According to the WHO, anaemia affects 68 % of pre-school age children (pre-SAC) and 48 % of non-pregnant women of reproductive age (WRA) in Africa( Reference McLean, Cogswell and Egli 1 ). The aetiology of anaemia in sub-Saharan Africa is multifactorial( Reference Crawley 2 ) and although Fe deficiency is probably the most common cause, it may also be associated with inflammation, acute and chronic infections( Reference Yip and Dallman 3 ), other micronutrient deficiencies, especially of folate, vitamin B12 and vitamin A( Reference Suharno, West and Muhilal 4 ), and genetically inherited Hb traits. The health and social consequences of anaemia and Fe deficiency are well characterized and include impaired cognition, poorer educational achievement, increased morbidity and mortality in children, and reduced work capacity and poor pregnancy outcomes in WRA( Reference Gilgen and Mascie-Taylor 5 , Reference Boccio and Iyengar 6 ). Important strategies to combat anaemia include Fe supplementation, Fe fortification of staple foods, which has been shown to be efficacious and cost-effective( Reference Hurrell 7 ), and infection control to increase Fe uptake and absorption( 8 ).
In 1996, a survey conducted in Côte d'Ivoire covering four regions found anaemia in 50 % and 42 %, and Fe deficiency in 63 % and 41 %, of pre-SAC and WRA, respectively( Reference Staubli Asobayire, Adou and Davidsson 9 ). The Ministry of Health adopted a strategy to reduce anaemia including Fe supplementation, dietary counselling, food diversification, food fortification and public health measures to combat malaria and other parasitic diseases.
In 2005, the ‘Communauté Economique des Etats de l'Afrique de l'Ouest’ estimated that 42 % of children were at risk of vitamin A deficiency (VAD) and adequate control could decrease the prevalence by 25 %( Reference Aguayo and Baker 10 ). A quasi-national survey in Côte d'Ivoire covering three rural and one urban area reported severe VAD (serum retinol <0·35 μmol/l) in 24 % and 7 %, and moderate VAD (serum retinol <0·7 μmol/l) in 56 % and 21 %, of pre-SAC and WRA, respectively( Reference Staubli-Asobayire 11 ). Diets are monotonous and based on cereals and legumes that are poor sources of vitamin A. VAD is found associated with low intake and/or absorption of vitamin A and with inflammation( Reference Thurnham, McCabe and Northrop-Clewes 12 ) and was a public health concern in Côte d'Ivoire, but its magnitude seemed to vary across social and geographical strata. In 2007, the twice annual supplementation of pre-SAC with vitamin A capsules was not linked to the immunization programme( 13 ) and the postnatal supplementation of women was having limited reach. Fortification of vegetable oil with vitamin A was in place, and 50–70 % of commercialized vegetable oil was estimated to be fortified in 2007.
By 2007, the socio-economic situation in Côte d'Ivoire had worsened due to the internal conflict, and there were indications that nutritional and health status had deteriorated along with an increase in poverty( Reference Minoiu and Shemyakina 14 ). Existing sub-national data on the prevalence of anaemia, vitamin A and Fe deficiencies were from the period before the conflict in Côte d'Ivoire and no literature on folate and vitamin B12 deficiencies was found. It was therefore proposed to carry out a representative cross-sectional survey in the nine eco-regions of Côte d'Ivoire (rural and urban), to obtain nationally representative data with the statistical power to compare eco-regions and to determine the prevalence of anaemia, vitamin A and Fe deficiencies and the influence of inflammation on their interpretation among pre-SAC and non-pregnant WRA, and the prevalence of vitamin B12 and folate deficiencies in WRA.
Materials and methods
Survey design and participant enrolment
The design and participant enrolment procedure is described in detail elsewhere( Reference Rohner, Tschannen and Northrop-Clewes 15 ). In brief, a nationally representative cross-sectional survey was conducted in Côte d'Ivoire in July/August 2007. The country was disaggregated into nine eco-regions, and the sampling strategy, using a proportionate-to-population size approach, was based on the general population census of 1998( 16 ). In total, there were sixty clusters with equal numbers from rural and urban areas. In each cluster, sixteen households were selected using the Expanded Programme on Immunization (EPI) method. Within the households, the study focused on children aged 6–59 months and non-pregnant (by self-declaration) WRA aged 15–49 years. Only one subject per age group was selected using the Kish table for random selection( Reference Kish 17 ), resulting in an expected sample size of 960 pre-SAC and 960 non-pregnant WRA.
After the household selection and consent to take part in the survey, all consenting and eligible subjects were registered (household, sex, age, date, ID number). The respondents answered questions on demographics, health and socio-economic status (housing quality, access to water, electricity and transport) and eligible household members were then invited to go to the nearest health facility to have anthropometric measurements taken and give a blood sample.
Ethics and consent
Approval for the study was granted by the Comité National d'Ethique des Sciences de la Vie et de la Santé, Abidjan, Côte d'Ivoire (clearance number 4027).
Inclusion in the survey was dependent on the household head being willing to participate. Written informed consent was also sought from the participating WRA or the parent or legal guardian of the participating child. Illiterate but consenting WRA or parents/guardians were asked to provide a fingerprint in lieu of a signature. Exclusion criteria included refusal to participate or the presence of medical contraindications for blood drawing.
Anthropometry
Anthropometric measurements were taken by trained field staff using standard anthropometric techniques. If children were >2 years of age, standing height measurement was done; for younger children their length was assessed. Weight was recorded to the nearest 0·1 kg and height to the nearest 0·5 cm.
Blood sampling and analysis
Intravenous blood samples were collected from an antecubital vein of WRA and children >12 months of age; for younger children blood was collected from the heel. The venous blood samples (7·5 ml from WRA, 2·5 ml from children) were drawn into EDTA-treated evacuated tubes (Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA). Immediately after mixing, the Hb concentration was determined using a Hemocue photometer (Hb 201+; HemoCue AB, Angelholm, Sweden), the results noted and also reported to the respondent.
After phlebotomy and on-site diagnostics, the remaining whole blood was stored on ice and protected from direct light until further processing. Later the same day, malaria smears were prepared, then the whole blood was centrifuged and the plasma aliquoted and stored at –25°C for later transportation and analysis.
For analysis of Hb concentration, the Hemocue photometer was used. Normal and low level controls, provided by the supplier, were used to test the Hemocue twice daily.
After completion of a cluster, thick and thin blood films for malaria testing were stained with Giemsa and dried for storage. Malaria slides were examined under a microscope for species-specific Plasmodium infection by experienced technicians. Parasites were counted against 200 leukocytes (if fewer than ten parasites were identified, counting was continued up to 500 leukocytes). Counts were converted to the number of parasites/μl of blood, assuming a leukocyte count of 8000/μl( 18 ).
Plasma was analysed for retinol-binding protein (RBP), ferritin, soluble transferrin receptor (sTfR), C-reactive protein (CRP) and α1-acid glycoprotein (AGP) at the VitA-Iron Tech laboratories (Wilstaett, Germany), in one run, from <100 μl plasma, using the sandwich ELISA method of Erhardt et al.( Reference Erhardt, Estes and Pfeiffer 19 ). The laboratory participates in the US Centers for Disease Control and Prevention (CDC) VITAL-EQA inter-laboratory comparison rounds and has a rigorous internal quality control system.
The Swiss Vitamin Institute analysed plasma samples from WRA for folate concentrations using the microbiological assay and Lactobacillus caseii ATCC 7469( Reference Horne and Patterson 20 ). On a sub-sample (random selection of half of the samples), vitamin B12 was analysed using the same method but working with Lactobacillus leichmanii (ATCC 7830). The laboratory regularly participates in the CDC inter-laboratory comparison rounds.
Data management and analysis
All field data were doubly entered using a pre-programmed data-entry screen (Microsoft® Access, version 2000), merged and cross-checked. Laboratory data were either auto-generated or doubly entered (Microsoft® Excel, version 97–2003).
Socio-economic classification
The poverty index was based on household characteristics and assets including: quality of housing (type of roof, wall); access to electricity and water; possession of electronic equipment; and transport. Analysis was done based on a principal component analysis-based asset index( Reference Tabachnick and Fidell 21 ), using a method and formula by Gwatkin et al.( Reference Gwatkin, Rustein and Johnson 22 ), and missing data were replaced prior to analysis with the mean of the asset. The discontinuous poverty index variable was then divided along predefined quintiles to assign each household to a socio-economic quintile, where 1 is the poorest and 5 the wealthiest quintile.
Anthropometry
Using the WHO 2006 growth standards, each child's Z-score for height-for-age (HAZ), weight-for-age (WAZ) and weight-for-height (WHZ) was determined. Z-scores have an overall mean set to 0 and sd of 1. A child who has HAZ of ≤2·00 is classified as stunted, a child with WAZ of ≤2·00 is classified as underweight and a child with WHZ of ≤2·00 is classified as wasted.
For WRA, BMI was calculated as [weight (kg)]/[height (m)]2 and four BMI categories were generated: underweight, BMI<18·5 kg/m2; normal, BMI = 18·5–24·9 kg/m2; overweight, BMI = 25·0–29·9 kg/m2; and obese, BMI ≥30·0 kg/m2( Reference Shetty and James 23 ). Both Z-scores and BMI were analysed as continuous and categorical variables.
Blood parameters
Anaemia is defined as Hb concentration of <110 g/l in children aged 6–59 months and Hb concentration of <120 g/l in non-pregnant WRA. At the population level, a ≥40 % prevalence of Hb concentrations below the cut-off indicates a severe public health problem, prevalence of 20·0–39·9 % indicates a moderate public health problem and prevalence of 5·0–19·9 % a mild public health problem; where the prevalence is <4·9 %, the population is described as normal( 8 ). Plasma sTfR concentrations reflect the intensity of erythropoiesis and the demand for Fe. The sTfR concentration rises in Fe-deficiency anaemia and is a marker of the severity of Fe insufficiency, but only when Fe stores have been exhausted and provided there are no other causes of abnormal erythropoiesis; concentrations of >8·3 mg/l are considered abnormal( Reference Erhardt, Estes and Pfeiffer 19 ) and are classified as Fe-deficient erythropoiesis. Fe-deficiency anaemia is defined as those with anaemia and Fe deficiency (low ferritin, as defined below). Low plasma folate status is indicated by folate concentrations <10 nmol/l and vitamin B12 deficiency by vitamin B12 concentrations <150 pmol/l( Reference Allen, de Benoist and Dary 24 ).
Ferritin concentrations of <12 μg/l in children aged <5 years, of <15 μg/l in all people aged >5 years in the absence of infection and of <30 μg/l in the presence of infection indicate Fe deficiency( 8 ). Ferritin concentrations are increased by inflammation and hence the prevalence of Fe deficiency in populations is generally underestimated. Therefore, to interpret ferritin in the presence of inflammation, the WHO suggests that one or more acute-phase proteins should be measured to detect the presence of inflammation but no instruction on how to use acute-phase proteins to interpret ferritin is provided( 25 ). Thurnham and colleagues( Reference Thurnham, McCabe and Haldar 26 ) described a method to estimate the increase in ferritin caused by inflammation in apparently healthy people using two acute-phase proteins, CRP and AGP, and calculated factors to adjust ferritin for the influence of inflammation( Reference Thurnham, McCabe and Northrop-Clewes 12 ): (i) no elevated acute-phase proteins, no correction; (ii) CRP concentration of >5 mg/l, correction factor of 0·77; (iii) CRP concentration >5 mg/l and AGP concentration >1 g/l, correction factor of 0·53; and (iv) AGP concentration >1 g/l, correction factor of 0·75.
VAD is defined as a serum retinol concentration <0·7 μmol/l( 27 ) and in populations a prevalence of ≥2–<10 % indicates a mild, prevalence of ≥10–<20 % a moderate and prevalence of ≥20 % indicates a severe public health problem( 28 ). Retinol concentrations are reduced by the presence of inflammation and hence prevalence of VAD is overestimated. A similar correction to that for ferritin can also be done for retinol concentrations( Reference Thurnham, McCabe and Northrop-Clewes 12 ). In the present study, RBP concentrations were measured, and not retinol; but because there was an excellent correlation between the retinol and RBP concentrations in the CDC VITAL-EQA programme, the same thresholds were used. The correction for inflammation proposed for serum retinol was applied to adjust RBP concentrations: (i) no inflammation, no correction; (ii) elevated CRP, correction factor of 0·13; (iii) elevated CRP and AGP, correction factor of 0·24; and (iv) elevated AGP, correction factor of 0·11.
Statistical analysis
Data management and analysis were performed with the statistical software package IBM SPSS Statistics 19. All continuous data were checked for skewness using the Cox test (coefficient of skewness divided by the standard error of skewness) as well as by examination of the frequency distribution with a normal curve. All analyses took into account characteristics of the cluster sampling design. Dichotomous variables are expressed as percentages. Continuous variables are expressed as arithmetic means and standard deviations, except for non-normally distributed data that were log-transformed before statistical analysis and expressed as geometric means. Associations between prevalence and residency (urban/rural) were assessed using univariate logistic regression models. Associations between continuous variables and region or residency were assessed using univariate linear regression models. The first type error rate was set at 0·05. Wherever it is stated that the comparison yields a difference, this refers to a significant difference.
Logistic regression models with micronutrient prevalence as the response variable were used to assess the effect of the different factors (urban or rural area, socio-economic class and age class for children).
Results
The questionnaire reported data from 990 households of which 447 were in the rural area. Apart from ownership of a motorcycle, there were urban/rural differences among all the other demographic categories (Table 1).
Table 1 Household characteristics of the study sample by residency (rural/urban), Côte d'Ivoire, 2007

Women of reproductive age
Information was available on 928 non-pregnant WRA whose mean age was 27·7 (sd 8·0) years. There were no differences in mean age by residency (urban/rural; Table 2). Weight, BMI, BMI categories and use of a bed net, but not height, were different by residency among the WRA.
Table 2 Age, anthropometry and bed-net use in the study sample by residency (rural/urban): women of reproductive age (aged 15–49 years) and pre-school age children (aged 6–59 months), Côte d'Ivoire, 2007

HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; WHZ, weight-for-height Z-score.
Prevalence of malaria parasitaemia (5·1 %) was low but there were rural/urban differences (P < 0·007; Table 3). Inflammation, as indicated by the elevation of the acute-phase proteins CRP and AGP, was found in 33·7 % of the women, but there were no differences in exposure to inflammation according to residency.
Table 3 Vitamin and mineral status and prevalence of deficiencies by residency (rural/urban): women of reproductive age (aged 15–49 years), Côte d'Ivoire, 2007
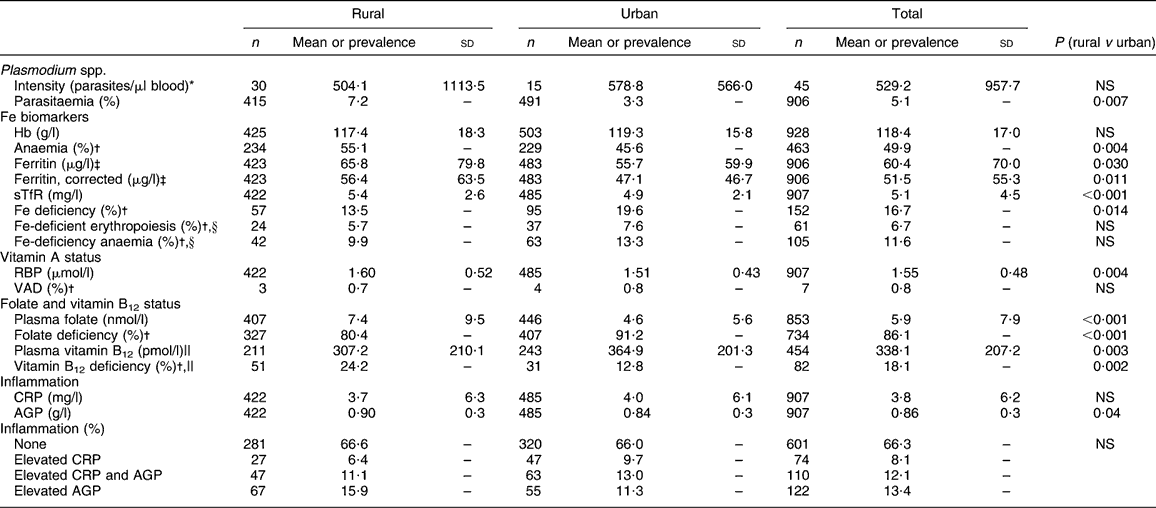
sTfR; soluble transferrin receptor; RBP, retinol-binding protein; VAD, vitamin A deficiency; CRP, C-reactive protein; AGP, α1-acid-glycoprotein.
*Geometric mean.
†Anaemia, Hb <120 g/l; Fe deficiency, ferritin <15 μg/l; Fe-deficient erythropoiesis, sTfR >8·3 mg/l; Fe-deficiency anaemia, ferritin <15 μg/l and Hb <120 g/l; VAD, RBP <0·7 μmol/l; folate deficiency, plasma folate <10 nmol/l; vitamin B12 deficiency, plasma vitamin B12 <150 pmol/l.
‡Median.
§Using corrected ferritin values.
||Random sub-sample of half of women of reproductive age.
The prevalence of anaemia was 49·9 % and there were differences in prevalence of anaemia by residency, with more anaemia in rural (55·1 %) compared with urban (45·6 %) areas (P = 0·004; Table 3). Prevalence of anaemia in WRA was different by eco-region with those in the south having the highest prevalence (63·1 %, P = 0·001; Table 4).
Table 4 Prevalence of deficiencies of iron, vitamin A, folate and vitamin B12 by eco-region: women of reproductive age (aged 15–49 years), Côte d'Ivoire, 2007

VAD, vitamin A deficiency; sTfR; soluble transferrin receptor; RBP, retinol-binding protein.
*Anaemia, Hb <120 g/l; Fe deficiency, ferritin <15 μg/l; Fe-deficient erythropoiesis, sTFR >8·3 mg/l; Fe-deficiency anaemia, ferritin <15 μg/l and Hb <120 g/l; VAD, RBP <0·7 μmol/l; folate deficiency, plasma folate <10 nmol/l; vitamin B12 deficiency, plasma vitamin B12 <150 pmol/l.
†Adjusted for inflammation.
‡Random sub-sample of half of women of reproductive age.
There were differences in both the uncorrected and corrected ferritin concentrations by residency and the prevalence of Fe deficiency, using the corrected data, was higher in the urban areas (19·6 %) compared with the rural areas (13·5 %, P = 0·014; Table 3). Fe deficiency and Fe-deficiency anaemia were not different by eco-region (Table 4).
The mean RBP concentration was higher in the rural (1·60 μmol/l) compared with the urban (1·51 μmol/l) areas (P = 0·004; Table 3). There were no differences in VAD by eco-region (Table 4).
Mean folate concentrations were less than the defined cut-off of 10 nmol/l( Reference Allen, de Benoist and Dary 24 ) and both folate levels and the prevalence of folate deficiency were worse (both P < 0·001) in the urban (4·6 nmol/l and 91·2 %, respectively) than rural areas (7·4 nmol/l and 80·4 %, respectively; Table 3). The prevalence of folate deficiency varied by eco-region, with populations in the north-west and Abidjan having the highest prevalence (∼96 %, P < 0·001). Folate concentrations were not significantly associated with Hb concentrations (data not shown).
Mean concentrations of vitamin B12 were in the normal range (>150 pmol/l), but the prevalence of those with vitamin B12 deficiency was higher in rural (24·2 %) compared with urban (12·8 %) areas (P = 0·002; Table 3). The prevalence of vitamin B12 deficiency was markedly different by eco-region (P < 0·001), especially between the north (48·6 %) and south (6·2 %; Table 4).
Pre-school age children
Data were available from 879 pre-SAC, of whom 47·3 % were female (sex ratio, male:female = 1·08:1; Table 5). The mean age of the pre-SAC was 30·6 (sd 15·1) months and there were no differences in mean age by residency (urban/rural; Table 2). The prevalences of stunting (46·2 %), wasting (14·1 %) and underweight (29·0 %) were high (Table 2). Use of bed nets and all anthropometric measures, apart from categories of wasting, were different by residency (P < 0·001; Table 2).
Table 5 Vitamin and mineral status and prevalence of deficiencies by residency (rural/urban): pre-school age children (aged 6–59 months), Côte d'Ivoire, 2007

sTfR; soluble transferrin receptor; RBP, retinol-binding protein; VAD, vitamin A deficiency; CRP, C-reactive protein; AGP, α1-acid-glycoprotein.
*Geometric mean.
†Anaemia, Hb <110 g/l; Fe deficiency, ferritin <12 μg/l; Fe-deficient erythropoiesis, sfTR >8·3 mg/l; Fe-deficiency anaemia, ferritin <12 μg/l and Hb <110 g/l; VAD, RBP <0·7 μmol/l.
‡Median.
§Using corrected ferritin values.
Children had high loads of Plasmodium spp. parasites (Table 5) and the prevalence of parasitaemia was higher in the rural (38·7 %) than the urban areas (14·5 %, P=0·0 0 1); however, when age group and residency were combined, there were no differences in prevalence (Fig. 1). Mean CRP and AGP concentrations were higher than the defined cut-offs, indicating the presence of inflammation, with more inflammation in rural (78·5 %) than urban (56·9 %) children (P < 0·001; Table 5).
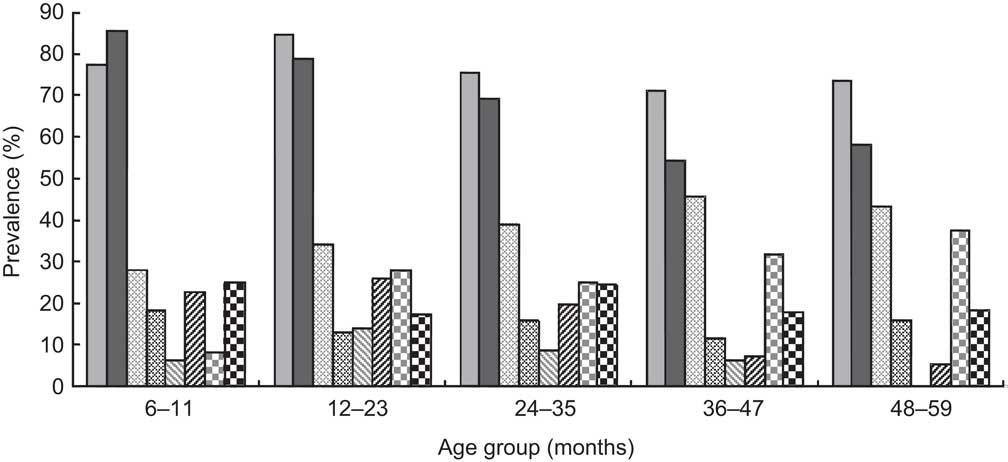
Fig. 1 Prevalence of anaemia (![]() , rural;
, rural; ![]() , urban), malaria parasitaemia (
, urban), malaria parasitaemia (![]() , rural;
, rural; ![]() , urban), iron deficiency (
, urban), iron deficiency (![]() , rural;
, rural; ![]() , urban) and vitamin A deficiency (
, urban) and vitamin A deficiency (![]() , rural;
, rural; ![]() , urban) by age and residency in pre-school children aged 6–59 months, Côte d'Ivoire, 2007. Anaemia, Hb <110 g/l; iron deficiency, ferritin <12 μg/l; vitamin A deficiency, retinol-binding protein <0·7 μmol/l
, urban) by age and residency in pre-school children aged 6–59 months, Côte d'Ivoire, 2007. Anaemia, Hb <110 g/l; iron deficiency, ferritin <12 μg/l; vitamin A deficiency, retinol-binding protein <0·7 μmol/l
Mean Hb concentrations were <110 g/l and children in the rural areas had lower mean concentrations (95·9 g/l) than those in the urban areas (101·5 g/l, P < 0·001; Table 5). Prevalence of anaemia was also higher in the rural (76·0 %) compared with the urban (68·2 %) areas (P = 0·009; Table 5). In both urban and rural areas and in all eco-regions, anaemia was classified as a severe public health problem( 8 ). Prevalence of anaemia was different (P < 0·001) by age group in urban but not rural children (Fig. 1). Anaemia was also different by eco-region, as those in the north had the highest prevalence (86·4 %; Table 6) compared with the west where the prevalence was lowest (59·6 %; P < 0·001).
Table 6 Prevalence of anaemia and deficiencies of iron and vitamin A by eco-region: pre-school age children (aged 6–59 months), Côte d'Ivoire, 2007

VAD, vitamin A deficiency; sTfR; soluble transferrin receptor; RBP, retinol-binding protein.
*Anaemia, Hb < 110 g/l; Fe deficiency, ferritin <12 μg/l; Fe-deficient erythropoiesis, sTfR >8·3 mg/l; Fe-deficiency anaemia, ferritin <12 μg/l and Hb < 110 g/l; VAD, RBP < 0·7 μmol/l.
†Adjusted for inflammation.
The geometric mean of the ferritin concentrations, whether corrected or uncorrected, was >12 μg/l( 8 ) and those in rural areas had significantly higher concentrations (60·5 μg/l) than those in urban areas (42·9 μg/l, P < 0·001; Table 5). Additionally, children with inflammation and Plasmodium parasites had mean adjusted ferritin concentrations that were 12 μg/l higher than in children without Plasmodium parasites. Fe deficiency was different by age group in both the rural (P = 0·005) and urban areas (P < 0·001; Fig. 1). Prevalence of Fe-deficiency anaemia was considerably higher in the urban (17·5 %) than rural (6·3 %) areas (P < 0·001; Table 5) and there was a big variation by eco-region, from 3·6 % in the west to 27·8 % in the north-west (P < 0·001; Table 6).
Children with Fe deficiency had sTfR concentrations that were higher (n 87, mean 13·8 mg/l) than those without (n 695, mean 10·3 mg/l; P < 0·001). Children with anaemia and Plasmodium parasites had higher sTfR concentrations (14·3 mg/l) than children without (10·4 mg/l; P < 0·001). Fe-deficient erythropoiesis was higher in the urban than rural areas (P < 0·001; Table 5) but did not differ among eco-regions (Table 6).
The prevalence of RBP concentrations <0·7 μmol/l, the population-specific threshold, was 24·1 %, which according to the WHO criteria makes VAD a severe public health problem in this age group. There were differences in RBP concentrations between urban (0·99 μmol/l) and rural (0·87 μmol/l) populations (P = 0·012; Table 5) and there were differences in VAD by age group in rural areas (P = 0·014; Fig. 1). VAD also differed by eco-region, with children in the south-east having the best status (12·4 %, P < 0·001; Table 6).
Further examination of the biochemical and anthropometric data by the poverty index quintiles, age, sex and residency using multiple regression analysis found an association between increase in age and Hb concentration of +0·294 g/l per month. There was also an association between wealth quintile and Hb concentration. Using quintile 5 as the reference group, those in the other quintiles had lower Hb concentrations: quintile 1, –16·1 g/l (n 176); quintile 2, –31·5 g/l (n 170); quintile 3, –12·8 g/l (n 161); and quintile 4, –12·8 g/l (n 161; P < 0·001). Finally, there was an association between quintile, residency and prevalence of Plasmodium spp. (P < 0·001), with those pre-SAC in the poorest rural households having more Plasmodium infection than those in other quintiles (Fig. 2).
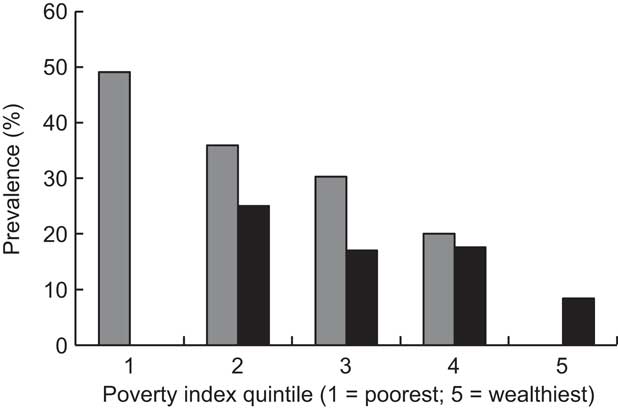
Fig. 2 Prevalence of Plasmodium spp. by poverty index and residency (![]() , rural;
, rural; ![]() , urban) in pre-school children aged 6–59 months, Côte d'Ivoire, 2007
, urban) in pre-school children aged 6–59 months, Côte d'Ivoire, 2007
Discussion
In the present survey, demographic, nutritional and anthropometric data were collected from pre-SAC and WRA in a cross-sectional survey in Côte d'Ivoire subsequent to the internal conflict of 2002–2007 but prior to the civil war in 2010.
From the demographic data, analysis showed that there were significant differences between urban and rural households in the construction of their houses and the availability of electricity. Hygiene is affected by environmental and living conditions, and in an affluent urban homes, it is possible that food can be stored and prepared in a way that minimizes the dangers of contamination because of the presence of a refrigerator, a cooking stove and tap water in the house. For the poorer rural households, where for the majority there is no refrigerator, food is cooked outside over a wood fire and water is carried from a central water pump, appropriate food hygiene is much more difficult to achieve. Personal hygiene is also more problematic in rural areas, where 56 % of the respondents are defecating in the open and there is no convenient access to water to wash their hands. The consequence of these environmental conditions may be that vulnerable poorer households will be exposed to more pathogenic organisms than affluent households, which might lead to e.g. more diarrhoeal episodes in children. Although the effect of environmental conditions was not tested specifically in the present survey, there were significant differences in the prevalence of elevated CRP and AGP concentrations, which were higher in rural than urban pre-SAC, suggesting a greater exposure to inflammation in rural areas. Furthermore, others have found evidence that the presence of a latrine, especially in combination with access to clean water, has a positive effect in reducing the incidence of diarrhoea( Reference Root 29 , Reference Esrey, Habicht and Casella 30 ).
Pre-school age children
Prevalence of stunting was 46 %, indicating a critical situation associated with chronic malnutrition, and the results suggest inappropriate infant and young child care practices and a lack of access to health facilities( 31 ). Wasting (14·1 %) and underweight (29·0 %) were also at worrying levels( 32 ). In comparison with data from a survey in Côte d'Ivoire in 2003/04, where the prevalence of stunting in rural pre-SAC was 27·7 % (cf. 46·2 % in 2007) and in urban pre-SAC 15·2 % (cf. 35·5 % in 2007)( 33 ), this represents a substantial increase in chronic malnutrition, probably due to the poor food security situation and collapse of the economy due to politico-military conflict. However, the most striking differences were in the prevalence of severe stunting and underweight (HAZ < –3 and WAZ < –3, respectively) in rural pre-SAC, which was twice that of the urban children. Anthropometric indicator Z-scores below −3 are associated with increased risk to health: based on WHO data, such children have nine times the risk of death than a child with a WHZ > –1( 34 ). Further, data from Deen et al. ( Reference Deen, Walraven and von Seidlein 35 ) suggest that stunted children experience more episodes of malaria than those who are not stunted (relative risk = 1·35; 95 % CI 1·08, 1·69). However, the authors found malaria had no further detrimental effect on nutritional status across a malarial season, suggesting that chronically malnourished children were at higher risk for developing malaria episodes. The inference from their study is that reducing the prevalence of stunting may be of great value in reducing the number of episodes and, therefore, the impact of malaria in young children.
The prevalence of Plasmodium spp. parasitaemia and of elevated acute-phase proteins was 2·7 and 1·7 times higher, respectively, in rural than urban children. The presence of inflammation, as identified by elevated CRP and/or AGP, had a significant effect on the plasma concentrations of ferritin, which were higher in children in rural areas, even after correction using the meta-analysis method of Thurnham et al.( Reference Thurnham, McCabe and Haldar 26 ). Furthermore, children with inflammation and Plasmodium parasites had mean ferritin concentrations that were 12 μg/l higher than those of children without Plasmodium parasites, even after adjustment, suggesting an additional detrimental effect of Plasmodium parasites on ferritin concentrations. Therefore in malaria-endemic areas, correction of ferritin concentrations in pre-SAC using the Thurnham meta-analysis method may not be enough and this will need to be investigated further.
Regardless of residency, anaemia prevalence was higher than the WHO threshold, classifying anaemia as a severe public health problem in Côte d'Ivoire( 8 ). It is commonly assumed that about half of anaemia is due to Fe deficiency( 36 ) but in the present survey, a much smaller proportion of the anaemia coincided with Fe-deficiency anaemia, especially in the rural children. To understand the aetiology of the anaemia in this population is difficult but it is probably multifactorial: resulting from Fe, vitamin A and folate deficiencies, haemoglobinopathies and/or the presence of Plasmodium parasites. Haemoglobinopathies were identified in only a small percentage of a Côte d'Ivoire population in a study by Staubli-Asobayire( Reference Staubli-Asobayire 11 ) (sickle-cell trait 9 %, Hb-C 9 %, all other haemoglobinopathies <1 %) and were not tested in the present survey. Folate is essential in haematopoiesis, and where it is limiting in the diet megaloblastic anaemia can develop. However, we did not measure red cell width or folate concentrations in the pre-SAC, but can speculate that it was present, as folate deficiency was found in 86 % of the WRA.
Anaemia was present in 76·0 % of rural and 68·2 % of urban children, but Fe deficiency was found in only 8·6 % of rural compared with 22·1 % of urban children. Because of the difference in exposure to inflammation and Plasmodium parasites, it is likely that the anaemia in the rural children was due to a larger extent to inflammation rather than Fe deficiency. This hypothesis is further supported when considering the percentage of children with Fe-deficient erythropoiesis; in the rural children only 7·0 % had concentrations ≥8·3 mg/l but in urban children the percentage was 15·3 %. The WHO/CDC Consultative Group( 25 ) in 2007 proposed that where the percentage of Fe deficiency (defined by low ferritin concentration) in a population group is ≥20 % and the percentage with Fe-deficient erythropoiesis is ≥10 %, then Fe deficiency is prevalent. Conversely, when the percentage of Fe deficiency is <10 % and of Fe-deficient erythropoiesis is <20 %, then Fe deficiency is not present. Thus the data presented herein suggest that Fe deficiency was present in the urban children but not in the rural children. Therefore, the likely mechanism whereby inflammation is contributing to anaemia in the rural children is by the production of inflammatory cytokines, such as IL-6, on a key mediator of hypoferraemia in inflammation, hepcidin, which can suppress both haematopoiesis by inhibition of erythropoietin production and erythropoiesis by inhibition of Fe mobilization. In addition, the presence of Plasmodium parasites (38·7 % prevalence in rural children) can result in haemolysis, when potentially tissue-damaging Hb is released into the plasma and is bound by the protective acute-phase protein, haptoglobin( Reference Weiss 37 ). The haptoglobin–Hb complex is rapidly cleared from the plasma on circulating macrophages. The Fe-loaded macrophages migrate to the reticulo-endothelial system where they can lodge for long periods, making the Fe unavailable until the inflammatory response subsides and the Fe component can be recycled for erythropoiesis.
Concentrations of sTfR can be stimulated by a number of factors, e.g. cytokines( 25 ), and so have to be interpreted with caution. The relationship between Fe status and sTfR concentrations in anaemia is inversely dependent on the degree of inflammation, since inflammation will depress erythropoietin production and marrow activity. However, the cyclical effect of Plasmodium parasites on disrupting red cell metabolism may be associated with sudden changes in Fe status, which can stimulate or depress erythropoiesis. Verhoef et al. found that concentrations of sTfR were increased in infants with both Fe-deficiency anaemia and malaria, but that there was a significantly greater increase in concentrations of sTfR in those with anaemia and malaria( Reference Verhoef, West and Kraaijenhagen 38 ). In the present survey, children with Fe deficiency had significantly higher sTfR concentrations than those without, and sTfR concentrations were significantly higher in children with both anaemia and Plasmodium parasites compared with those who had neither, suggesting an increase in sTfR concentrations was an attempt to increase erythropoiesis.
VAD is a severe public health problem nationally in Côte d'Ivoire, but there were differences in VAD among pre-SAC by eco-region; those in the central (13·0 %) and south-eastern (12·4 %) regions had a lower prevalence. Apart from the impact of VAD on the health of the child, significantly increasing the risk of severe illness from common childhood infections such as diarrhoeal disease and measles, vitamin A also appears to influence Fe metabolism but the precise mechanism is not yet elucidated( Reference Semba and Bloem 39 ).
Women of reproductive age
The majority of women were of normal BMI, with <5 % obese and <12 % underweight, but there were urban/rural differences as urban women had higher BMI and weight, but not height, than those in rural areas. There was a lower prevalence of Plasmodium parasites and a lighter parasite load in women than children, although there were similar rural/urban differences.
Anaemia was a severe public health problem in the population( 8 ) and there were significant urban/rural and eco-regional differences, with those in the rural, south, south-east and northern areas being worst affected. Whether the internal conflict was an underlying reason is hard to say, although most conflict events between 2002 and 2007 took place in the south, the central-west and the north of the country( Reference Minoiu and Shemyakina 14 ).
Ferritin concentrations were in the normal range, again with significant urban/rural differences, but as with the children, there were more WRA with Fe deficiency in urban than rural areas. Although the prevalence of Plasmodium parasites was lower in the women than in the children, there was still a rural/urban difference with rural women having more parasitaemia, which could be the underlying reason for the higher ferritin concentrations in those women. There were no significant differences in the prevalence of Fe-deficient erythropoiesis or Fe-deficiency anaemia between the rural and urban women.
Mean folate concentrations were below the accepted cut-off( Reference Allen, de Benoist and Dary 24 ). Folate deficiency was high and differed significantly between rural and urban populations, and among the eco-regions. Fresh green vegetables are a good source of natural folate but losses during harvesting, storage and cooking can be high, as can losses from cooking animal products, while staples such as rice and wheat are low in folate and may contribute to poor folate status( 40 ). In contrast, vitamin B12 status was good, with mean values above the recommended cut-off( Reference Allen, de Benoist and Dary 24 ), although there was a difference in prevalence of deficiency between the rural (24·2 %) and urban areas (12·8 %). The rural/urban difference may be due to differences in dietary intake of vitamin B12, which might be lower in the rural communities, but without dietary intake data it is impossible to know. Vitamin B12 is produced by bacteria and is naturally occurring in animal products. However, contamination of other non-animal foods with bacteria may be a possible source of vitamin B12 for the rural populations, where animal foods may be less affordable( Reference Herbert 41 ).
Mean RBP concentrations were good, although lower in the rural population. There was very little VAD and there were no differences in VAD prevalence among the eco-regions or between rural and urban areas.
Conclusions
The micronutrient interventions under consideration by the Government of Côte d'Ivoire include the fortification of the staple wheat flour with Fe and folic acid, and an improvement in the coverage of vegetable oil fortified with vitamin A. Folate deficiency was present in 86 % of WRA, indicating that fortification of flour with folic acid could be an effective public health policy, particularly for WRA with market access to fortified products. VAD was not prevalent among WRA and therefore fortified vegetable oil is not a priority for WRA, but it could be of benefit to pre-SAC, especially the older children eating complementary or family foods. Younger children are dependent on breast milk and as the women's vitamin A status is good, breast milk vitamin A concentrations should also be adequate. Lower vitamin A concentrations seen in the children in the survey were most likely due to inflammation and especially the presence of Plasmodium parasites.
Acknowledgements
Sources of funding: The Global Alliance for Improved Nutrition (GAIN), UNICEF, Helen Keller International (HKI), the Swiss Federal Institute of Technology (ETH Zurich), the Institut National de Santé Publique en Côte d'Ivoire (INSP), Map International, the Centre Suisse des Recherches Scientifiques and Unilever provided financial, technical or in-kind contributions to this study, a support that is gratefully acknowledged; none of the commercial funders had any role in the design, analysis or writing of this article. Conflicts of interest: None of the authors have a conflict of interest to declare. Authors’ contributions: F.R., A.B.T., V.K.-G., P.E.B. and M.B.Z. designed the study; F.R., A.B.T., V.K.-G., P.E.B., J.G.E. and M.B. conducted the research; F.R., C.N.-C., A.B.T. and C.G.N.M.-T. analysed the data; F.R., C.N.-C. and C.G.N.M.-T. wrote the first draft of the manuscript; all authors contributed to, read and approved the final manuscript.










