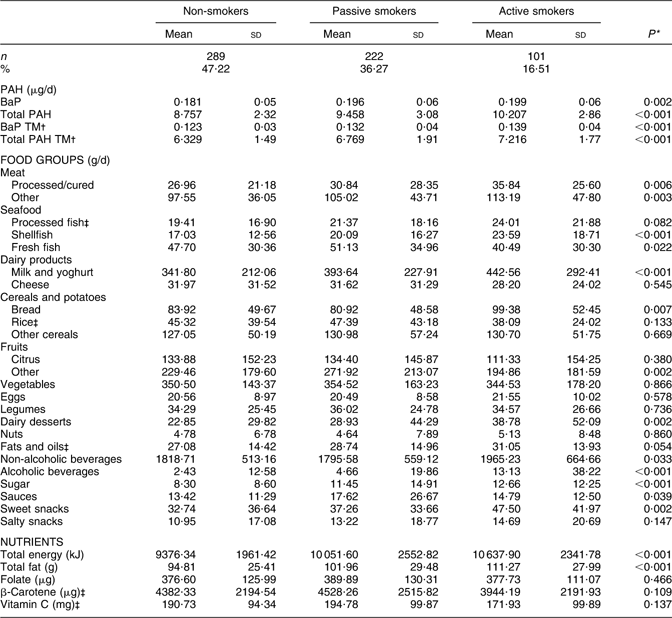
BaP, benzo(a)pyrene; PAH, polycyclic aromatic hydrocarbons; TM, trimmed mean.
PAH intakes based on concentrations in individual foods derived from all eligible published values.
*P values from ANOVA tests comparing all three groups.
†Estimated intakes based on the mean of published concentrations after excluding the highest and the lowest values.
‡t test P value <0·05 for non-smokers and passive smokers combined v. active smokers.
Table 2 Association (coefficients and se) between dietary intakes of BaP and total PAH, and sociodemographic and lifestyle factors, among pregnant women from the INMA–Sabadell cohort
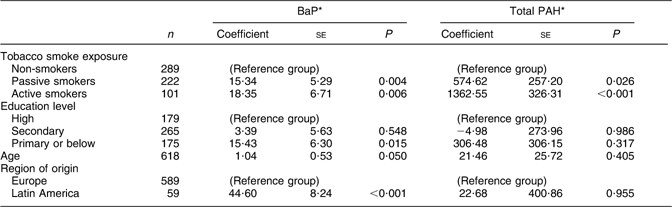
BaP, benzo(a)pyrene; PAH, polycyclic aromatic hydrocarbons.
Results from multivariate linear regression models predicting BaP and total PAH, adjusting simultaneously for all variables shown in the table.
*BaP and total PAH values converted to 10 μg/g.
Given that different dietary patterns were observed for passive and active smokers, food groups that were major contributors to PAH intake also differed to some extent among women in these smoking groups (Table 3). For BaP, the main contributors were vegetables (16·61 % and 16·27 % of total intake) and fruit (15·86 % and 15·26 %) in non-smokers and passive smokers, respectively, v. seafood and vegetables in active smokers. The major contributors to total PAH intake among women in each smoking group were processed/cured meat, cereals/potatoes and seafood (Table 3). For three of the five major contributors to PAH intake (fruit, vegetables and cereals/potatoes), the corresponding food group intake (see Table 1) was also relatively high. In contrast, the mean food intake of the other principal dietary PAH sources – shellfish and processed/cured meats – was relatively low.
Table 3 Contribution of food groups to intakes of BaP and total PAH among pregnant women with different smoking habits from the INMA–Sabadell cohort
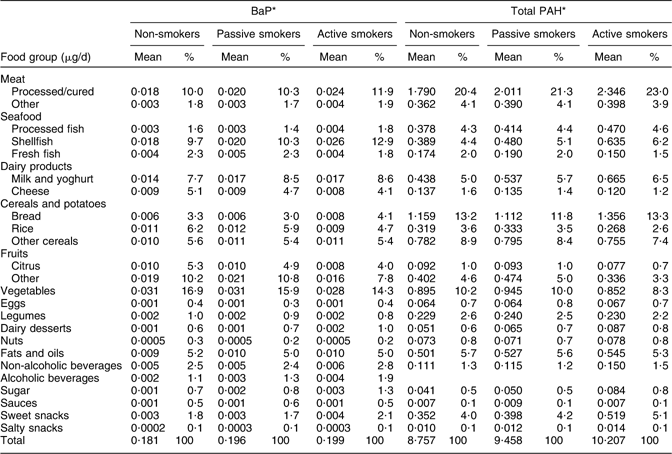
BaP, benzo(a)pyrene; PAH, polycyclic aromatic hydrocarbons.
*Percentage of BaP and total PAH intake within each smoking group.
The food groups that most strongly predicted high intake of both BaP and total PAH were shellfish and processed/cured meats (Table 4; coefficients with their standard errors for shellfish and processed/cured meats were 1·57 (se 0·06) and 0·76 (se 0·03), respectively, for BaP v. coefficients ranging from −0·11 (se 0·05) to 0·49 (se 0·06) for other food groups). Fruit, vegetables and cereals/potatoes, though important food sources of PAH, explained a relatively small portion of variability in intake, and were thus weak predictors of elevated intake.
Table 4 Food group predictors of high dietary intake of BaP and total PAH
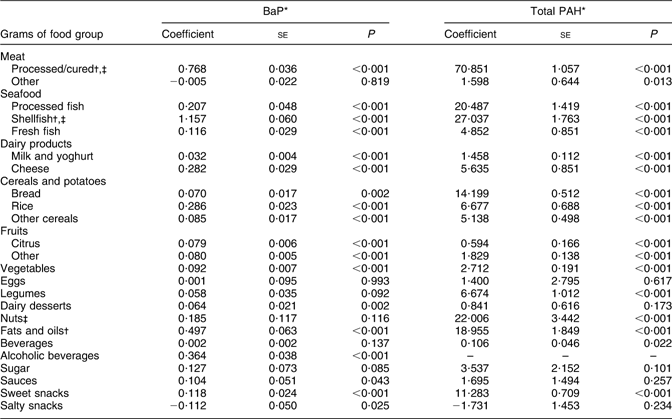
BaP, benzo(a)pyrene; PAH, polycyclic aromatic hydrocarbons.
*BaP and total PAH values converted to 10 μg/g.
†Top three predictors of BaP dietary intake.
‡Top three predictors of total PAH dietary intake.
Finally, the first trimester intake of BaP, but not total PAH, was associated with lower birth weight after adjusting for potential confounders including cigarette smoke exposure. The mean birth weight was 142·73 g lower for the fourth than the first quartile of dietary BaP exposure (P value <0·05). The dietary intake of PAH in the third trimester was not associated with birth weight.
Discussion
In this cohort of pregnant women from Spain, women who smoked actively or were exposed to passive smoke had higher exposure to dietary PAH during pregnancy than did unexposed non-smokers. Higher intake in women exposed to tobacco smoke was due mainly to their higher intake of processed meats and shellfish, both important sources of PAH exposure. Moreover, dietary BaP in the first trimester was associated with a small, but significant reduction in birth weight. Given this finding and previous evidence of possible adverse child health outcomes such as low birth weight and poor neurodevelopment associated with PAH exposure during pregnancy(Reference Choi, Jedrychowski and Spengler8–Reference Perera, Rauh and Whyatt10), the dietary contribution to PAH exposure in this population – particularly in women already exposed to PAH via tobacco smoke – is of concern. In addition to smoking habits, older maternal age, primary-school education and Latin American origin were associated with higher dietary exposure to BaP.
Estimates of daily intake of both BaP (0·19 μg/d) and total PAH (9·28 μg/d) in this population of pregnant women were in a range comparable to levels previously reported in Spain(Reference Ibanez, Agudo and Berenguer13, Reference Marti-Cid, Llobet and Castell14, Reference Falco, Domingo and Llobet17) and other European countries(Reference Lodovici, Akpan and Evangelisti3, 24–Reference Dennis, Massey and McWeeny26). For example, our estimates of dietary BaP intake were slightly lower than the estimates in the United Kingdom, Finland and the upper bound in the Netherlands (ranging from 0·25 to 0·29 μg/d) and higher than the estimates in France, Greece, Germany, Italy, Norway and previous studies from Spain (0·06–0·12 μg/d). Similarly, estimates of total PAH were lower than the upper bound in the Netherlands (17 μg/d) and slightly higher than the estimates reported in the United Kingdom, Italy, Finland and other studies from Spain (ranging from 3·00 to 8·80 μg/d)(Reference Lodovici, Akpan and Evangelisti3, Reference Ibanez, Agudo and Berenguer13, Reference Marti-Cid, Llobet and Castell14, Reference Falco, Domingo and Llobet17, 24–Reference Falco, Domingo and Llobet27). Such differences could be explained by disparities in dietary habits or the populations studied (all adults, adult women v. pregnant women), but may also be due to differences in the methods used to assess dietary intake or estimates of PAH concentrations.
In this population, cereals, processed/cured meat, shellfish, fruit and vegetables were the major contributors to total dietary daily intake of PAH. Previous Spanish studies(Reference Ibanez, Agudo and Berenguer13, Reference Marti-Cid, Llobet and Castell14, Reference Falco, Domingo and Llobet27), and other European studies(Reference Lodovici, Akpan and Evangelisti3, 24), have also found cereals to be the highest contributor to total PAH intake. However, it is important to note that while the high contribution of cereals and vegetables was due to high levels of intake (mean intake of 260·925 and 350·479 g/d for cereals and vegetables, respectively), the high contribution of processed meat and shellfish to PAH intake was due mainly to the high concentrations of these compounds in these foods, as the intake of these foods was quite low (30·137 and 19·111 g/d, respectively). Indeed, the high intake of shellfish and processed meats was more strongly predictive of the high intake of BaP and total PAH than was the high intake of cereals and vegetables. Thus, substantial reductions in PAH intake could be achieved by reducing the intake of processed meats and shellfish during pregnancy, which is in keeping with the dietary guidelines for pregnant women(Reference Kaiser and Allen28).
The results of the present study indicate that smoking during pregnancy is associated with higher dietary PAH intake in our population. However, in an earlier study in Spain, Ibáñez et al.(Reference Ibanez, Agudo and Berenguer13) did not find significant differences in PAH intake associated with smoking habits in the general population. The reasons for this difference are uncertain, although lower levels of smoking in pregnant women (16·51 % in the present study v. 25·07 % in the study of Ibáñez et al.)(Reference Ibanez, Agudo and Berenguer13) or differences in food intake in the general population v. pregnant women may be contributory factors. Although it is accepted that, barring occupational exposure, smoking is the main source of PAH exposure in active smokers, diet is a significant source of PAH exposure in both smokers and non-smokers. Previous studies have estimated the total PAH intake from cigarettes to be in the range of 0·806–0·934 μg per cigarette(Reference Ding, Yan and Jain29). Consequently, for the typical smoking woman in the present study, with a mean consumption of five cigarettes per day, the smoking-related exposure may be approximately 4·03–4·67 μg/d, and the contribution of dietary PAH (mean 10·2 μg/d) on the order of 68–72 % of total exposure. Moreover, dietary PAH has been associated with higher levels of BaP–DNA adducts in both smokers and non-smokers(Reference Pavanello, Pulliero and Saia30) and dietary intake more strongly correlated than ambient air with urinary metabolites of PAH exposure in earlier studies(Reference Suzuki and Yoshinaga6).
Passive smoke may be a substantial source of PAH exposure; it has been found that PAH concentrations are approximately 2- to 4-fold higher in sidestream smoke than in mainstream smoke(Reference Besaratinia, Kleinjans and Van Schooten31). Moreover, PAH–DNA adducts have been found in the placenta of women passively exposed to cigarette smoke during pregnancy. This suggests that involuntary exposure to tobacco smoke could affect the functions of the placental cells and consequently be a source of genotoxicity during fetal development(Reference Sanyal, Mercan and Belanger32). For this reason, the relatively high dietary exposure to PAH found not only among active smokers, but also among women with substantial passive exposure to tobacco smoke, is of concern.
Similar to our findings, numerous studies have shown that smokers generally have poorer-quality diets than non-smokers, with food choices that tend to favour energy-dense foods high in dietary fats and relatively poor in nutrient content(Reference Palaniappan, Jacobs and O’Loughlin33). Besides the higher intake of foods with high concentrations of PAH, smokers and women exposed to passive smoke also consumed lower levels of antioxidant-rich foods such as fruit and vegetables, as well as having a higher intake of dietary fat and total energy. Both the estimated intake of β-carotene and vitamin C and plasma measures of vitamin C were significantly lower in active smokers than in women who did not smoke during pregnancy. Given the potential for these nutrients to help reduce or counter the adverse effects of PAH exposure(Reference Mooney, Madsen and Tang11), this dietary pattern is an additional concern.
Since PAH concentrations in food, particularly meat, increase depending on the cooking method used(Reference Phillips5, 7), the fact that data on the use of different cooking methods were not collected is a limitation of the present study. However, estimates of the use of cooking methods associated with the highest levels of PAH, such as barbecuing(Reference Kazerouni, Sinha and Hsu34), are relatively low (1 %) in the Spanish population(Reference Rohrmann, Linseisen and Becker35). We calculated a weighted mean of BaP and total PAH for meat items based on the proportions of use of different cooking methods in the Spanish population estimated in the European Prospective Investigation into Cancer and Nutrition study(Reference Rohrmann, Linseisen and Becker35).
The strength of the present study is that we estimated the intake of dietary PAH based on concentrations in individual foods, which enabled us to report the intake for fairly refined food subgroups. Focusing on more refined food subgroups enabled us to quantify the relevance of more specific food sources of PAH exposure, such as processed/cured meats and shellfish, rather than of meats or seafood as a whole. In addition, we included an analysis to identify food groups predictive of high levels of intake, which facilitates the development of recommendations to aid pregnant women to reduce their exposure to these compounds.
In conclusion, the present study provides an estimate of dietary exposure to BaP and total PAH in pregnant Spanish women, and identifies population subgroups with the highest levels of exposure, as well as the food groups that predict dietary exposure to these compounds. The finding of higher intake among women exposed to tobacco smoke suggests that, in addition to confounding by smoking habits in research on reproductive health effects of dietary PAH exposure, dietary PAH exposure may also be an important source of additional risk in studies on effects of prenatal cigarette smoke exposure.
Acknowledgements
The present study was funded by grants from the Spanish Ministry of Health (FIS-PI041436), Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041) and the Generalitat de Catalunya-CIRIT (1999SGR 00241). None of the authors has potential conflicts of interest related to this manuscript, financial or otherwise. T.D.S. performed the literature review, statistical analysis, interpretation of findings and wrote the manuscript. M.A.M. provided substantial conceptual contributions and worked with the first author at the conceptualisation of the hypotheses and analysis plan, interpretation of the findings and writing of the manuscript. V.P. participated in the development of the PAH food composition tables. M.G., I.A., M.K. and J.S. provided substantial conceptual contributions and revisions of the manuscript drafts. J.S. is the principal investigator of the study. All authors have read and approved the final manuscript. The authors are grateful to Silvia Fochs, Anna Sànchez, Maribel López and Nuria Pey for their assistance in contacting the families and administering the questionnaires. A full roster of the INMA–Sabadell Study Investigators can be found at http://www.proyectoinma.org.
Appendix 1
Barranco A, Alonso-Salces RM, Crespo I et al. (2004) Polycyclic aromatic hydrocarbon content in commercial Spanish fatty foods. J Food Prot 67, 2786–2791.
Chen BH, Wang CY & Chiu CP (1996) Evaluation of analysis of polycyclic aromatic hydrocarbons in meat products by liquid chromatography. J Agric Food Chem 44, 2244–2251.
Dennis MJ, Massey RC, Cripps G et al. (1991) Factors affecting the polycyclic aromatic hydrocarbon content of cereals, fats and other food products. Food Addit Contam 8, 517–530.
De Vos RH, Van Dokkum W, Schouten A et al. (1990) Polycyclic aromatic hydrocarbons in Dutch total diet samples (1984–1986). Food Chem Toxicol 28, 263–268.
EU Director-General Health and Consumer Protection (2004) SCOOP Report: Collection of Occurrence Data on Polycyclic Aromatic Hydrocarbons in Food.
Falco G, Domingo JL, Llobet JM et al. (2003) Polycyclic aromatic hydrocarbons in foods: human exposure through the diet in Catalonia, Spain. J Food Prot 66, 2325–2331.
Fontcuberta M, Arques JF, Martinez M et al. (2006) Polycyclic aromatic hydrocarbons in food samples collected in Barcelona, Spain. J Food Prot 69, 2024–2028.
Garcia-Falcon MS & Simal-Gandara J (2005) Determination of polycyclic aromatic hydrocarbons in alcoholic drinks and the identification of their potential sources. Food Addit Contam 22, 791–797.
Garcia Falcon MS, Gonzalez AS, Lage Yusty MA et al. (1996) Enrichment of benzo(a)pyrene in smoked food products and determination by high-performance liquid chromatography-fluorescence detection. J Chromatogr A 753, 207–215.
Garcia Falcon MS, Gonzalez AS, Lage Yusty MA et al. (1999) Determination of benzo(a)pyrene in some Spanish commercial smoked products by HPLC-FL. Food Addit Contam 16, 9–14.
Gomaa EA, Gray JI, Rabie S et al. (1993) Polycyclic aromatic hydrocarbons in smoked food products and commercial liquid smoke flavourings. Food Addit Contam 10, 503–521.
Guillen MD & Sopelana P (2004) Occurrence of polycyclic aromatic hydrocarbons in smoked cheese. J Dairy Sci 87, 556–564.
Kayali-Sayadi MN, Rubio-Barroso S, Cuesta-Jimenez MP et al. (1998) Rapid determination of polycyclic aromatic hydrocarbons in tea infusion samples by high-performance liquid chromatography and fluorimetric detection based on solid-phase extraction. Analyst 123, 2145–2148.
Kayali-Sayadi MN, Rubio-Barroso S, Cuesta-Jimenez MP et al. (1999) A new method for the determination of selected PAHs in coffee brew samples by HPLC with fluorimetric detection and solid-phase extraction. J Liq Chromatogr Relat Technol 22, 615–627.
Kazerouni N, Sinha R, Hsu CH et al. (2001) Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 39, 423–436.
Llobet JM, Falco G, Bocio A et al. (2006) Exposure to polycyclic aromatic hydrocarbons through consumption of edible marine species in Catalonia, Spain. J Food Prot 69, 2493–2499.
Lodovici M, Dolara P, Casalini C et al. (1995) Polycyclic aromatic hydrocarbon contamination in the Italian diet. Food Addit Contam 12, 703–713.
Marti-Cid R, Llobet JM, Castell V et al. (2008) Evolution of the dietary exposure to polycyclic aromatic hydrocarbons in Catalonia, Spain. Food Chem Toxicol 46, 3163-3171.
Menichini E, Bocca A, Merli F et al. (1991) Polycyclic aromatic hydrocarbons in olive oils on the Italian market. Food Addit Contam 8, 363–369.
Pupin AM & Toledo MC (1996a) Benzo(a)pyrene in Brazilian vegetable oils. Food Addit Contam 13, 639–645.
Pupin AM & Toledo MCF (1996b) Benzo(a)pyrene in olive oils on the Brazilian market. Food Chem 55, 185–188.
Rodríguez-Acuña R, del Carmen Pérez-Camino M, Cert A et al. (2008) Polycyclic aromatic hydrocarbons in Spanish olive oils: relationship between benzo(a)pyrene and total polycyclic aromatic hydrocarbon content. J Agric Food Chem 56, 10428–10432.
Speer K, Steeg E, Horstmann P et al. (1990) Determination and distribution of polycyclic aromatic-hydrocarbons in native vegetable-oils, smoked fish products, mussels and oysters, and bream from the River Elbe. J High Resolut Chromatogr 13, 104–111.
Teixeira VH, Casal S & Oliveira MB (2007) PAHs content in sunflower, soybean and virgin olive oils: evaluation in commercial samples and during refining process. Food Chem 104, 106–112.
Vazquez TS, Garcia Falcon MS, Gonzalez AS et al. (2000) Enrichment of benzo[a]pyrene in vegetable oils and determination by HPLC-FL. Talanta 51, 1069–1076.
Yurchenko S & Mölder U (2005) The determination of polycyclic aromatic hydrocarbons in smoked fish by gas chromatography mass spectrometry with positive-ion chemical ionisation. J Food Compost Anal 18, 857–869.
Appendix 2 Food grouping used to construct the estimates of benzo(a)pyrene and total polycyclic hydrocarbons shown in Figs 1A and 1B








