Obesity is a worldwide public health problem and affects around 12 % of adults. Prevalence rates are higher in the female sex, and especially for those at childbearing age, obesity represents a great concern(Reference Afshin, Forouzanfar and Reitsma1,Reference Vézina-Im, Nicklas and Baranowski2) . Excessive body adiposity in early pregnancy has been associated with several gestational complications, contributing to maternal morbidity and infant mortality(Reference Vézina-Im, Nicklas and Baranowski2,Reference Black, Victora and Walker3) , as well as to increased risk of CVD(Reference Afshin, Forouzanfar and Reitsma1).
As far as the offspring is concerned, maternal pre-pregnancy BMI was shown to be associated with adiposity later in life, resulting in a ‘vicious cycle of obesity’(Reference Kaar, Crume and Brinton4–Reference Chaparro, Koupil and Byberg8). Genetic components could partly explain this association(Reference Silventoinen, Rokholm and Kaprio9), but it is known that epigenetic mechanisms, through metabolic programming during the first 1000 d of life, play an important role on this issue(Reference Battista, Hivert and Duval10,Reference Fernandez-Twinn, Constância and Ozanne11) . Maternal lifestyle, mainly related to dietary intake habits, may influence offspring behaviours and risk for outcomes(Reference Silventoinen, Rokholm and Kaprio9,Reference Eshriqui, Folchetti and Valente12) . In this context, our group found that maternal pre-pregnancy BMI was associated with offspring adherence to a processed and prudent dietary pattern in adulthood(Reference Eshriqui, Folchetti and Valente12).
Several studies reported an association of maternal pre-pregnancy BMI and offspring adiposity in childhood(Reference Kaar, Crume and Brinton4–Reference Cadenas-Sanchez, Henriksson and Henriksson7), but few were conducted in adults(Reference Chaparro, Koupil and Byberg8,Reference Reynolds, Osmond and Phillips13) , and none assessed all body compartments including the visceral adipose tissue (VAT). Increased VAT generates systemic inflammation and has been considered a strong predictor of cardiovascular events, independent of BMI(Reference Figueroa, Takx and MacNabb14). There is some evidence that excessive visceral adiposity can deteriorate metabolic profile even in apparently healthy non-obese young adults(Reference De Larochellière, Côté and Gilbert15), indicating that assessment of VAT in this age group could be useful to anticipate detection of those at-risk and the prevention of cardiometabolic disorders.
Associations of maternal pre-pregnancy BMI with offspring fat-free mass in childhood(Reference Castillo, Santos and Matijasevich5,Reference Cadenas-Sanchez, Henriksson and Henriksson7) and in early adulthood(Reference Chaparro, Koupil and Byberg8) were less investigated and provided controversial results. The Avon Longitudinal Study of Parents and Children showed that maternal pre-gestational BMI was directly associated with bone mass in infancy, but it was mainly explained by child’s height and weight(Reference Macdonald-Wallis, Tobias and Smith16). It is unknown whether this association would be maintained in adulthood. To the best of our knowledge, no study has tested associations of maternal pre-pregnancy BMI with all three body compartments of the offspring in apparently healthy adults, using a reliable technique and software to identify VAT. Dual-energy x-ray absorptiometry (DXA) provides accurate measures of body composition and advantages of low X-ray exposure, cost and scanning time against computed tomography or MRI(Reference Kaul, Rothney and Peters17). We investigated whether maternal pre-pregnancy BMI was associated with offspring muscle, bone and adipose (general and visceral) body compartments assessed by DXA in early adulthood before adiposity-related disorders.
Materials and methods
This is a retrospective cohort of women who participated at the baseline of the Nutritionists’ Health Study (NutriHS). Briefly, the NutriHS is a cohort conducted in Sao Paulo, Brazil, with non-pregnant undergraduates of Nutrition courses or nutritionists (graduates) recruited between 2014 and 2017(Reference Folchetti, Silva and Almeida-Pititto18). All participants registered on the NutriHS website, electronically signed the informed consent term and answered structured questionnaires regarding early life events and current aspects of health, socio-economic background and lifestyle.
The sample of the present study was composed of female participants aged 20–45 years who agreed to attend a face-to-face interview and to a body composition assessment. To be eligible, women should not have diabetes or cancer diagnoses nor exceed the limits of the densitometer (1·87 m and 201 kg). Those with missing information of maternal pre-pregnancy BMI (our main exposure) (n 51) were excluded, resulting in a final sample of 150 apparently healthy young women for the present study.
Before answering the early life events questionnaire available on NutriHS website, participants were oriented to ask for their mothers’ help, or to consult birth cards. Maternal pre-pregnancy BMI was obtained as continuous variable and further categorised as <25 or ≥25 kg/m2. Other maternal characteristics, such as age at conception (≤19, >19 and <35, ≥35 years), education (<11, ≥11 years), parity (0, ≥1 pregnancy), gestational diabetes and smoking during pregnancy (no, yes), were obtained. Information of delivery (vaginal, C-section) and birth weight (<2·5, 2·5 and <4·0, ≥4·0 kg) were also collected. Participant’s current life data, such as age, skin colour (white, non-white), leisure physical activity practice (no, yes), smoking habit (no, yes), family income (<6, ≥6 minimum wages) and education level (undergraduates attending the first-half of nutrition courses, undergraduates attending the second-half of nutrition courses, graduates), were also obtained.
During visits to the university health care centre, weight was measured using a digital scale (Filizola®) with 0·1 kg of precision and height by a fixed stadiometer with 0·1 cm precision. A flexible and inelastic tape was used to assess waist circumference at the midpoint between the last rib and the iliac crest. Body composition was assessed using DXA by a researcher (A.M.M.V.) certified by the Brazilian Association of Bone and Osteometabolism. The equipment (GE Lunar iDXA®) and software provide measurements of the three body compartments (adipose, muscle and bone), differentiating the VAT. Measurements obtained were total fat (kg and %), android and gynoid fat (used to calculate android-to-gynoid fat ratio), VAT (cm3), appendicular skeletal muscle mass (kg) (ASM), total bone and femur mineral content (g) and density (g/cm2). Fat mass index and ASM index (kg/m2) were calculated dividing total fat and ASM per squared height, respectively. According to the manufacturer’s instructions, regions of interest were determined manually and the densitometer was routinely calibrated.
A random sub-sample (n 117) was submitted to a clinical examination. Blood pressure was assessed in sitting position after 5 min resting using an automatic device (Omron model HEM-712C®; Omron Health Care Inc.). Systolic and diastolic blood pressure (mmHg) were obtained in triplicate, and the mean value between the last two measures was considered. After overnight fasting, blood sample was collected for biomarkers determinations. Glucose (mg/dl) was determined by the glucose oxidase method; total cholesterol, HDL-cholesterol and TAG (mg/dl) by the enzymatic colorimetric method and LDL-cholesterol were calculated by Friedewald formula(Reference Friedewald, Levy and Fredrickson19). High-sensitivity C-reactive protein and 25-hydroxyvitamin D were measured by chemiluminescence assay (Elecsys 2012, Roche Diagnostics).
Statistical analyses
The Kolmogorov–Smirnov test was used to verify normal distribution of continuous variables, which were described as mean (sd) or median (interquartile range). Categorical variables were described as frequencies. Student’s t test or Mann–Whitney U test was used to compare central distribution values of body composition measurements according to maternal pre-gestational BMI categories.
The shapes of the associations between maternal pre-pregnancy BMI and offspring body composition parameters were illustrated with scatterplots (see online supplementary material, Supplemental Figure S1). Linear regression models were performed to verify the association between maternal pre-pregnancy BMI (kg/m2) and body composition measurements (outcomes). ASM index and VAT were log-transformed for analyses. Model 1 was adjusted for maternal age, maternal education and parity, according to the set of minimal sufficient adjustments recommended by the directed acyclic graph (DAG) (see online supplementary material, Supplemental Figure S2). DAG is a causal graph, which consists of variables (vertices/rectangle in Supplemental Figure S2) connected with edges oriented by arrows, denoting a direct causal relationship, with no feedback loops. Complex hypothetical situations, which could be represented by bi-directional arrows, are simplified considering temporality of the association and then replaced by unidirectional arrows. DAG approach is not a statistical technique that estimates effect, but considering a rigorous mathematical methodology, it suggests subsets of covariates that should be included in traditional statistical approaches (minimal sufficient adjustment) to potentially minimise the magnitude bias. Epidemiological biases can be categorised as either lack of conditioning on a common cause (confounding bias) or conditioning on a common effect (or a descendant of the common effect; known as selection bias or collider bias). DAG allows representing both situations. Thus, they are valuable tools for guiding epidemiological analysis, once they help to translate a causal hypothetical model into a statistical model in the light of observed data(Reference la Bastide-Van Gemert, Stolk and van den Heuvel20–Reference Shrier and Platt22). We used an online and free access software, named DAGitty, to construct the DAG of the present study. Minimally sufficient adjustment set was outputted instantly by DAGitty, reflecting any possible changes made to the diagram(Reference Textor, Hardt and Knüppel23). Model 2 included variables considered in model 1 plus height (for measurements of adiposity and bone as outcomes).
Sensitivity analysis was performed to test possible differences between our main results, with the whole sample (n 150), and results considering: (i) the sub-sample with clinical data available (n 117) (see online supplementary material, Supplemental Table 1a) or (ii) participants whose mothers reported maternal pre-pregnancy BMI ≥ 18·5 kg/m2 (n 139) (see online supplementary material, Supplemental Table 1b).
Statistical significance was considered at the level of 5 %. Stata statistical software version 12.0 (release 12, 2011, StataCorp LP) was used for analyses.
Results
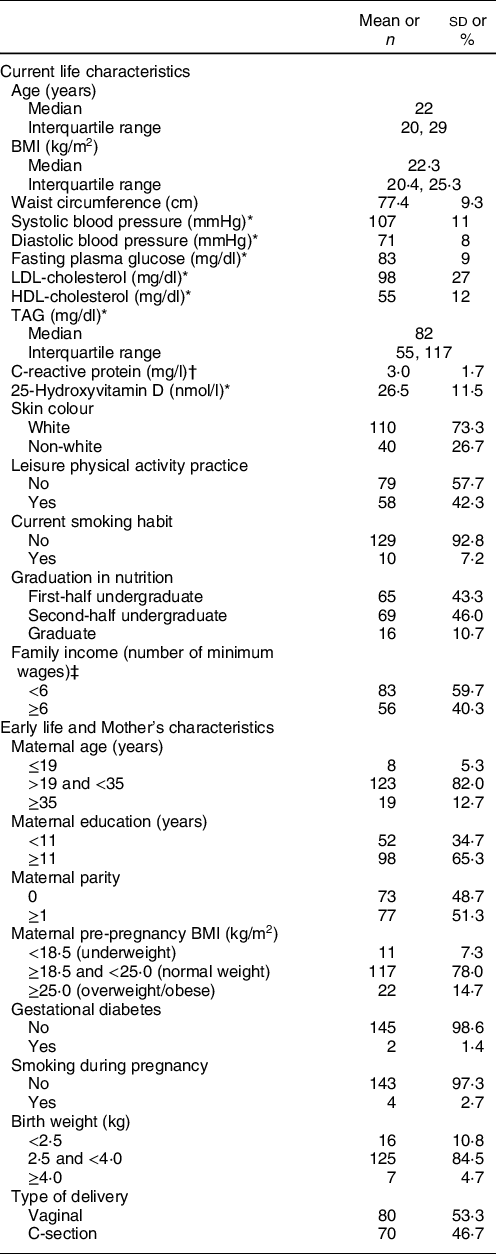
One hundred and fifty participants had body composition assessed by DXA. Median age was 22 years (interquartile range 20–29 years), and mean values of blood pressure, plasma glucose and lipids biomarkers, as well as C-reactive protein and 25-hydroxyvitamin D were within normal ranges. Most participants were undergraduates (89·3 %) and reported white skin colour (73·3 %) (Table 1). The majority had normal weight (64·0 %), 6·7 % were underweight, 22 % overweight and 7·3 % had obesity.
Table 1 Current and early life characteristics of Nutritionists’ Health Study (NutriHS) participants included in the present study (n 150) and their mothers’ data
* n 117.
† n 111.
‡ One minimum wage is equivalent to 724·0 Brazilian real (or US$ 212·9).
Regarding maternal characteristics and early life events, most mothers had at least 11 years of education (65·3 %). Excessive pre-pregnancy weight was reported by 14·7 %. Frequencies of gestational diabetes and smoking during pregnancy were 1·4 and 2·7 %, respectively. A slight predominance (53·3 %) of vaginal delivery was reported, and most participants were born with adequate weight (84·5 %) (Table 1).
Measurements of the three body compartments are shown in Table 2. Higher mean values of VAT, android-to-gynoid ratio and fat mass index were verified in participants whose mothers had excessive pre-pregnancy BMI compared with those with eutrophic mothers before pregnancy. ASM index and bone compartment measurements did not differ according to maternal pre-pregnancy BMI.
Table 2 Means and sd of body composition measurements according to maternal pre-pregnancy BMI (n 150)
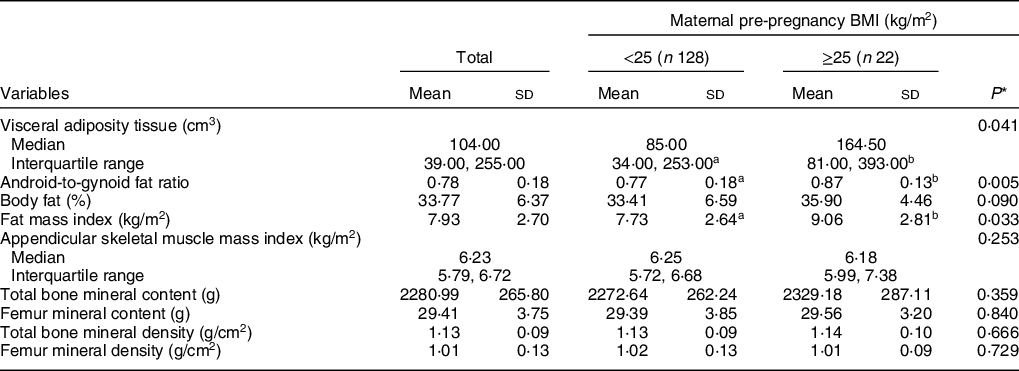
a,bMean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Refers to Mann–Whitney U test or Student’s t test.
Maternal pre-pregnancy BMI was directly associated with all adiposity measurements of their daughters (Table 3). Both, measurements of general adiposity, such as percentage of body fat (β = 0·31; P = 0·050) and fat mass index (β = 0·17; P = 0·015), and of central adiposity, such as VAT (β = 0·09; P = 0·011), as well as android-to-gynoid ratio (β = 0·01; P = 0·005), showed increasing values as maternal pre-pregnancy BMI increased. Maternal pre-pregnancy BMI was also associated with ASM index (β = 0·007; P = 0·039), but not with any bone compartment measurements. Results did not change after adjustment for height (model 2), nor in sensitivity analysis performed considering only the participants with clinical data available (n 117) (see online supplementary material, Supplemental Table 1a). Sensitivity analysis was also performed excluding participants whose mothers reported maternal pre-pregnancy BMI lower than 18·5 kg/m2 (n 11) (see online supplementary material, Supplemental Table 1b). The only change was the loss of the statistical significance of the previous association detected with appendicular skeletal muscle mass index.
Table 3 Linear regression analysis for the association between maternal pre-pregnancy BMI and offspring’s DXA-determined body composition measurements (n 150)
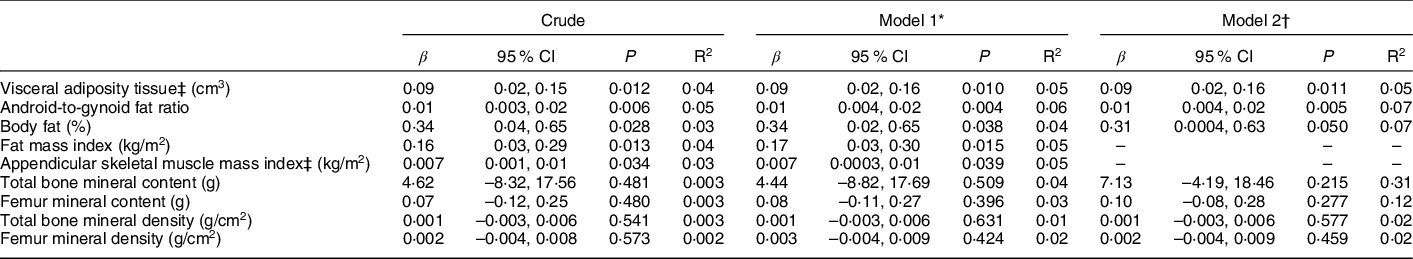
* Adjusted by maternal age (≤19, >19 and <35, ≥35 years), maternal education (<11, ≥11 years) and parity (0, ≥1), as recommended by DAG.
† Adjusted by variables included in model 1 plus height (cm).
‡ Variables were log-transformed.
Discussion
Principal findings of the study
A detailed and accurate evaluation of the three body compartments was performed at early adulthood when cardiometabolic profile is still normal. In a sample of 150 apparently healthy young women, using DXA, our findings indicated a direct association between maternal pre-pregnancy BMI and measurements of offspring adiposity, both general (fat mass index and percentage of bod fat) and central (android-to-gynoid ratio and VAT) fatness. A weaker direct association with muscle compartment was also identified, but it was statistically significant only when underweight mothers were considered. Maternal pre-pregnancy BMI was not associated with offspring bone compartment.
Strengths and limitations
This is the first study to evaluate the association between maternal pre-pregnancy BMI and visceral adiposity of the young adult offspring. Several clinical data confirmed that our sample had not adiposity-related disorders, representing a unique opportunity to investigate usefulness of body composition measurements even before the occurrence of metabolic diseases. The use of a DXA equipment able to identify VAT is a strength of our study, considering that accumulation of this particular tissue is involved in the pathophysiology of the most important public health problem nowadays.
Another strength was related to statistical analyses including adjustments oriented by DAG. This approach has been valued due to its adequacy to test associations of interest minimising confounding bias and avoiding over adjustments that might introduce bias(Reference Shrier and Platt22,Reference Textor, Hardt and Knüppel23) . Another advantage of using the minimal sufficient adjustment suggested by DAG regards to increased statistical efficiency of the analysis (with fewer covariates in the model, there are more df)(Reference la Bastide-Van Gemert, Stolk and van den Heuvel20–Reference Shrier and Platt22).
Although there is possibility that women could self-report pre-pregnancy weight with reasonable accuracy almost 30 years later(Reference Chin, Baird and McConnaughey24), the recall of maternal pre-pregnancy BMI implies recall bias that should be considered a limitation of the present study. Additionally, our small and homogenous sample, composed of young, apparently healthy and educated women (and their mothers), implies limitation regarding external validity; that is, generalisation of our results to different samples with different characteristics is not appropriate. Majority of participants’ mothers seems to be healthier when get pregnant; thus, memory (self-reported early life data could lead to misclassification) and selection bias should be considered as limitations of the current study. Especially, the frequency of mothers who reported gestational diabetes was lower in our study (1·4 %) compared with other Brazilian studies conducted in 90’s(Reference Coutinho and Silva Júnior25) and 00’s(Reference Dode and Santos26), in which prevalence rates were 2·4 and 2·9 %, respectively. Similarly, the frequency of maternal pre-pregnancy overweight/obesity was lower in the present study (14·7 %) compared with others, in which prevalence was around 45 %(Reference Dode and Santos26,Reference Reichelt, Weinert and Mastella27) .
Although external validity is a concern in our study, internal validity could be reasonably assured due to the accurate method for outcomes assessment and to the inclusion of an educated sample with skills to manage research tools and aware of the relevance of scientific research, that enhanced chances of reliable answers and good quality of reported data.
Results in the context of what is known
The direct association between maternal pre-pregnancy BMI and offspring adiposity agreed with previous studies that also assessed body composition using indirect methods, such as DXA and air-displacement plethysmography in childhood(Reference Kaar, Crume and Brinton4–Reference Cadenas-Sanchez, Henriksson and Henriksson7,Reference Castillo-Laura, Santos and Quadros28) . We found three studies assessing offspring adiposity in adulthood, but only one considered maternal pre-pregnancy BMI(Reference Chaparro, Koupil and Byberg8), while the others assessed maternal weight at early or late pregnancy(Reference Reynolds, Osmond and Phillips13,Reference Eriksson, Sandboge and Salonen29) . One of them was conducted with 276 adults in Scotland whose skinfold-estimated body fatness was predicted by maternal early pregnancy BMI(Reference Reynolds, Osmond and Phillips13). Similar to our findings, a Swedish study assessed body composition of 226 participants aged 19–21 years using DXA and identified that maternal pre-pregnancy BMI was directly associated with fat mass of daughters, but not sons. However, VAT measurement was not available(Reference Chaparro, Koupil and Byberg8).
The association between maternal pre-pregnancy BMI and offspring central adiposity in young adult women, expressed by the VAT volume and the android-to-gynoid ratio in our study, had not been reported, but agrees with studies conducted with children in the USA(Reference Kaar, Crume and Brinton4,Reference Tan, Roberts and Catov6) . The latter studies verified that offspring of overweight or obese mothers had higher visceral adiposity compared with children born to women with pre-pregnancy BMI lower than 25 kg/m2.
In contrast to others, we highlight that our sample included a low proportion of participants with overweight or obese mothers before pregnancy and still such associations were present, suggesting being maintained until adulthood. It could be argued that the epigenetic impact of mothers’ adiposity on their offspring occurs in a continuum during the life cycle of their daughters.
We reinforce that participants of our study had on average normal BMI and were apparently healthy, that is, without disturbances of glucose and lipid metabolism, low-grade inflammation or vitamin D deficiency. It is known that vitamin D contributes to reduce inflammation, which has an important role for cardiometabolic disease development(Reference Moukayed and Grant30). Normal mean values of C-reactive protein and 25-hydroxyvitamin D support that our sample is at low cardiometabolic risk. Even so, findings suggest that general and visceral fat contents could be impacted by maternal adiposity. Thus, our study calls attention to the importance of nutritional care during the pre-gestational period. Avoiding maternal weight excess at conception should contribute to preventing increase of offspring visceral adiposity, which is known to be an important trigger of insulin resistance and cardiometabolic diseases(Reference Figueroa, Takx and MacNabb14,Reference De Larochellière, Côté and Gilbert15) . We emphasise these aspects against the current scenario of worldwide prevalence of overweight women of 38 % and even higher in Brazil(Reference Ng, Fleming and Robinson31), favouring a vicious cycle of obesity transmission.
Intergenerational transmission of body adiposity has been explained by three main mechanisms. First, by a genetic component, considering a report that around 60 % of fat mass may be hereditable(Reference Elder, Roberts and McCrory32). Second, by epigenetic mechanisms, that is, maternal adiposity could induce adaptive response during pregnancy that is a period of plasticity. According to the Developmental Origins of Health and Disease, this period comprises the first thousand days of life, starting at conception. Developmental Origins of Health and Disease proposes that insults during this period (e.g. fetal overnutrition) might trigger epigenetic changes, such as DNA methylation, resulting in phenotypic alterations that could increase the risk of disease development later in life(Reference Fernandez-Twinn, Constância and Ozanne11,Reference Barnes, Heaton and Goates33,Reference Gluckman, Hanson and Low34) . The third mechanism refers to common environmental factors, transmitted from mother to offspring, such as dietary habits(Reference Silventoinen, Rokholm and Kaprio9,Reference Elder, Roberts and McCrory32) . Our previous study(Reference Eshriqui, Folchetti and Valente12) showed that daughters of overweight mothers had lower chance to adhere to a prudent diet, characterised by healthy foods such as fruits, vegetables and whole grains, and higher chance to adhere to a processed diet, composed of high energy density foods, such as fast foods, sweets and fried food, which could favour body fat accumulation(Reference Cespedes Feliciano, Tinker and Manson35).
Our study also showed that maternal pre-pregnancy BMI seemed to impact offspring ASM, but this association lost statistical significance when participants whose mothers were underweight were excluded. Different from ours, the Swedish study(Reference Chaparro, Koupil and Byberg8) verified that maternal pre-pregnancy BMI was inversely associated with daughters’ lean mass in early adulthood, but the association was not significant for sons. On the other hand, studies in childhood using air-displacement plethysmography have reported direct association between maternal BMI and offspring lean mass(Reference Castillo, Santos and Matijasevich5,Reference Cadenas-Sanchez, Henriksson and Henriksson7) . A meta-analysis of studies that assessed body composition at childhood using indirect methods also reported that maternal pre-pregnancy BMI seems to be directly associated with offspring lean mass, although high heterogeneity between studies included(Reference Castillo-Laura, Santos and Quadros28). Controversies could be attributed to different approaches to assess body composition and subjects’ characteristics. We highlight that, as composed by Brazilians, our study sample is characterised by high miscegenation, which could also limit comparability with other studies.
Our results do not support an impact of maternal pre-gestational BMI on offspring bone mass in adulthood. In contrast, results from Avon Longitudinal Study of Parents and Children indicated a direct association between maternal BMI and offspring bone size and density in childhood; however, the association was similar when considering paternal BMI instead of maternal. Also, this relation was mainly explained by child’s height, thus suggesting that the influence of maternal pre-pregnancy BMI on offspring bone mass was not explained by intrauterine environment but seemed to be driven by family shared environmental and genetic factors(Reference Macdonald-Wallis, Tobias and Smith16). We speculate that these common environmental characteristics become weaker during adolescence and adulthood, when subjects are more independent and acquire their individuality.
The hypothesis that maternal pre-pregnancy BMI impacts her daughters’ visceral adiposity in early adulthood which could contribute to the development of cardiometabolic diseases needs to be investigated in the follow-up of NutriHS participants and in populations with different characteristics.
Conclusion
We conclude that maternal pre-pregnancy BMI seems to be long-termly associated with female offspring general and visceral adiposity, even among apparently healthy young women. These findings reinforce the relevance of nutritional care for women at childbearing age (especially when planning pregnancy) as an attempt to prevent excess of maternal adiposity during pregnancy and the propagation of the vicious cycle of obesity to offspring. Interventions in early life must be prioritised once it shows potential to drive favourable health trajectories with less effort, compared with actions aiming change of habits in later life.
Acknowledgements
Acknowledgements: We thank the NutriHS Research Team. Financial support: The present study was supported by the São Paulo Foundation for Research Support (FAPESP), Brazil (grant numbers: S.R.G.F. – 2015/10045-7; I.E. and S.R.G.F. – 2017/13087-8). Conflict of interest: None. Authorship: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by I.E., A.M.M.V., L.D.F. and B.de.A.-P. The first draft of the manuscript was written by I.E. and critically reviewed by S.R.G.F. Funding acquisition and supervision were performed by S.R.G.F. All authors commented on previous versions of the manuscript, read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Research Ethics Committee of the School of Public Health of the University of São Paulo (CAAE 12455313.8.0000.5421). Written informed consent was obtained from all subjects/patients. Disclosure Statements: FAPESP had no role in the design, analysis or writing of this article.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020005285






