Findings of validation and comparative risk studies indicate that the standard dietary assessment instrument in large-scale prospective studies since the 1980s, the FFQ, might not provide the dietary data needed to investigate diet–disease relationships properly(Reference Bingham, Luben and Welch1, Reference Subar, Kipnis and Troiano2). Therefore, researchers argue about whether to replace or supplement the error-prone FFQ by short-term dietary assessment instruments, such as food records or repeated 24 h dietary recalls (24-HDR)(Reference Kristal, Peters and Potter3). Compared with an FFQ that enquires about diet over a relatively long period of time, short-term instruments may have a potential cognitive advantage and provide quantitative and detailed dietary data(Reference Thompson and Subar4). Furthermore, especially the 24-HDR has the ability to obtain standardized dietary information in culturally diverse groups or multi-centre studies(Reference Slimani, Deharveng and Charrondiere5). However, the variance of dietary estimates based on repeated 24-HDR is inflated by intra-individual variance(Reference Tarasuk and Beaton6, Reference Beaton, Milner and Corey7). In addition, the estimation of dietary intakes of irregularly consumed foods, if not supplemented, may be biased, since the 24-HDR usually includes a substantial proportion of participants reporting zero intakes during the recorded days. Long-term instruments such as FFQ or food propensity questionnaires (FPQ – an FFQ that considers only the frequency of consumption) may have the potential to identify habitual users of such foods(Reference Subar, Dodd and Guenther8). Recent statistical advances in estimating usual dietary intakes therefore combine the advantages of both quantitative and rich 24-HDR data and non-quantitative FFQ/FPQ information about habitual consumers while minimizing their limitations (e.g. adjusting for intra-individual variation in the 24-HDR data)(Reference Illner, Nothlings and Wagner9). Promising results have already been obtained from methodological and simulation studies showing an increased precision when including the reported frequency of consumption on an FFQ or FPQ as a covariate in the model (J Haubrock, U Nöthlings, J-L Volatier et al., unpublished results)(Reference Kipnis, Midthune and Buckman10, Reference Kipnis, Shumakovich and Midthune11). Nevertheless, future challenges of these statistical combination methods include exploring empirically the optimal number of repeated short-term measurements, and also testing the feasibility of data collection, including the mode of enquiry to collect multiple dietary information, especially in culturally diverse populations. Particularly, FFQ or FPQ are suited to be adapted to Internet technologies and to being applied as web-based applications(Reference Beasley, Davis and Riley12, Reference Matthys, Pynaert and De13). However, knowledge about their practical feasibility and acceptance is still limited.
In the present paper, we report about the feasibility of applying three monthly telephone-administered 24-HDR in conjunction with a standardized FPQ in a multi-centre pilot study involving five ongoing European cohort populations. The FPQ (hereafter named European Food Propensity Questionnaire (EFPQ)) was created to be self-administered and available as both web- and paper-based versions. To evaluate the feasibility of the approach, we present response and participation rates, reasons for non-participation, percentages of completion of the instruments, number of contact attempts and data automatically recorded by web analysis.
Subjects, study design and methods
Subjects and study design
Between January and April 2009, a random sample of 400 active study participants was generated from five ongoing cohorts, according to age ranges and sex distributions in the vital source cohorts. An upper age limit of 75 years was applied. Thus, eighty participants were recruited from the European Prospective Investigation into Cancer and Nutrition (EPIC)-San Sebastian, Spain (vital source population: n 7973; 3833 men and 4140 women), EPIC-Florence, Italy (n 13 597; 3514 men and 10 083 women), EPIC-Potsdam, Germany (n 25 784; 10 335 men and 15 449 women) and the Norwegian Women and Cancer study, Norway (NOWAC; 165 772 women). In the cohort of the Estonian Genome Center (EGC), University of Tartu, Estonia, eligible subjects were invited during the ongoing baseline recruitment of participants into the cohort. In November 2010, EGC included almost 50 000 men and women. All cohorts were based on the general adult populations residing in a given geographical area.
Subjects were invited to complete the single, self-administered EFPQ, either in web-based (web-EFPQ) or paper format (paper-EFPQ), and to answer to the three non-consecutive, unannounced 24-HDR telephone interviews. Subjects were contacted by regular mail. The mail included the study invitation letter, information material and a response form on which reasons for non-participation, preferences for the EFPQ-administration modes and for the timing of the 24-HDR could be indicated (except in NOWAC, in which questions about non-participation were not permitted by the local privacy law). Furthermore, user-specific instructions with individual username and password for the web-EFPQ were provided to each participant to guarantee data protection. Consent of participation was obtained when subjects accepted the invitation to participate in the pilot study by either sending back the response sheet or directly using the web-EFPQ. Responders with preferences for the paper-EFPQ received a printed personal questionnaire by mail. Participants were offered support on request for both administration modes. To measure the acceptance of the web-EFPQ in each study centre, users were encouraged to complete a web-based brief evaluation questionnaire that enquired their opinion on the clarity of questions and explanations, visual elements, user friendliness, time needed to complete the questionnaire and difficulties experienced in estimating their food intake. A six-item Likert scale from 1 = brilliant to 6 = abysmal was offered to rate each question and a free text field was given to add comments and suggestions. There was no evaluation questionnaire administered for the paper-EFPQ. After having completed the EFPQ, a pilot study-specific booklet was mailed in order to help subjects during the three 24-HDR in quantifying their food consumption. An attempt to contact those subjects who answered the web-EFPQ for the first 24-HDR was made no earlier than 5 d after mailing of the picture booklet, whereas those who preferred the paper-EFPQ were immediately contacted after the return of the completed questionnaire. The 24-HDR, randomly assigned by day of the week, was collected approximately 1 month apart from February to September 2009. In most centres, food intake on Saturday was assessed on Monday. For each subject's 24-HDR, contact attempts were documented.
To address potential sources of bias, an explicit study protocol for quality control was designed ensuring a standardized data collection and study management across the centres. Necessary deviations from the common study protocol regarded a different sampling procedure in EGC. Because of the ongoing recruitment, eligible Estonian subjects were asked whether they were interested to participate in the pilot study during the recruitment examinations. After a short oral explanation, subjects received a closed envelope containing the study material and signed the consent of agreement or refused to participate. The study was approved by the responsible ethics committee of the participating centres.
Dietary assessment instruments
The EPIC-SOFT program (Dietary Exposure Assessment Group, International Agency for Research on Cancer/WHO, Lyon, France) was used for the administration of the 24-HDR. In Europe, the multi-language EPIC-SOFT is the primary software aimed at assessing detailed and quantified dietary data of a single day in a standardized manner(Reference Slimani, Deharveng and Charrondiere5, Reference Slimani, Ferrari and Ocke14). Country-specific versions were available in all centres conducting the pilot study, except in EGC, where the English version was used. In the 24-HDR interviews, trained personnel asked subjects to report all food and beverages consumed during the day before the interview. The pilot study-specific picture booklet contained a selection of photographs of portion sizes and household measures (e.g. mugs, glasses and bowls) from the original EPIC-SOFT picture booklet.
The multilingual EFPQ was based on an existing validated FFQ covering 102 typical German food items that was applied in the EPIC-Potsdam cohort(Reference Noethlings, Hoffmann and Bergmann15). The EFPQ was developed using a web-based portal that allowed nutritionists of the pilot study centres to review and modify an English translated version of the German FFQ regarding its adequacy in reflecting the dietary habits of the selected European countries. The experts being familiar with the usual dietary habits in the local population under study could decide online with predefined choices whether or not each item should be kept, modified to a country-specific food or deleted. Additional comments could be entered into free text areas at the bottom of each web page. The common English version was then translated into the local languages: Italian, Norwegian, Basque, Spanish, Estonian and German. The final EFPQ enquired about the frequency of consumption of 116 foods and beverages during the previous 12 months. Portion sizes for most food items were graphically displayed with pictograms. Additional questions were asked about food preparation practices, specifications of several food items (e.g. preferred fat content, additions to hot beverages, such as dairy creamer) and the use of nutritional supplements and medications. Furthermore, summary questions about general consumption patterns and questions about body weight and body height were included. The web-based EFPQ is publicly available at https://nugo.dife.de/efbo/portal/en.
Statistical analysis
All analyses, unless otherwise specified, were performed after stratification by pilot study centre and ordered according to a geographical south–north gradient.
To investigate the feasibility and acceptance of the approach of combining instruments, overall and centre-specific numbers and percentages of responders, participants and non-participants were computed (response rate and participation rate). Participants were those who completed the EFPQ in order to take into account the initial adherence of subjects to the complete study protocol. Demographic and other lifestyle characteristics of participants and non-participants in each pilot study centre were displayed as arithmetic means and sd (for continuous variables) and frequencies (for categorical variables). Age at enrolment in the pilot study, sex and educational attainment were derived from the baseline assessment of each cohort and collected through standardized questionnaires(Reference Metspalu16–Reference Lund, Dumeaux and Braaten18). Pilot study enrolment was defined as the date of completion of the EFPQ for participants and date of invitation for non-participants. BMI (calculated as weight in kilograms divided by the square of height in metres (kg/m2), obtained from self-reported or measured data (according to study centre) and smoking status were obtained from the most recent data assessment of each cohort. The time interval varied between lifestyle data collection and pilot study by centre from 1 d (only for EGC, where recruitment was ongoing) to 6 years. Reasons for non-participation, percentage of completion of the 24-HDR and the administration modes of the EFPQ and the mean number of contact attempts for each 24-HDR were analysed. To specifically assess the practical feasibility of the web-EFPQ data from the evaluation questionnaire, further background data that were automatically recorded by the software when the website was used were also evaluated. Demographic and lifestyle characteristics of subjects with preferences for the web- or paper-EFPQ were displayed. Differences were tested for statistical significance with the unpaired differences t test (for continuous variables) and χ 2 test (for categorical variables). P values were two-sided and a significance level of P < 0·05 was applied. All analyses have been performed using the SAS statistical software package version 9·13 (SAS Institute, Cary, NC, USA).
Results
Of the 400 eligible participants, 350 responded (overall response rate: 87·5 %). The centre-specific response rate was 75·8 % in NOWAC (n 63), 83·8 % in EPIC-Florence (n 67), 87·5 % in EPIC-San Sebastian (n 70), 92·5 % in EPIC-Potsdam (n 74) and 95·0 % in EGC (n 76). Where reported on the response form, reasons for non-participation included no interest (n 16), not available, no time (n 24), health constraints (n 15) and other reasons such as no Internet (n 9); however, the majority of the non-participants did not state any reason. From the 350 respondents, 281 persons agreed to participate in the pilot study and completed the EFPQ, sixteen accepted initially but failed to complete the EFPQ and an additional four persons were subsequently excluded because of health constraints or non-compliance. Thus, 261 participants returned a completed EFPQ, resulting in a total participation rate of 65·3 %. The respective centre-specific participation rates were 62·5 % in EPIC-Florence (n 50), 68·8 % in EPIC-San Sebastian (n 55), 70·0 % in EPIC-Potsdam (n 56) and 87·5 % in EGC (n 70). In NOWAC, only 37·5 % (n 30) of the invited women (n 80) participated. Overall, participants had a mean age of 55·2 (sd 15·5) years (minimum age: 19·4 years, maximum age: 82·4 years). Demographic and selected lifestyle characteristics of participants and non-participants in the study centres are shown in Table 1. Except in EPIC-Potsdam and EGC, participants were younger than non-participants, but the difference was statistically significant only in EPIC-San Sebastian (P = 0·018) and NOWAC (P = 0·013). In addition, in all centres the proportion of higher-educated subjects was larger in participants than in non-participants (ranged from 10·9 % in EPIC-San Sebastian to 48·3 % in NOWAC), but this difference was not significant. With regard to BMI and smoking status, no consistent pattern across centres was observed: differences in mean BMI between participants and non-participants were only significant in EPIC-San Sebastian (P = 0·017) and a higher proportion of never-smokers among participants was seen in three of the five centres (except EPIC-Florence and EGC).
Table 1 Characteristics of study participants (P) and non-participants (NP), by study centre, IDAMES pilot study 2009 (n 400)

IDAMES, Innovative Dietary Assessment Methods in Epidemiological Studies and Public Health; EPIC, European Prospective Investigation into Cancer and Nutrition; EGC, Estonian Genome Center; NOWAC, Norwegian Women and Cancer study.
Data are number and percentage or mean and standard deviation.
*P values <0·05 for differences between P and NP.
†Number of participants for BMI calculation varies because of item non-response (EPIC-San Sebastian: forty-five P and fourteen NP; EPIC-Potsdam: fifty-five P and twenty-three NP; NOWAC: twenty-nine P and forty-eight NP).
‡Number of participants for smoking status varies because of item non-response (EPIC-Potsdam: fifty-three P and twenty-three NP).
Out of the 400 eligible subjects, 252 subjects completed only one recall (63·0 %), 241 completed two (60·3 %) and 225 three 24-HDR (56·3 %). In all pilot study centres, the completion of the 24-HDR was highest for the first 24-HDR and lowest for the third 24-HDR (Table 2). In EPIC-Florence and EGC, the first 24-HDR could be administered among all study participants, whereas in NOWAC only 86·7 % of the women were interviewed. Although completion of the 24-HDR decreased across the recall rounds, the assumption that contact attempts would increase could not be confirmed. The respective mean number of contact attempts was 3·9 (sd 0·3), 2·8 (sd 0·2) and 2·6 (sd 0·4) for the first, second and third 24-HDR, respectively, in EPIC-San Sebastian; 1·0 (sd 0·3), 1·1 (sd 0·2) and 1·1 (sd 0·3) in EPIC-Florence; 2·4 (sd 0·3), 2·9 (sd 0·2) and 3·6 (sd 0·3) in EPIC-Potsdam; 2·0 (sd 0·3), 1·9 (sd 0·2) and 1·9 (sd 0·3) in EGC and 3·2 (sd 0·5), 2·6 (sd 0·3) and 3·2 (sd 0·5) times in NOWAC.
Table 2 Completion of the three 24-HDR among all study participants and by study centre, IDAMES pilot study 2009 (n 261)
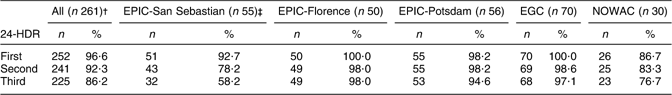
24-HDR, 24 h dietary recall; IDAMES, Innovative Dietary Assessment Methods in Epidemiological Studies and Public Health; EPIC, European Prospective Investigation into Cancer and Nutrition; EGC, Estonian Genome Center; NOWAC, Norwegian Women and Cancer study.
†Study participants were defined as having completed the European Food Propensity Questionnaire.
‡The rounds were not completed because of vacation period.
Of the 261 participants enrolled in the pilot study, 143 participants completed the web-EFPQ (54·8 %) and 118 completed the paper-EFPQ (45·2 %). Completion of either the paper-EFPQ or the web-EFPQ differed across centres (presented in Table 3). In EGC, 92·9 % of participants (mean age of 34·6 (sd 13·2) years) chose the web-EFPQ (n 65). In contrast, there was a preference for paper-EFPQ in EPIC-Potsdam and EPIC-San Sebastian, with 66·1 % (n 37) and 74·5 % (n 41) completion, respectively. German and Spanish participants were on average 65·6 (sd 6·9) and 61·9 (sd 6·5) years old. In EPIC-Florence, there was no clear preference for one administration mode. Except in EPIC-San Sebastian, web users were significantly younger and had attained a higher education in all centres. Overall, support for completing the web-EFPQ was requested by seven of the 143 participants, whereas ten of 118 persons needed help to answer the paper-EFPQ. The support was provided by telephone, directly in the study centre, or through a visit to the participant's home. In addition, three participants had to complete their web-EFPQ twice because of technical errors. Furthermore, an overall moderate subjective acceptability of the web-EFPQ was found in 109 of 143 web-EFPQ users who completed the evaluation questionnaire (EPIC-San Sebastian: n 9, EPIC-Florence: n 20, EPIC-Potsdam: n 9, EGC: n 61 and NOWAC: n 10). In all, 52·7 % rated both the clarity of questions and explanations and the visual elements (colours, font, font size, pictures, placing of the elements) and 68·8 % considered the user friendliness (clarity, navigation, logical structure) as very good or excellent. In addition, 34·1 % indicated having difficulties in reporting their usual food intake, particularly for the intake of fruit, vegetables and alcoholic beverages. The automatically recorded time to complete the web-EFPQ (median) ranged from 27·0 min (EGC) and 30·8 min (NOWAC) to 38·2 min (EPIC-Potsdam), 45·1 min (EPIC-Florence) and 56·5 min (EPIC-San Sebastian). Overall, the majority of participants (52·2 %) were logged in for 20–39 min. The percentage of subjects who completed the web-EFPQ within one login session varied from 22·2 % (EPIC-San Sebastian) and 36·8 % (EPIC-Potsdam) to 54·2 % (EPIC-Florence) and 66·7 % (NOWAC).
Table 3 Characteristics of web- and paper-EFPQ users, by study centre, IDAMES pilot study 2009 (n 261)

EFPQ, European Food Propensity Questionnaire; IDAMES, Innovative Dietary Assessment Methods in Epidemiological Studies and Public Health; EPIC, European Prospective Investigation into Cancer and Nutrition; EGC, Estonian Genome Center; NOWAC, Norwegian Women and Cancer study; web, web-based EFPQ; paper, paper-based EFPQ.
Data are number and percentage or mean and standard deviation.
*P values <0·05 for differences between web- and paper-EFPQ users.
†Number of participants for BMI calculation varies because of item non-response (EPIC-San Sebastian: n 45; EPIC-Potsdam: n 55; NOWAC: n 29).
‡Number of participants for smoking status varies because of item non-response (EPIC-Potsdam: n 53).
Discussion
The main objective of our pilot study was to investigate the feasibility of applying three 24-HDR interviews conducted by telephone in combination with a uniform, self-administered FPQ in different European countries, making use of Internet technologies. The overall participation rate of about two-thirds suggests that this approach is feasible in the study population and indicates that there is a potential for this approach to be applied in ongoing and new epidemiological studies.
Our findings are in line with one previous American study of combining different assessment instruments in which 60 % of subjects completed four telephone-administered 24-HDR and one paper-based frequency instrument(Reference Subar, Dodd and Guenther8). In the present pilot study, more than half of the eligible subjects completed all three 24-HDR and the EFPQ, whereas almost two-thirds had dietary data from at least two or one 24-HDR and the EFPQ. We found differences in the centre-specific participation rates. Although almost all cohorts share certain common features, such as recruitment of subjects from the general population and the targeted age range of 34–69 years, study designs are distinct. For example, in EPIC-Potsdam, the active 2-year internal follow-up included a second dietary assessment using an FFQ that was mailed to each study participant between 2002 and 2003(Reference Noethlings, Hoffmann and Bergmann19). In NOWAC, by contrast, follow-up of exposure information is carried out with approximately 5-year intervals using follow-up questionnaires with four pages asking for detailed information on dietary habits(Reference Lund, Dumeaux and Braaten18), whereas in EGC genes are the primary research topic instead of diet(Reference Metspalu16). Further details about other pilot study centres can be found elsewhere(Reference Jakszyn, Agudo and Berenguer20, Reference Pala, Sieri and Palli21). Hence, factors relative to diet that spur subjects to participate may vary across centres, e.g. personal interest in diet or desired weight loss. In our study, personal contact with study staff had also a marked influence on response rates, since the highest participation rate was observed in EGC, in which eligible pilot study participants were recruited during the baseline examination for the local study.
To prevent potential bias introduced by financial incentives or changes in usual dietary habits, our pilot study design implied no financial reward or personalized dietary information for participation, except in EPIC-Florence in which a financial reward was offered at the end of data collection that was not mentioned in any other phase of the study. The participation rate may be higher if some well-thought benefits (e.g. personalized feedback, gift card, etc.) are offered to participants(Reference Edwards, Roberts and Clarke22, Reference Hunt and White23). This issue needs consideration, particularly in terms of maintaining long-term compliance in studies with multiple and follow-up dietary assessments. In addition, ‘no time’ was the most reported reason for non-participation on the response form; this indicates that improved dietary assessment methods allowing more completion time flexibility could diminish the proportion of non-participants. In general, non-participation was not systematically associated with age, gender, BMI or smoking status; however, in all centres, the proportion of subjects with a higher educational attainment was higher in participants than in non-participants. Overall, the effect of non-participation in approaches of combining different dietary assessment instruments requires further examination in larger study populations.
The advanced statistical methods of combining dietary data from different instruments require repeated 24-HDR, at least two of every individual in the analytical study population, in order to obtain an estimate of intra-individual variance (J Haubrock, U Nöthlings, J-L Volatier et al., unpublished results)(Reference Kipnis, Shumakovich and Midthune11). A third 24-HDR seems to yield to an increased precision of the individual-level intake estimates, as indicated by unpublished research in a German study population (S Knueppel, personal communication). Similarly, a recent study comparing energy intake from seven 24-HDR with the doubly labelled water method pointed out that, although the first of three recalls was likely to underestimate dietary intake, more than three 24-HDR did not significantly improve the estimation of energy intake(Reference Ma, Olendzki and Pagoto24). Our study adds to the current discussion by showing the feasibility of obtaining dietary data from three short-term measurements from more than 50 % of a culturally diverse European sample. In all study centres, participation was high but decreased slightly with each further telephone interview; in EPIC-San Sebastian, in which the waves could not be completed because of the start of the national summer vacation period before the end of the study, the trend seems to be similar. Given that particularly the third recall appears to be an issue of concern in all centres, the incorporation of additional less-burdensome short-term instruments might be advantageous. In a recent validation study, about 80 % of the 235 enrolled subjects fully completed four measurements of a shorter, self-administered, web-based version of the DASH (Dietary Approaches to Stop Hypertension) questionnaire (DASH Online Questionnaire) that assesses dietary intake over the previous 24 h(Reference Apovian, Murphy and Cullum-Dugan25). By contrast, in another study in undergraduate students, imprecise individual dietary intake estimates were obtained by four measurements with a 1 d online Food Recall Checklist compared with dietary estimates provided by a 4 d food diary, although time efficiency of the web-based tool was shown(Reference Comrie, Masson and McNeill26). This suggests that a comprehensive 24-HDR computer-assisted interview, such as EPIC-SOFT, is still the primary instrument to gather accurate detailed and quantified dietary data, particularly for a standardized assessment. How new self-administered web-based automated 24-HDR programmes (e.g. the US Automated Self-Administered 24-Hour Dietary Recall (ASA24; National Cancer Institute, Bethesda, MD, USA)(Reference Subar, Thompson and Potischman27) or French MXS (Medical Expert Systems, Paris, France)(Reference Hercberg, Castetbon and Czernichow28)) will impact the feasibility of administering repeated recalls in large-scale epidemiological studies remains to be evaluated.
With respect to the feasibility of the web-EFPQ, it needs special mention that our study population was not selected on the basis of Internet access and experience. We approached participants of ongoing cohort studies who were used to classical instruments of data collection such as questionnaires or personal interviews on diet. Therefore, our figures cannot be directly compared with a situation in which study participants are recruited on the basis of Internet access/literacy. According to official European statistics from 2009, Internet access in households differed substantially between European countries, with an average of 65 %, reflecting a relatively high access in northern and central countries such as Norway (86 %), Estonia (63 %) and Germany (79 %), but a modest access in the south (e.g. Spain (54 %) and Italy (47 %))(Reference Lööf and Seybert29). Although not directly comparable to the present study because of the survey methodology, the present completion rates of the web-EFPQ in EPIC-San Sebastian, EGC and NOWAC may therefore partly reflect the country-specific situations regarding Internet penetration, but not for Germany and Italy. Similarly, a population-based study in Sweden – a country in which Internet access is estimated to be about 80 % – reported a lower response rate for a web-based questionnaire with optional questions about dietary habits than for the corresponding paper-and-pencil version(Reference Balter, Balter and Fondell30, Reference Ekman, Dickman and Klint31). Thus, further factors should be considered that may influence the response to online questionnaires, beyond the availability of an Internet access. In line with the officially reported highest educational disparities in the regular use of the Internet in the age group of 55–74 years(Reference Lööf and Seybert29), we found a larger proportion of subjects with a university degree among web-EFPQ users of all centres. In addition, unlike the Swedish study that did not identify differences in response rates by age(Reference Balter, Balter and Fondell30), the present findings showed that in all pilot study centres web-EFPQ users were younger than those who selected to complete the paper-EFPQ, in line with results from non-observational studies in middle-aged or elderly study populations(Reference Klovning, Sandvik and Hunskaar32, Reference Verheijden, Jans and Hildebrandt33). Age is also likely to contribute to between-centre differences in selecting the web-EFPQ, since study participants from the EPIC cohorts were, on average, older than subjects participating in NOWAC and EGC and may be somehow conditioned to self-report using paper-and-pencil questionnaires about diet. It therefore poses a major challenge to develop and apply easy-to-use and understandable technically advanced tools, tailored to the population under study, in order to reduce selection bias. On the basis of the moderate results of the evaluation questionnaire, we hypothesize further that the current design of the EFPQ might need adaptations in order to be more easily answered by elderly or less-educated subjects. In Native Americans, for example, a simplified design, introductory and HELP screens as well as audio features were advantageous to self-complete a computerized version of the Dietary History Questionnaire(Reference Edwards, Slattery and Murtaugh34). Nevertheless, the self-administered web-EFPQ had the advantage of being able to obtain complete information in a geographically dispersed European study population without questions being skipped, in a reasonable amount of time and with good compatibility with different screen configurations, operating systems and browsers. In addition, the organizational constraints and costs in performing a multi-centre study appeared to be reduced because of the direct and secure electronic data transfer to a central database, automatic control for missing and implausible data and facilitated self-management of the local dietary assessment, consistent with findings from other research(Reference Ekman, Dickman and Klint31).
The strength of the present pilot study is that it is the first study investigating the feasibility of combining multiple dietary assessment instruments, including Internet technologies, in a well-characterized multi-centric diverse European study sample, suitable to investigate the feasibility of the approach within a broader perspective. However, major limitations are the limited generalizability and statistical power. All cohorts were not designed to be representative of their countries, although NOWAC is characterized by a high external validity(Reference Lund, Kumle and Braaten35). Indeed, EPIC-San Sebastian included members of local blood donor associations and employees of selected enterprises; women participating in EPIC-Florence were invited for a local population-based breast cancer screening programme; and EPIC-Potsdam study participants were of higher socio-economic status and were healthier than the general German population(Reference Riboli, Hunt and Slimani17, Reference Boeing, Korfmann and Bergmann36). Consequently, extrapolation of the present study to general populations should be made with caution. Furthermore, the small sample size may imply that the compliance and ability to complete the EFPQ and the three 24-HDR might be higher in those volunteers who decided to participate in the pilot study than in all the source cohorts, despite the random selection procedure. These aspects could have led to artefactual between-centre differences regarding the feasibility of the approach. Compared with the original cohorts, participation rates in the present study were substantially higher (e.g. EPIC-Potsdam originally 22·7 %(Reference Boeing, Korfmann and Bergmann36), NOWAC originally 57·5 %)(Reference Lund, Kumle and Braaten35). One could infer from the results that this approach might solely be feasible for established cohorts, but the high participation rate in EGC indicates the potential of the approach for newer studies.
In conclusion, the present pilot study design and features of participation showed that the combination of dietary assessment instruments is a feasible approach for assessing diet in different European study populations. For the target age group, it was necessary to offer subjects a paper and an online version of the long-term instrument in order to avoid selection bias.
Acknowledgements
The present pilot study was funded by a grant issued by the European Union (no. 2006315 ‘IDAMES’) to explore new avenues for dietary assessment in epidemiological studies and public health (www.idames.eu). None of the authors had a conflict of interest. No supplementary online material has been submitted. A.-K.I., U.H., G.T., S.S., P.A., T.K., D.E., M.B. and H.B. designed the research; A.-K.I., U.H., G.T., S.S., E.B., P.A., T.K., D.E. and M.B. conducted the research; H.W. and N.S. provided essential materials; A.-K.I. and U.H. performed the statistical analysis; A.-K.I., H.W., M.B., G.T., S.S., D.E., T.K., P.A. and H.B. wrote the paper; H.B. had primary responsibility for the final content; D.P., A.M., E.L. and K.W. were members of the IDAMES project involved in the overall research plan. All authors read and approved the final manuscript. The authors thank all the study participants. They also thank everyone who participated actively in the development of the EFPQ and in the field work of the pilot study, as well as those in charge of each centre's technical assistance: Herbert Piechot, Ellen Kohlsdorf, Elektra Polychrondidou (Germany), Paolo Aretini (Italy).





