Metabolic syndrome (Mets), despite ambiguity on the precise definition, is a combination of interrelated risk factors including central obesity, elevated blood pressure, dyslipidaemia and hyperglycaemia(Reference Alberti, Eckel and Grundy1). It is generally accepted a high (20–30 % in most countries) and increasing prevalence of Mets, regardless of the Mets prevalence estimates may differ depending on the definition used(Reference O’Neill and O’Driscoll2,Reference Grundy3) . Several studies have emphasised Mets can trigger a series of poor prognosis, such as CVD, diabetes, cardiovascular and all-cause mortality, and has become one of the main threats to human health(Reference Wilson, D’Agostino and Parise4–Reference Mottillo, Filion and Genest8). The occurrence of Mets is related to various factors especially aging, geography, ethnicity and lifestyle(Reference Alberti, Eckel and Grundy1,Reference O’Neill and O’Driscoll2,Reference Zhao, Yan and Yang9–Reference Liang, Yan and Song11) . The northeast China, specifically rural area, has its own geography, climate and lifestyles that differ from other regions of China. For example, a high-salt and high-carb diet leads to high prevalence of hypertension and obesity, and a decline in exercise levels caused by prolonged snow, those may trigger a higher prevalence of Mets. Therefore, it is critical to understand the prevalence of Mets in the general population in northeast China.
In the case of rapid progression of lifestyle westernisation and population aging in rural China, understanding updated prevalence trends may be paramount given the potential effect of the Mets and its associated health complications on them. Although previous researches stated the prevalence of Mets in rural northeast China, all the results were not accurate. Respecting definition, Mets was diagnosed using currently taking dyslipidaemia medication, rather than specific treatment for raised TAG and/or increased HDL-cholesterol. This disadvantage could lead to overestimation of the prevalence of Mets(Reference Yu, Guo and Yang10). Alternatively, only hypertensive residents were recruited decade ago(Reference Zhang, Sun and Zhang12) or including partial urban population(Reference Wang, Kong and Sun13).
To gain a comprehensive and accurate understanding of Mets among rural northeast Chinese population, the current study, involving 9790 participants aged at least 40 years older, used three widely accepted definitions (modified Adults Treatment Panel (ATP) III criteria in 2005(Reference Grundy, Cleeman and Daniels14), International Diabetes Federation (IDF) criteria in 2005(Reference Alberti, Zimmet and Shaw15) a harmonized definition in 2009(Reference Alberti, Eckel and Grundy1), Table 1) to report and compare the prevalence of Mets and identified its determinants by multivariate stepwise logistic regression models.
Table 1 Diagnosis criteria of metabolic syndrome used in current study
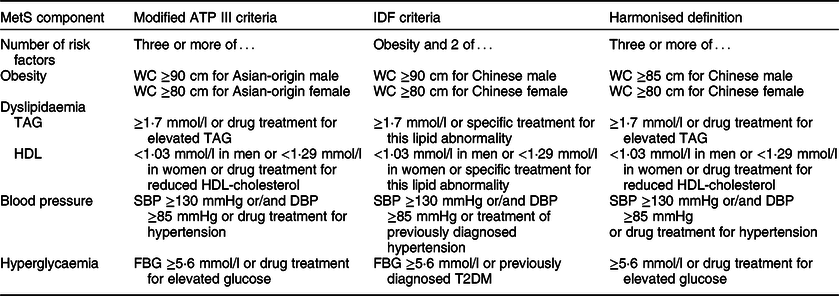
SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose.
Methods
Study population
This cross-sectional study was undertaken from September 2017 to May 2018 in rural areas of Liaoning Province. A multi-stage, stratified and cluster random sampling method was used to ensure that the sample was representative. Four counties (Chaoyang, Lingyuan, Liaoyang and Donggang) were randomly selected from the central, eastern and western regions of Liaoning Province. Thereafter, nineteen rural villages were randomly selected from these four counties. All permanent residents aged ≥40 years in each village (n 12 808), except those who were pregnant or had a mental disorder, were eligible to participate; 10 926 (85·3 %) completed the study. We further excluded participants did not provide blood specimens (n 26) and with CVD (n 1110). Eventually, data from a total of 9790 (89·6 %) subjects (3866 men and 5924 women) were analysed.
Data collection and measurement
Data were collected by a team consisting of specialists trained in the prevention and control of chronic disease, cardiologists and neurologists, using a face-to-face interview and a self-administered questionnaire during a single clinic visit. All the team members underwent rigorous knowledge training before data collection and completed pilot interviews (performed with volunteers). Further instruction and support were provided by study authors during data collection.
Demographic and clinical data, including data on age, sex, socioeconomic status (education, occupation, marital status and annual household income), lifestyle (smoking, drinking and physical activity), comorbidities (such as hypertension, diabetes and dyslipidaemia) and other medical history (such as coronary artery disease, cerebrovascular disease) were collected using a self-administered questionnaire. To ensure that data were recorded truthfully and carefully (with an accuracy of at least 98 %), trained staff scanned the questionnaires, and relevant information was manually extracted, with double entry included as a quality check.
Current smoking was defined as more than one cigarette per day and cumulative smoking more than 6 months. Participants who met the above criteria in the past but did not smoke during the interview were defined as former smokers. Participants who were unable to meet the above conditions were defined as none-smokers(Reference Zhao, Yan and Yang9). Participants were asked whether they regularly consumed alcohol, their average alcohol consumption per day and the number of days per month that they consumed alcohol. They were divided into four categories: never drank, moderate drinkers, heavy drinkers and former drinkers. One drink was defined as containing 15 g of ethanol(Reference Qiao, Shi and Hou16). Moderate drinking was defined as up to 1 drink/d for women and up to 2 drinks/d for men; heavy drinking was defined as >1 drink/d for women and >2 drinks/d for men(Reference Li, Guo and Liu17). Regular exercise was defined as moderate intensity exercise (equivalent to walking) for ≥30 min and ≥3 times per week, which moderate and heavy manual workers fulfil due to their work(Reference Zhang, Chen and Wang18).
Each participant was recorded as having medical history of a specific disease (such as diabetes, coronary artery disease) if he/she answered ‘Yes’ to the question ‘Have you been diagnosed with [specific disease] by a certified doctor?’. Cerebrovascular diseases (such as ischemic or haemorrhagic stroke) were diagnosed by a neurologist according to the WHO recommendations and confirmed with computed tomography (CT) and/or MRI(Reference Hatano19). The diagnosis of CHD was based on self-reported history, and angina was recognised for hospitalisation of secondary and above medical institutions. Participants were asked during interview whether they had taken prescription medication for blood pressure, lipid control, glucose control or certain diseases in the past 2 weeks. Those who answered ‘yes’ were asked to report the name, dose and frequency of each drug if known. Those who did not remember the exact dose were asked the number of pills taken. We cleaned the data and matched all the names given to those of generic drugs in the China Pharmacopoeia 2015, with a 95 % success rate.
Physical measurements, including height, weight and waist circumference (WC), were obtained during the interview. WC was measured at a point midway between the lowest rib and the iliac crest in a horizontal plane using non-elastic tape. These parameters were measured to the nearest 0·1 kg and 0·1 cm, as appropriate, with participants wearing lightweight clothes without shoes. The BMI was calculated as weight (kg) divided by the square of the height (m2). A central steering committee with a subcommittee for quality control was established to ensure that data were obtained according to standardised protocols. For each participant, blood pressure was measured three times at 2-min intervals after at least 5 min of rest in a seated position using a standardised automatic electronic sphygmomanometer (J30; Omron). Hypertension was defined as a mean systolic blood pressure (SBP) ≥140 mmHg or a mean diastolic blood pressure (DBP) ≥90 mmHg, and/or self-reported use of antihypertensive medication in the past 2 weeks(Reference Chobanian, Bakris and Black20).
Fasting blood samples were collected from participants after at least 8 h of overnight fasting. The samples were obtained from an antecubital vein using BD Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA; Becton, Dickinson and Co.), and serum was subsequently isolated from whole blood. Thereafter, serum samples were frozen at −20 C for storage. Subsequently, the biochemical parameters, comprising fasting blood glucose (FBG), glycosylated Hb (HbA1c), serum lipid profiles including total cholesterol (TC), TAG, serum HDL-cholesterol and LDL-cholesterol were measured using an Abbott Diagnostics C800i auto-analyzer (Abbott Laboratories) with commercial kits. The laboratory tests were carried out at three laboratories. In addition, we randomly selected 10 % of specimens from each laboratory for centralised re-testing by China’s Ministry of Health’s National Center for Clinical Laboratory to ensure that the testing was accurate. Reproducible indices for lab examination were more than 95 %. Diabetes mellitus was diagnosed as an FBG ≥7·0 mmol/l or HbA1c ≥6·5 %, and/or self-reported diagnosis that was previously determined by a physician(Reference Chamberlain, Rhinehart and Shaefer21).
Statistics analysis
Continuous variables are reported as means and SDs, and categorical variables are reported as frequencies and percentages in each subgroup. Student’s t test and χ 2 tests were used, as appropriate, to compare differences between male and female. We calculated the prevalence of each component of the Mets. The overall, age- or/and sex-specific prevalence of Mets was analysed. The Sixth China Population Census data were used to standardise the prevalence of Mets. In addition, stepwise multivariate logistic regression analyses were carried out to evaluate the risk factors for Mets. For the regression models, multivariable adjustment was performed for known demographic characteristics (age, sex) and certified clinical variables (BMI, level of education, marital status, smoke status, annual income, drinking status, exercise regularly and occupation) in previous studies. Regarding importance of menopause on metabolic profiles, we evaluated age independent role of menopause status in female subjects in the model. Finally, participants with CVD were included, and the association between Mets with CVD was evaluated by logistic regression. ORs and 95 % CIs are presented for the logistic regression analyses. Statistical analyses were performed using the statistical software package IBM SPSS statistics software version 22.0 (SPSS Inc.); P < 0·05 were considered statistically significant.
Results
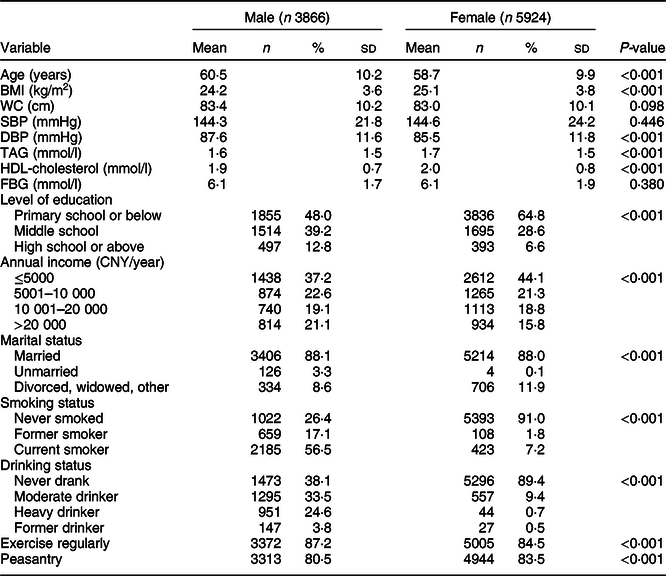
Our study included 9790 subjects (mean age 59·4 ± 10·1, males 39·5 %). Clinical characteristics by sex are shown in Table 2. Compared with female subjects, male participants were significantly older and had significantly higher DBP (all ps < 0·05). However, male had significantly lower BMI, TAG and HDL-cholesterol (all ps < 0·05). In addition, male comprised a significantly higher proportion of high education level, with prevalence of smokers, drinkers and individuals who exercised regularly (all ps < 0·05).
Table 2 Characteristics of the study sample

WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose.
Data are presented as mean (sd) or n (%), as appropriate
The prevalence of single component of the Mets was shown in Fig. 1 according to modified ATP III criteria, IDF criteria and harmonised definition. Elevated blood pressure (≥130/85 mmHg or/and drug treatment for hypertension) was the most common component of Mets in both sexes. However, the prevalence of reduced HDL-cholesterol (<1·03 mmol/l in men or <1·29 mmol/l in women or/and specific treatment for this lipid abnormality) was the lowest (6·3 % in men v. 16·0 % in women) among these individual components.

Fig. 1 Prevalence of each component of the metabolic syndrome based on three definitions. ![]() , men;
, men; ![]() , women
, women
According to modified ATP III criteria, IDF criteria and harmonised definition, the overall prevalence of Mets was 41·3 % (4040/9790), 34·2 % (3345/9790) and 44·1 % (4316/9790), respectively. The age- and sex-adjusted prevalence of Mets was 38·2, 30·9 and 41·8 %. There was a higher age-adjusted prevalence of Mets in women than in men (modified ATP III criteria: 32·4 v. 44·2 %, IDF criteria: 22·7 v. 39·9 %, harmonised definition: 39·6 v. 44·2 %). Regardless of the adapted definitions, the prevalence of the Mets increased slightly with age until about 70 years of age and then decline. Participants aged 60–69 years had the highest prevalence, while the lowest prevalence appeared in participants aged 40–49 years. Related details are shown in Table 3.
Table 3 Prevalence of metabolic syndrome stratified by age and sex among the study participants

ASSR = age- and sex-standardised rates.
Percentages represent the number of subjects with metabolic syndrome among the total number of subjects; data are presented as n (%).
* ASSRs by China census population 2010.
Table 4 shows the potential risk factors for Mets, as identified by the multivariate logistic regression models. Whatever definition of Mets was used, stepwise multivariate logistic regression analysis manifested that the risk of Mets increased by 33–41 % for each 10-year increase in age. The risk of Mets in female subjects was significantly higher than that in male subjects according to modified ATP III criteria (OR 2·05, 95 % CI 1·82, 2·31), IDF criteria (OR 3·14, 95 % CI 2·75, 3·60) and harmonised definition (OR 1·41, 95 % CI 1·39, 1·44). Meanwhile, lower-annual income, higher BMI and non-peasantry worker were equally significant independent risk factors. However, levels of education, marital status, smoke status and exercise status were excluded in regression analysis. Besides, drinking status also showed a consistent correlation in three Mets definitions.
Table 4 The risk factors of metabolic syndrome by stepwise logistic regression

Level of education, marital status, smoke status and exercise regularly were excluded in three diagnosis criteria.
Discussion
In the current study, we found the age- and sex-adjusted prevalence of Mets was 38·2, 30·9 and 41·8 % in rural northeast China, according to modified ATP III criteria, IDF criteria and harmonised definition, respectively. Elevated blood pressure (≥130/85 mmHg or/and drug treatment for hypertension) was still the most common component of Mets. Whatever definition of Mets was used, stepwise multivariate logistic regression analysis demonstrated that higher age, female sex, non-peasantry worker and higher BMI and annual income were independent risk factors of Mets.
In terms of northeast rural population, the prevalence of Mets in our current study was similar to Yu etal.’s study which was based on modified ATP III criteria (32·4 v. 31·4 % in male, 44·2 v. 45·6 % in female, 38·2 v. 39·0 % in total)(Reference Yu, Guo and Yang10). According to IDF criteria, our result was slightly lower than Zhang etal.’s lecture in hypertension participants (34·7 %)(Reference Zhang, Sun and Zhang12), but higher than the outcome another study based on urban and rural residents (22·4 %)(Reference Wang, Kong and Sun13). In the first two studies, sample encompassed individuals aged ≥35 year, which is considerably lower, and individuals with hypertension in the latter study. Further, there were a slice of deviations from the diagnostic criteria in the former study. Accordingly, we have reasons to infer that the prevalence of Mets may still be on the rise.
The current prevalence of Mets was similar to the results of Nantong’s participants in 2013 based on harmonised definition (39·6 v. 39·8 % in male, 44·2 v. 45·0 % in female, 41·8 v. 42·6 % in total)(Reference Xiao, Wu and Gao22). However, the prevalence of Mets was higher in our study than the published estimates of the prevalence of Mets. The prevalence among rural northwest adults in China was 15·1 % (modified ATP III criteria) and 10·8 % (IDF criteria), respectively(Reference Zhao, Yan and Yang9). The age standardised prevalence based on IDF criteria and harmonised definition was 21·33 and 26·50 %, respectively, in Xinjiang rural areas(Reference Guo, Gao and Ma23). A meta-analysis from mainland China stated that the prevalence of Mets in rural areas was just 19·2 % (IDF criteria)(Reference Li, Li and Lun24). Even if some studies were conducted in urban areas or including urban areas, the prevalence of Mets was lower than ours. Results of The CHPSNE Study manifested that the overall prevalence of Mets was 27·4 % (men 27·9 % and women 26·8 %) according to modified ATP III criteria(Reference Song, Zhao and Liu25). Gu etal. asserted prevalence of Mets in nationally representative participants aged 35–74 years was 13·7 % (men 9·8 % and women 17·8 %)(Reference Gu, Reynolds and Wu26). Another nationally representative sampling from the China Health and Nutrition Survey shown 21·3 % (modified ATP III criteria), 18·2 % (IDF criteria) of characters have Mets(Reference Xi, He and Hu27). Among the Northeast Chinese people, their daily diet contains excessive salt, such as pickles and preserved beancurd. Long-term high salt intake is an important environmental factor involved in the aggravation of hypertension, which may be a possible explanation for the high prevalence of Mets found in our study. However, the prevalence of Mets was higher than ours in CHAP study in Shandong: 58 % using IDF criteria, 61·6 % using harmonised definition and it might be because the participants were older(Reference Liang, Yan and Song11). Meanwhile, the prevalence of Mets was similar to other countries. The Iran national study conducted in 2007 suggested that age-standardised prevalence of Mets was 37·4 % (IDF criteria) and 41·6 % (modified ATP III criteria). The prevalence of Mets was 28·8 % (male 23·1 % and female 33·5 %) according to IDF criteria in Turkey(Reference Gundogan, Bayram and Capak28). In the USA, the prevalence based on modified ATP III criteria was 33 % (men 30·3 % and women 35·6 %), and the age-standardised prevalence of the Mets was 35·0 % (men 33·7 % and women 36·0 %) according to harmonised definition(Reference Heiss, Snyder and Teng29). Compared with the results of the above surveys, the present study revealed Mets was still a healthy challenge in northeast rural areas.
In our study, whatever definition was selected, a sex difference in the prevalence of Mets was found, being higher in females than in males. Stepwise logistic regression models also indicated that being female was independent risk factor. This conclusion was consistent with some researches(Reference Zhao, Yan and Yang9–Reference Zhang, Sun and Zhang12,Reference Guo, Gao and Ma23,Reference Gu, Reynolds and Wu26) , but different from a previous study (27·9 % in men and 26·8 % in women)(Reference Song, Zhao and Liu25). Post-menopausal status may have effects on Mets because of insulin resistance and central obesity after menopause(Reference Fujimoto, Bergstrom and Boyko30). Our results also indicated that the risk of Mets increased by approximately 75 % for post-menopausal female (see online supplementary material, Supplemental Table 1). Besides, the multivariate logistic regression models also showed that prevalence increased with age in three different definitions, which was similar to previous studies(Reference Zhao, Yan and Yang9,Reference Yu, Guo and Yang10,Reference Guo, Gao and Ma23,Reference Heiss, Snyder and Teng29) . BMI also showed a positive correlation with Mets. This was subject to explaining because BMI, such as WC, was a manifestation of obesity. In subjects with BMI > 30 kg/m2, central obesity could be assumed and WC did not need to be measured(Reference Alberti, Zimmet and Shaw15). Of course, the results suggested also exercise regularly, healthy dietary program and other healthy behaviours and lifestyles would be helpful to maintain the optimal standardised weight, thereby lowering the prevalence of MetS(Reference Xi, He and Hu27). Moreover, annual income was negatively correlated with Mets, while the result was opposite to previous studies(Reference Zhao, Yan and Yang9,Reference Yu, Guo and Yang10,Reference Song, Zhao and Liu25) . The result suggests subjects with high income had begun to concern their health in northeast rural areas. Peasantry had a lower prevalence than non-peasantry, which was due to heavy physical labour and light diet habit.
The most frequent component of Mets was elevated blood pressure in the current study, which coincided with previous studies(Reference Zhao, Yan and Yang9,Reference Yu, Guo and Yang10) . However, it was out of keeping with a recent meta-analysis in mainland China, in which hypertension was the most common component in men, whereas central obesity was the most common feature in women(Reference Li, Li and Lun24). Similarly, central obesity was the most common component in the USA 2009–2010 (56·07 %), and the prevalence of central obesity was increasing(Reference Beltran-Sanchez, Harhay and Harhay31). The demographic characteristics of cold climate and high-salt diet may be the crucial reasons in our study.
Interestingly, according to modified ATP III criteria and harmonised definition, heavy drinking had positive relation to Mets, which further certified these results of studies(Reference Sun, Ren and Liu32,Reference Hirakawa, Arase and Amakawa33) . Besides, participants with CVD (n 1110) were included, and we analysed the associations between Mets with CVD by logistic regression. The results suggested that the association between Mets and cerebrovascular disease and coronary artery disease was similar under modified ATP III criteria and harmonized definition, which were significant prior to IDF criteria (see online supplementary material, Supplemental Table 2). Overall, results suggested that modified ATP III criteria and harmonised definition might be better definitions of Mets.
There are some limitations in our study. First, the cross-sectional design of the study only allowed assessment of the associations between Mets and risk factors rather than causal links. Second, although our study included a large number of subjects, these participants were from northeast China and over 40 years old. This may reduce the applicability of our results to other populations. Finally, some confounding factors proved in other studies such as sleep duration, number of child, beans, bean products and tea intake, as well as various pressures are not collection in our study. Large-sample prospective studies are needed to confirm the results.
Conclusion
The current study confirms that the accurate prevalence of Mets is increasingly high among a general population from rural areas of northeast China, especially in the female population. It demonstrates that females need more attention to prevent and control Mets. The final definition of a Mets is needed to identify and intervene patients as early as possible based on relevant risks.
Acknowledgements
Acknowledgements: The authors acknowledge assistance from CDC of Chaoyang, Lingyuan, Liaoyang, Dandong and Donggang. The study sponsor had no role in study design; collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication. Financial support: Research reported in this publication was supported by the National Key R&D Program of China (grant no. 2017YFC1307600). Conflict of interest: There is no conflict of interest. Authorship: Z.D. takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. L.X., Y.T. and L.J. take responsibility for research design and data collection. M.L., H.Y., B.Z., S.L. and S.Y. take responsibility for data collection. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Central Ethics Committee at the China National Center for Cardiovascular Disease. Written informed consent was obtained from all subjects.
Supplementary Material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019004166.