Breast-feeding is the natural and preferable way to provide ideal food for newborns and infants. Breast milk gives infants all the nutrients they need for healthy growth and development. Several nutrition committees recommend exclusive breast-feeding for the first 6 months of life( 1 – 3 ). Complementary feeding (i.e. solid foods and liquids other than breast milk or infant formula, and follow-on formula) should not be introduced before 17 weeks and after 26 weeks of age( Reference Agostoni, Decsi and Fewtrell 2 ).
One relevant aspect of infant feeding is the timing of the introduction of unmodified cow’s milk. Current recommendations on the optimal age for the introduction of cow’s milk into the infant diet in industrialized countries are conflicting. The WHO allows introducing cow’s milk simultaneously with complementary foods during the weaning period when babies are about 6 months or older( 4 ). Some recommendations allow limited cow’s milk consumption (i.e. small volumes to be added to complementary foods) before the age of 12 months( Reference Agostoni, Decsi and Fewtrell 2 , 5 , Reference Koletzko, Bauer and Brönstrup 6 ). However, there are other recommendations that strongly advise against cow’s milk consumption before 9–12 months of age( 7 ) or the age of 12 months( 8 , Reference Baehler, Baenziger and Belli 9 ).
Although cow’s milk is a good source of protein, Ca and other nutrients( 10 , Reference Souci, Fachmann and Kraut 11 ), concerns exist about cow’s milk consumption in early infancy due to possible negative health effects. Fe deficiency is one of the main issues to be considered when weighing the pros and cons of the effects of unmodified cow’s milk v. formula. Fe is a critical nutrient in the first year of life and besides the fact that cow’s milk is a poor source of Fe, it is known to inhibit the absorption of non-haem Fe through components like Ca and casein( Reference Fleischer Michaelsen, Weaver and Branca 12 , Reference Ziegler 13 ). In addition, a child may require even more Fe than usual if he/she suffers from occult gastrointestinal blood loss which can occur after cow’s milk ingestion( Reference Oski 14 ). How much cow’s milk an infant ingested daily can become quite a significant question in light of Fe deficiency concerns. This is especially relevant if the infant is consuming greater amounts of cow’s milk in place of other complementary nutrient-rich foods that would be better suited to ensure adequate intake of all required nutrients( 8 , Reference Michaelsen, Hoppe and Lauritzen 15 ).
The main aim of the present review was to summarize the best available evidence for short- and long-term health effects of cow’s milk intake and to identify areas for future research. Our main research question was: what are the health effects of cow’s milk consumption in healthy, full-term infants up to 3 years of age? Further questions related to the timing of introduction and dose–response relationships.
Methods
We followed the guidelines of the Cochrane Collaboration for undertaking and reporting the results of the present systematic review( Reference Higgins and Green 16 ).
Search strategy
We identified references by searching electronic databases and reference lists from pertinent articles and existing guidelines on the topic. The search strategy was developed by the authors in conjunction with a senior information specialist. We searched the electronic databases MEDLINE (via PubMed), EMBASE and the Cochrane Library, between 1960 and July 2013, with a combination of MeSH (medical subject headings) terms and free-text keywords for relevant interventions (milk, milk products, follow-on formula, infant formula, breast milk) and outcomes. We limited the search to human studies in English or German language. The detailed search strategy is available in the online supplementary material. In addition, we checked reference lists from existing guidelines on cow’s milk consumption and pertinent articles.
All citations were imported to and managed in a bibliographic database (Endnote X·6·0·1) and duplicates were deleted.
Study eligibility criteria
Criteria for the consideration of studies for the present review were: (i) healthy infants aged between 17 weeks and 3 years; (ii) pasteurized animal milk, milk products or follow-on formula; (iii) patient-relevant outcomes; and (iv) randomized and non-randomized controlled studies and observational studies. These criteria are described in more detail in Table 1.
Table 1 Eligibility criteria for inclusion of studies in the present systematic review and meta-analysis

Study selection
Two researchers independently reviewed abstracts and full-text articles. If both reviewers agreed that the study did not meet eligibility criteria, we excluded it. Investigators resolved disagreements about inclusion or exclusion by consensus or by involving a third reviewer. When articles contained insufficient information to assess their eligibility or to extract relevant data, the corresponding author was contacted for further information. Studies reported only in abstract form were excluded.
Data extraction
Trained reviewers abstracted data from each included study into a structured data extraction sheet and assigned an initial quality rating. Investigators extracted data relating to: (i) the aims and duration of the study; (ii) observation period; (iii) study design and sample size; (iv) inclusion and exclusion criteria; (v) the intervention; (vi) outcome measurements; (vii) description of the study population; and (viii) results of the study relating to our research questions.
A senior reviewer evaluated completeness and correctness of data abstraction and confirmed the quality rating. Study authors were contacted to obtain details concerning the formula used.
Study quality
We evaluated the study quality for different study designs separately with standardized assessment forms. To assess the risk of bias of intervention studies, we used predefined criteria based on those developed by the Cochrane Collaboration( Reference Higgins and Green 16 ) and the Centre for Reviews and Dissemination of the University of York( 17 ). For the assessment of observational studies we applied the characteristics that were deemed essential by Deeks et al.( Reference Deeks, Dinnes and D’Amico 18 ).
Data analyses
A meta-analysis of included studies was not justified for most outcomes; therefore we synthesized the evidence on the majority of outcomes qualitatively according to study design, exposure and health outcome. If data were sufficient, we conducted random-effects (DerSimonian and Laird) meta-analyses to estimate pooled effects. We used relative risks (RR) as outcome measures. We tested for heterogeneity with Cochrane’s Q test and used the I 2 index to estimate the magnitude of heterogeneity. We explored the impact of study duration on Fe-deficiency anaemia (IDA) using meta-regression. We determined publication bias employing funnel plots and Kendell’s test. Because of the small number of included studies and investigated children, results of these tests have to be viewed cautiously. We conducted all statistical analyses using the Comprehensive Meta Analysis software version 2·2·064.
Rating the quality of evidence
We rated the quality of evidence for each outcome based on the approach suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group( Reference Balshem, Helfand and Schunemann 19 ). GRADE specifies four categories for the quality of a body of evidence: high, moderate, low and very low. According to the study design the basic rating is high for randomized controlled studies (RCT) and low for observational studies. Several criteria were then taken into account for down- or upgrading of the quality of evidence. For downgrading: risk of bias, inconsistency, indirectness, imprecision and publication bias; for upgrading: dose–response gradient, effect size and confounding in opposite direction( Reference Guyatt, Oxman and Kunz 20 – Reference Guyatt, Oxman and Vist 23 ).
Public posting of protocol
The study protocol was posted on the project website (www.richtigessenvonanfangan.at) for two weeks in November 2010 to gather public comments.
Results
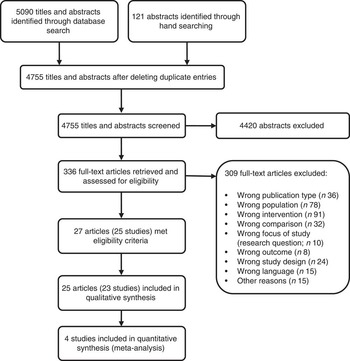
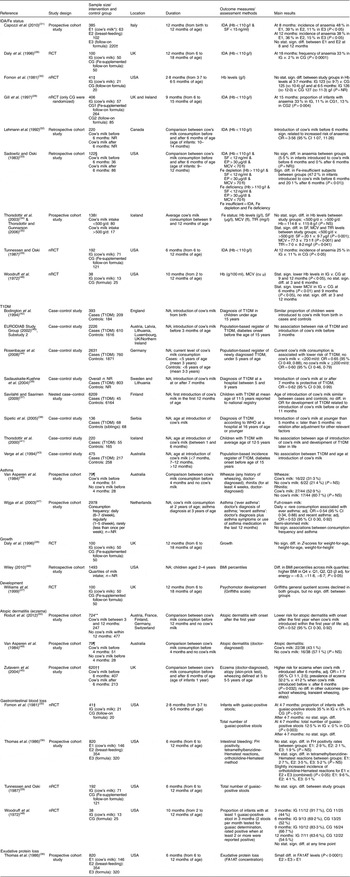
After screening 4755 titles and abstracts and assessing 336 full-text articles for eligibility, we identified twenty-seven publications (describing twenty-five studies) that met our inclusion criteria (see Fig. 1). Two studies were excluded from the qualitative analysis because of high risk of bias( Reference Koopman, Turkish and Monto 24 , Reference Skrodeniene, Marciulionyte and Padaiga 25 ). The remaining twenty-five publications (describing twenty-three studies of fair quality)( Reference Daly, MacDonald and Aukett 26 – Reference Roduit, Frei and Loss 50 ) investigated various health effects of cow’s milk intake and are presented in Table 2. Out of the twenty-three studies included in the review, one study was a randomized controlled trial (RCT)( Reference Daly, MacDonald and Aukett 26 , Reference Williams, Wolff and Daly 27 ), four were non-randomized controlled trials (nRCT)( Reference Gill, Vincent and Segal 28 – Reference Fomon, Ziegler and Nelson 30 , Reference Woodruff, Wright and Wright 48 ), seven studies were prospective cohort studies( Reference Capozzi, Russo and Bertocco 31 , Reference Thorsdottir, Gunnarsson and Atladottir 34 – Reference Thomas, McGilligan and Carlson 36 , Reference Zutavern, Von Mutius and Harris 45 , Reference Wijga, Smit and Kerkhof 47 , Reference Van Asperen, Kemp and Mellis 49 , Reference Roduit, Frei and Loss 50 ), three studies were retrospective cohort studies( Reference Lehmann, Gray-Donald and Mongeon 32 , Reference Sadowitz and Oski 33 , Reference Wiley 46 ) and eight were case–control studies( Reference Savilahti and Saarinen 37 – Reference Rosenbauer, Herzig and Giani 44 ). In the following sections, we present the most important results. Table 2 provides a more detailed summary of the results of individual studies.
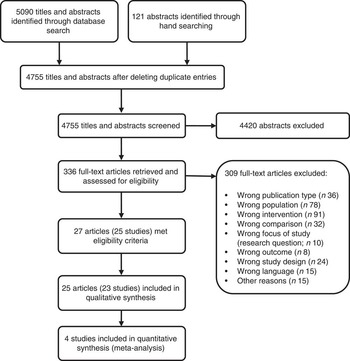
Fig. 1 Flow diagram for the study selection process
Table 2 Description of the studies investigating the health effects of cow’s milk intake
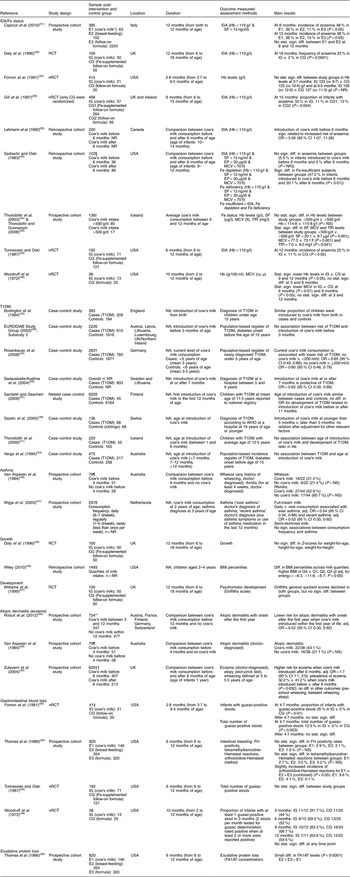
IDA, Fe-deficiency anaemia; T1DM, type 1 diabetes mellitus; RCT, randomized controlled trial; nRCT, non-randomized controlled trial; CG, control group; E, exposure group; IG, intervention group; NR, not reported; NA, not applicable; SF, serum ferritin; EP, erythrocyte porphyrin; MCV, mean corpuscular volume; TfR, serum transferrin receptors; cu μ, cubic micron calculated according to Wintrobe; FH, faecal Hb; FA1AT, faecal α1-antitrypsin; stat., statistically; sign., significant; diff., difference; Q, quartile; adj., adjusted.
* Cow’s milk consumption is always whole cow’s milk unless otherwise stated.
† Children in both groups also received breast milk during the first 2 months of life.
‡ We report only data on boys, because girls received either pasteurized cow’s milk or extensively heat-treated cow’s milk, but no infant formula.
§ We report only data on white infants.
|| Blood samples were only available for ninety-seven children.
¶ The original cohort included ninety-two children.
** In total, 1041 children were included in the study, but the analyses were restricted only to children having no atopic dermatitis in the first year (n 724).
†† The original cohort included 642 children.
Fe-deficiency anaemia
Nine studies, one RCT( Reference Daly, MacDonald and Aukett 26 ), four nRCT( Reference Gill, Vincent and Segal 28 – Reference Fomon, Ziegler and Nelson 30 , Reference Woodruff, Wright and Wright 48 ), two prospective cohort studies( Reference Capozzi, Russo and Bertocco 31 , Reference Thorsdottir, Gunnarsson and Atladottir 34 , Reference Thorsdottir and Gunnarsson 35 ) and two retrospective cohort studies( Reference Lehmann, Gray-Donald and Mongeon 32 , Reference Sadowitz and Oski 33 ), addressed the risk of developing IDA in infants who regularly consumed cow’s milk over a longer period of time, providing data on 1642 children between the ages of 0 and 18 months. Overall, seven out of eight studies reported a substantially greater risk of developing IDA in infants who were fed cow’s milk compared with those who received Fe-fortified follow-on formula. Another nRCT( Reference Woodruff, Wright and Wright 48 ) showed lower Hb levels at 9 and 12 months in infants fed cow’s milk starting from 2 months of age compared with infants who received non-Fe-fortified formula. The only study that did not find a statistically significant difference in Fe status markers compared twenty-one infants who received cow’s milk v. twenty infants who received formula with low Fe content. However, all infants in that study received Fe supplementation of 12 mg/d and vitamin C supplementation( Reference Fomon, Ziegler and Nelson 30 ). Four of the included studies indicated that the amount of cow’s milk or/and formula consumed was measured. Values were reported in three studies and ranged between 538 and 983 ml/d in infants between 3·7 and 18 months( Reference Daly, MacDonald and Aukett 26 , Reference Fomon, Ziegler and Nelson 30 , Reference Lehmann, Gray-Donald and Mongeon 32 ). In one study all study participants received an Fe supplement of 12 mg/d( Reference Fomon, Ziegler and Nelson 30 ) and in another study 11 % of infants reported the use of Fe supplements as drops( Reference Lehmann, Gray-Donald and Mongeon 32 ). In one study the use of non-dietary Fe supplements was not permitted( Reference Gill, Vincent and Segal 28 ). Only a single study specifically reported Fe intake for each study group( Reference Daly, MacDonald and Aukett 26 ); it concluded that follow-on formula contributed a substantial proportion of total Fe intake( Reference Daly, MacDonald and Aukett 26 ).
Two retrospective cohort studies( Reference Lehmann, Gray-Donald and Mongeon 32 , Reference Sadowitz and Oski 33 ) (342 infants) investigated the age of introduction of cow’s milk and the associated risk for IDA, finding an increased risk of IDA and Fe insufficiency in children exposed to cow’s milk before 6 months of age compared with those who were exposed after this age. One prospective cohort study( Reference Thorsdottir, Gunnarsson and Atladottir 34 , Reference Thorsdottir and Gunnarsson 35 ) explored a possible dose–response relationship in 138 infants before 12 months of age and found higher levels of the Fe status markers serum ferritin and mean corpuscular volume in infants consuming <500 g cow’s milk/d compared with those consuming >500 g/d, but no difference in Hb levels.
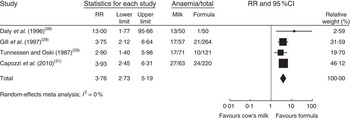
In a meta-analysis, we pooled data from one RCT( Reference Daly, MacDonald and Aukett 26 ), two nRCT( Reference Gill, Vincent and Segal 28 , Reference Tunnessen and Oski 29 ) and one prospective cohort study( Reference Capozzi, Russo and Bertocco 31 ) (with a total of 1083 study participants). The duration of cow’s milk exposure varied between 6 and 12 months, starting at birth or at 6 months of age. Pooled results rendered a more than three times higher risk of IDA for infants consuming cow’s milk compared with those drinking follow-on formula (RR=3·76; 95 % CI 2·73, 5·19; Fig. 2). In these four studies 25–38 % of infants consuming cow’s milk developed IDA compared with 2–15 % of those fed with Fe-fortified formula.
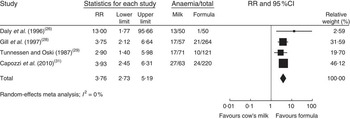
Fig. 2 Meta-analysis of the relative risk (RR) of iron-deficiency anaemia between cow’s milk and formula in healthy, full-term infants up to 3 years of age. The study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI
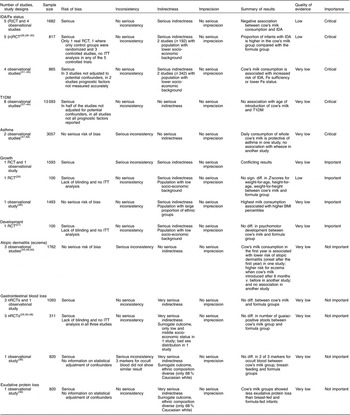
In a meta-regression we explored the impact of duration of exposure to cow’s milk (4–8 months) on the risk of developing IDA. Results showed no statistically significant association (P=0·85). We could not find any relationship between duration of cow’s milk consumption and IDA. Overall, we graded the quality of evidence that cow’s milk consumption in healthy, full-term infants up to 18 months of age leads to IDA as low, indicating substantial uncertainty about the estimate of the effect. The evidence base is limited by serious risk of bias due to the lack of randomization in three of four controlled studies, missing intention-to-treat (ITT) analysis in all four controlled studies and the lack of adjustment for potential confounders in three of four observational studies. Furthermore, half of the studies were conducted in families with low or very low socio-economic background, which limits the applicability to the general population (Table 3).
Table 3 Quality of evidence for health effects of cow’s milk consumption in infants up to 3 years old, for each outcome (according to GRADE)

GRADE, Grading of Recommendations Assessment, Development and Evaluation; IDA, Fe-deficiency anaemia; T1DM, type 1 diabetes mellitus; RCT, randomized controlled trial; nRCT, non-randomized controlled trial; ITT, intention-to-treat; sign., significant; diff., difference.
Type 1 diabetes mellitus
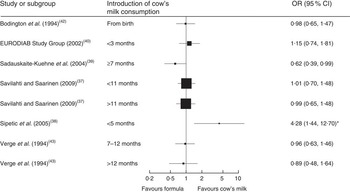
Seven case–control studies (with a total of 2007 cases and 8455 controls) investigated the association between the age of introduction of cow’s milk and type 1 diabetes mellitus (T1DM)( Reference Savilahti and Saarinen 37 – Reference Verge, Howard and Irwig 43 ). In the adjusted analyses, all but one study( Reference Sadauskaite-Kuehne, Ludvigsson and Padaiga 39 ) consistently showed no difference in the risk for T1DM due to early introduction of cow’s milk, starting from birth or after 3, 5, 7 or 11 months. Figure 3 depicts the results of all studies reporting odds ratios in a forest plot. Because of heterogeneity regarding age of study participants and durations of exposure to cow’s milk, we did not conduct a meta-analysis of these studies.
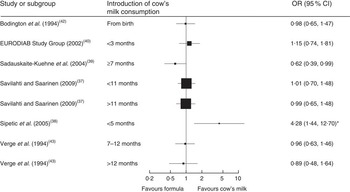
Fig. 3 Forest plot of studies investigating the association between the age of introduction of cow’s milk and type 1 diabetes mellitus in healthy, full-term infants up to 3 years of age. Only studies reporting OR are depicted. The study-specific OR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight. *The significant association disappeared after adjustment for other factors significantly related to diabetes
One case–control study( Reference Rosenbauer, Herzig and Giani 44 ) (670 cases, 1871 controls) found that current consumption of cow’s milk <200 ml/d or ≥200 ml/d compared with no consumption was associated with a lower risk of T1DM (OR=0·65; 95 % CI 0·46, 0·88 and OR=0·60; 95 % CI 0·46, 0·79, respectively).
The quality of evidence is very low, mostly because of the retrospective study design and the concomitant serious risk of bias of included studies. Half of the studies did not adjust for potential confounders and none of the studies reported all relevant prognostic factors (Table 3).
Asthma
One prospective cohort study (n 2978) investigated the effect of consumption frequency of cow’s milk on the prevalence of asthma( Reference Wijga, Smit and Kerkhof 47 ) and found that daily v. rare consumption of full-cream milk had a protective effect on asthma (‘ever asthma’: adjusted OR=0·54; 95 % CI 0·34, 0·88 and recent asthma: adjusted OR=0·53; 95 % CI 0·30, 0·92). No significant associations between consumption and frequency of asthma were found for semi-skimmed milk (Table 2). Another prospective cohort study (n 79) showed no significant association between the occurrence of wheeze or rhinitis and the introduction of cow’s milk in the first 4 months of life( Reference Van Asperen, Kemp and Mellis 49 ).
We rated the quality of evidence as very low, indicating great uncertainty about the validity of this finding, because it is based on a single observational study (Table 3).
Growth
One RCT( Reference Daly, MacDonald and Aukett 26 ) and one prospective cohort study( Reference Wiley 46 ) did not show a consistent effect of cow’s milk consumption on infants’ growth. The RCT reported no significant differences in growth parameters (weight-for-age, height-for-age and weight-for-height) between cow’s milk and follow-on formula intake. In the prospective cohort study a higher cow’s milk intake was associated with higher BMI percentiles. No conclusions can be drawn from these two conflicting studies. The quality of evidence is very low because of the lack of blinding and ITT analysis in the RCT, and there is limited applicability to the general population because of participants from low socio-economic background and large proportions of ethnic groups (Table 3).
Development
One RCT( Reference Williams, Wolff and Daly 27 ) showed no statistically significant difference in psychomotor development between the cow’s milk and the formula groups. The quality of evidence is very low because of serious risk of bias due to lack of blinding and ITT analysis, and external validity is low because the investigated population had a low socio-economic background which limits the applicability to the general population (Table 3).
Atopic dermatitis (eczema)
Three prospective cohort studies( Reference Zutavern, Von Mutius and Harris 45 , Reference Van Asperen, Kemp and Mellis 49 , Reference Roduit, Frei and Loss 50 ) investigated the effect of cow’s milk intake on atopic dermatitis with inconsistent results. One study (n 1041) showed that the introduction of cow’s milk in the first year of life reduced risk for the development of atopic dermatitis with onset after the first year of life compared with no cow’s milk (Table 2). Another study (n 642) reported a higher risk for eczema when cow’s milk was introduced after 6 months of age compared with before 6 months( Reference Zutavern, Von Mutius and Harris 45 ). A third study (n 79) showed no significant association between the occurrence of atopic dermatitis and the introduction of cow’s milk in the first 4 months of life( Reference Van Asperen, Kemp and Mellis 49 ). Because of the inconsistency of results, the inherent risk of bias and confounding, and the risk of chance findings because of low event rates, we rated the quality of evidence as very low (Table 3).
Gastrointestinal blood loss
Three nRCT( Reference Tunnessen and Oski 29 , Reference Fomon, Ziegler and Nelson 30 , Reference Woodruff, Wright and Wright 48 ) and one cohort study( Reference Thomas, McGilligan and Carlson 36 ) (with a total of 1091 study participants) showed no association between cow’s milk consumption and gastrointestinal blood loss after the age of 3 and 6 months, respectively. One of the three nRCT found a higher risk of faecal blood stools when children consumed pasteurized cow’s milk compared with follow-on formula at the age of 3·7 to 4·7 months. The quality of evidence is very low because of lack of blinding and ITT analysis in the controlled studies and no statistical adjustment in the observational studies. Furthermore, surrogate markers for gastrointestinal blood loss were used and applicability is hampered because of low socio-economic status and a diverse ethnic composition (Table 3).
Discussion
To our knowledge, the present study is the first systematic review and meta-analysis on the health effects of cow’s milk consumption in infants up to 3 years of age. The results of our meta-analysis with data from four studies indicate a more than three times higher risk for IDA at ages 8 to 18 months in infants who consume cow’s milk compared with those who consume Fe-fortified formula for duration of 6–12 months starting either at birth or at 6 months of age. This confirms what has been regarded as common knowledge until now: that consumption of cow’s milk in infancy leads to an increased risk for developing IDA. The quality of evidence is low due to the fact that several methodological flaws in the available studies introduce a serious risk of bias. Furthermore, some of the studies were carried out in populations with low socio-economic status which by itself is an independent risk factor for the development of Fe deficiency( Reference Oski 51 ). Nevertheless, the very large increase in risk attributable to cow’s milk that our meta-analysis found is unlikely to be caused exclusively by bias and confounding. Due to the fact that the group of infants included in our meta-analysis all received Fe-fortified formula, it is likely that the observed increase in risk for IDA in the group of infants fed cow’s milk is largely explained by differences in Fe intake. However, studies comparing different formulas with high and low Fe content showed no statistically significant difference in anaemia incidence, although in most studies infants in the group fed with high-Fe formula had higher ferritin levels than the infants fed low-Fe formula( Reference Domellöf, Braegger and Campoy 52 ). In the study by Gill et al.( Reference Gill, Vincent and Segal 28 ) a smaller proportion of infants receiving the non-fortified formula were anaemic compared with infants fed cow’s milk (13 % v. 33 %). It is therefore possible that cow’s milk exerts adverse effects on Fe status on its own, either by inhibition of non-haem Fe absorption or by another unknown mechanism or a combination of all( Reference Ziegler 53 ). Young children are most susceptible to Fe deficiency as a result of an increased Fe requirement related to rapid growth during the first 2 years of life( Reference Kleinman 54 ). However, little is known about whether cow’s milk is also detrimental if the diet otherwise provides sufficient Fe for the infant. Meat is a good source of Fe because it contains a high proportion of haem Fe which has a higher rate of absorption compared with non-haem Fe. Non-haem Fe found in plant foods and fortified food products has lower rates of absorption. Ascorbic acid and the concurrent consumption of meat are enhancers of non-haem Fe absorption. Tea, bran and milk tend to inhibit non-haem Fe absorption( Reference Kleinman 54 ). Cow’s milk generally tends to be less expensive than infant formula or follow-on formula, and should therefore be considered a possible alternative if an infant who consumes milk can still achieve a well-balanced diet along with Fe-rich sources. Efforts should be made to increase public awareness regarding the risk of anaemia associated with cow’s milk consumption in infants up to 18 months. Mothers who opt to feed their infants cow’s milk should be provided enough information to do so conscientiously by ensuring that the babies given cow’s milk also receive an Fe-rich diet with foods that enhance Fe absorption.
Cow’s milk exposure during infancy has been described as a possible risk factor for the later development of T1DM( Reference Gerstein and VanderMeulen 55 ). Earlier studies showed an increased risk of T1DM when an infant was exposed to cow’s milk at an early age and breast-feeding lasted less than 3 months( Reference Gerstein 56 ). A systematic review highlighted the role of exclusive breast-feeding as a protective factor against the development of T1DM( Reference Patelarou, Girvalaki and Brokalaki 57 ). In contrast to earlier results, our review shows no association between the age of introduction of cow’s milk and T1DM. The difference in results may be explained by the definition of cow’s milk exposure. Whereas in all studies that we included for the question regarding T1DM, the intake of pure cow’s milk was investigated, other reviews did not confine cow’s milk exposure in such a way and included all exposure to dairy proteins including cow’s milk-based formulas. Therefore, it seems likely that the duration of breast-feeding exhibits a protective effect and the concomitant introduction of cow’s milk may not be associated with the risk of developing T1DM later in life.
The evidence concerning the question if cow’s milk intake poses a risk for asthma, or is negatively associated with development, showed no negative effects of cow’s milk intake in infancy.
Although associations between high protein intake early in life and an increased risk of developing obesity later in life have been shown previously( Reference Michaelsen, Larnkjaer and Molgaard 58 ), our study did not find evidence for an association between cow’s milk intake in infancy and growth. With regard to atopic dermatitis, we found very-low-quality evidence that showed infants exposed to cow’s milk in the first year of life were less likely to develop atopic dermatitis than those infants who were not exposed to cow’s milk.
Our systematic review did not identify conclusive evidence for a dose–response relationship between the consumption of cow’s milk and any of the described outcomes.
Strengths and limitations of the review
The strength of our review is that it used state-of-the-art methods to objectively and systematically assess the benefits and risks of cow’s milk intake in healthy, full-term infants. We searched multiple scientific databases, hand searched reference lists and contacted authors to receive additional data or information about their studies. Nevertheless, some potential limitations of the review process exist. Naturally, it is possible that we did not identify all relevant publications. We may have introduced bias by excluding publications written in languages other than English or German.
The main limitation of our review, however, is that the strength of our conclusions is limited by the low methodological quality of the primary studies that are available for our questions of interest. The identified articles were very heterogeneous in terms of their study designs and methods used for measurement of exposure. In particular, our search did not identify sufficient high-quality controlled trials. Although it would be unethical to randomize mothers into groups who either breast-feed or feed their babies cow’s milk, researchers can conduct high-quality prospective studies using methods such as propensity score analyses to adjust for potential confounders. Finally, the conclusions of our systematic review apply only to healthy and term-born infants living in countries with a population comparable to European populations.
Implications for research
Further high-quality studies should be conducted to contribute sufficient high-quality evidence as grounds for public recommendations. Such studies should specifically address questions concerning the kinds and amounts of complementary food necessary to ensure that infants consuming cow’s milk have sufficient Fe intakes. Additional research should compare the effects of introducing cow’s milk into the diets of children who are younger than 6 months, between 6 months and 12 months old, and older than 1 year of age.
Implications for practice
According to the results of the present review, the only currently known risk associated with introducing cow’s milk to a young infant is that the child will develop Fe deficiency. This could occur if the child is given too much cow’s milk in place of alternative food sources that would support a well-balanced infant diet. Further research is needed to make concrete public recommendations. Parents and caregivers should have access to high-quality information regarding the effects of cow’s milk consumption on their babies. Recommendations regarding cow’s milk should provide parents with information regarding the ideal age to introduce a child to cow’s milk, the proper amount for that age, as well as the most effective dietary complements (Fe-source foods). In the absence of clear evidence answering questions about the optimal age to introduce a child to cow’s milk and the proper amount for that age, expert consensus is the best way to provide guidance to parents.
Acknowledgements
Financial support: This work was supported by the Austrian Agency for Health and Food Safety (AGES); the Austrian Federal Ministry of Health; and the Main Association of Austrian Social Security Institutions. The funders had no role in the design, analysis or writing of this article. Conflicts of interest: None. Authorship: A.W., A.H. and G.G. initiated and designed the study; U.G., M.U.B., C.K., B.D., B.M., K.S. and R.E. conducted the literature review (abstract and full-text review, data abstraction) and synthesized the data; G.G. performed the meta-analysis; U.G., C.K., A.H. and G.G. prepared drafts of the manuscript; U.G. had primary responsibility for the final content of the manuscript; and all authors read, critically revised and approved the final manuscript. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980015001354