Fortification of flour with Fe and folic acid is a strategy adopted by many countries to reduce Fe and folic acid deficiencies. Wheat flour fortification programmes have been adopted or are in the planning stages in seventy-eight countries( Reference Ranum and Wesley 1 ). In Brazil, mandatory fortification of wheat and maize flour began in 2004 with 150 µg of folic acid and 4·2 mg of Fe per 100 g of flour( 2 ). However, nearly 10 years after flour fortification started, there is a lack of evaluation nationwide on the impact of fortified products on the intakes of Fe and folic acid. There are a few studies, but they are restricted to the state capital cities of Brazil( Reference Marchioni, Verly-Jr and Steluti 3 , Reference Ferreira and Giugliani 4 ).
Although folic acid is readily distinguishable from food folate – the former is found only in supplements and fortified products, while the latter occurs naturally in leafy green vegetables, legumes, citrus fruits, liver and other meats( 5 ) – Fe from fortification cannot be distinguished from Fe from food. On the other hand, fortified flours include both Fe and folic acid, and Fe from fortification can be estimated using available data of folic acid intake.
Although large surveys, including blood collection, are required to assess the impact of food fortification programmes on anaemia, estimates of the amount of fortified products being consumed is an alternative first step to evaluate fortification programmes, as shown in the present communication.
Materials and methods
Study population
The study analysed data from the first National Dietary Survey (NDS), which was conducted along with the Household Budget Survey (HBS) 2008–2009 carried out by the Brazilian Institute of Geography and Statistics.
Briefly, HBS 2008–2009 adopted a two-stage cluster sampling. In the first stage, primary sampling units were selected by systematic sampling with probability proportional to the number of households according to the 2000 Census. For the systematic sampling, primary sampling units were stratified according to main geographical areas, urban or rural situation and the mean income of heads of households. The units sampled in the second stage of selection were the permanent households selected by simple random sampling without replacement within each sector. Primary sampling units were evaluated throughout 12 months of research in all strata of the research( 6 ).
In the 2008–2009 HBS, 68 373 households were selected. A sub-sample for the NDS was calculated at 25 % of the households in the HBS 2008–2009 sample and was organized so that one in every four households in each primary sampling unit was selected. In the data collection stage, 16 764 households (24·5 %) were sampled. There were 38 340 residents aged 10 years and above, in 13 569 households, who responded to the research, with a non-response rate of 19 %. A total of 34 032 individuals completed the data on food and/or drink intake (11 % non-response rate).
Details on sampling and data collection are available in elsewhere( Reference Barbosa, Brito and Junger 7 , Reference Bezerra, Souza and Pereira 8 ). The current analysis included data on food consumption of 15 700 men, 17 192 women (non-pregnant and non-lactating women) aged 10 to >60 years, and 328 pregnant and 783 lactating women aged 19–59 years.
Assessment of food intake
Dietary intake was collected from two non-consecutive food records, in which the individual recorded all foods and beverages consumed, including the time of intake, quantities consumed in portion sizes and preparation form. In order to guide adequate recording, each participant received instructional material with guidance on filling in the food record and photographs of utensils commonly used to serve the foods and drinks. When the interviewee was unable to complete the food records, or when the interviewee was illiterate, someone indicated by the interviewee completed the record.
At home, interviewers reviewed the records and entered the data into software that was developed specifically for the NDS. The database comprised approximately 1500 items (foods and beverages). In the analysis of food folate and folic acid, the Nutrient Data System for Research (NSDR) was used( 9 ). The nutrient compositions and portion sizes, which were specifically compiled for the analysis of foods and preparations cited on the HBS 2008–2009, were selected from the 5686 items registered in the food and drink database from the HBS 2002–2003.
When verifying the reliability of the data, twenty-nine individuals reporting fewer than five items whose energy consumption seemed unlikely were excluded. In addition, quantities considered to be unlikely were entered using the hot deck imputation procedure and this information was registered in the database( 6 ).
Details of the pre-test, training and validation of the data collection can be found elsewhere( Reference Barbosa, Brito and Junger 7 ).
Data analysis
The distribution of intake based on only two days is subject to day-to-day fluctuations (within-person variation). The method of the National Cancer Institute( Reference Tooze, Midthune and Dodd 10 ) was used to account for the within-person variance, allowing estimation of the usual dietary intake. This method consists of a two-part non-linear mixed model. The first part of the model estimates the probability of consumption using logistic regression, adjusting for the person-specific random effect. These probabilities were then used to calculate the intake percentiles of Fe and folic acid, since it was assumed that they are ubiquitously consumed. Analyses were performed using the statistical software package SAS version 9·1.
The mean intake of folic acid by sex and age groups was used to estimate the per capita intake of wheat and maize flour, since flour is distributed in many preparations, using the amount of folic acid proposed for mandatory fortification by the Ministry of Health of Brazil (150 µg of folic acid per 100 g of flour).
Based on the amount of flour consumed and since fortified flour includes both folic acid and Fe, we calculated the intake of Fe from fortification, using the value proposed by mandatory fortification (4·2 mg of Fe per 100 g of flour).
Fe intake from food was obtained through the difference between total Fe intake calculated by the National Cancer Institute method and Fe intake from fortification.
Finally, the absorption of folic acid, food folate, Fe from food and Fe from fortification was calculated considering 85 % absorption for folic acid, 50 % for natural folate and 15 % for Fe naturally present in foods( 5 , 11 ). For pregnant women, the value of absorption used for Fe from food was 25 %( 5 ).
Fe fortification can be done by using different Fe compounds( 2 ), although electrolytic Fe is the most commonly used including in Brazil. Few studies measured have absorption of electrolytic Fe( Reference Motzok, Verma and Chen 12 ). In a clinical trial( Reference Motzok, Verma and Chen 12 , Reference Forbes, Adams and Arnaud 13 ), electrolytic Fe absorption from the diet ranged from 3·4 % to 8·0 % (with ascorbic acid) and that of ferrous sulfate was 4·5 %. Thus, we adopted the value of 5 % as that for absorption of electrolytic Fe from the diet, based on absorption of restricted vegetarian diets( 11 ).
Ethics statement
The research protocol was approved by the Ethics Committee of the Medicine Social Institute of the State University of Rio de Janeiro (CAAE 0011.0.259.000-11).
Results
Mean intake, absorption of nutrients and estimated consumption of flour in men and women by age are shown in Tables 1 and 2.
Table 1 Mean intake, absorption of nutrients and estimated consumption of flour by age group per day among Brazilian women, first National Dietary Survey, 2008–2009
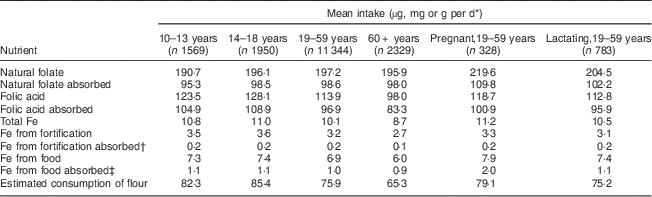
* Units used are micrograms for folic acid and folate, milligrams for Fe and grams for flour.
† 5 % absorption.
‡ 15 % absorption.
Table 2 Mean intake, absorption of nutrients and estimated consumption of flour by age group per day among Brazilian men, first National Dietary Survey, 2008–2009
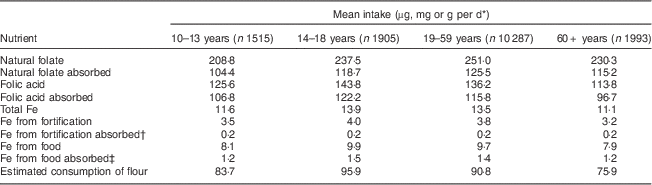
* Units used are micrograms for folic acid and folate, milligrams for Fe and grams for flour.
† 5 % absorption.
‡ 15 % absorption.
Mean intake and absorption of Fe from fortification (about 0·2 mg/d) is low in both men and women. This is due to the most frequently used Fe compound in flour fortification in Brazil (electrolytic Fe) and because the estimated flour consumption in Brazil is lower than planned when designing the fortification policy.
Discussion
We presented an inexpensive strategy to evaluate food fortification programmes. The strategy is simple, possible to be replicated in other contexts and allows evaluation of the quantities of Fe, folic acid and fortified flour consumed.
Our results show that the impact of fortified products in Brazil is small in relation to Fe intake, due to problems in the fortification planning process. It was planned that the intake of 100 g of fortified flour should provide at least 30 % of the RDA of Fe( 14 ). However, the reference to be used for planning the adequacy of estimated nutrient intakes of groups should be the Estimated Average Requirement (EAR). By using the RDA in assessing the diets of groups, the nutrient requirements will be overestimated. The EAR values were established by estimating the amount of Fe that should be ingested assuming absorption of 18 % (absorption for adults from the typical North American diet)( 11 ). It would be necessary to consume a greater amount of flour fortified with electrolytic Fe due to its low absorption (5 %)( 15 ). However, the high prevalence of overweight and obesity in Brazil and worldwide( 6 ) does not allow us to encourage an increased consumption of flour. A better alternative would be to use a form of Fe with higher absorption.
Brazilians’ intake of flour (about 80 g/d, according to the HBS 2008–2009) is somewhat lower than that planned by the programme (100 g/d). However, if the sub-report of about 20 % in this survey is taken in account( 6 ), the target amount of flour would almost be achieved. Although actual flour consumption is close to what was first planned, possible options to improve the impact of the fortification programme would be either to change the Fe compound that is used, as previously mentioned, or to choose another food vehicle for fortification. As the amount of flour consumed in Brazil is less than 150 g/d, electrolytic Fe seems not to be the most appropriate Fe compound to be used, because the high fortification levels required may cause sensory changes( Reference Hurrell, Ranum and de Pee 16 ). Ferrous sulfate is the most bioavailable Fe source, but it still has low absorption. Due to the sensory effects in many food vehicles that it produces( Reference Hurrell, Bothwell and Cook 17 ), it is usually less used( Reference Hurrell 18 ).
Fe bioavailability, which is a key factor in fortification programmes, depends on the nature and level of the Fe compound added to the food, the amount of fortified foods consumed, the Fe status of the consumer, and the presence of inhibitors and enhancers of Fe absorption in the overall diet( 11 ). The Brazilian diet does not favour the absorption of Fe since the intake of vitamin C is quite low( Reference Araujo, Bezerra and Barbosa 19 – Reference Fisberg, Marchioni and Castro 21 ).
It is known that the Fe content of the body is highly conserved. Adult men need to absorb only about 1 mg/d to maintain Fe balance. The average requirement for menstruating women is somewhat higher, approximately 1·5 mg/d, and for pregnant women, 4–5 mg/d is necessary to preserve Fe balance( 11 ). The contribution of Fe from fortification is about 0·2 mg/d in all groups, corresponding to about 20 % of the daily amount required for men, 13·3 % for menstruating women and 5 % for pregnant women. As seen in the results, most of the absorbable Fe comes from Fe present in foods, confirming the small contribution of Fe from fortification.
As another problem found in the analysis of the fortification programme, we highlight that many food manufacturers do not follow the amount of nutrients recommended by mandatory fortification in Brazil. It has been found that 51 % of flour samples contain less than 150 µg of folic acid per 100 g( Reference Alaburda, Almeida and Shundo 22 ). Another study found that the variation of folic acid in flour was from 73 to 558 µg/100 g and of Fe was 3·8–8·7 mg/100 g( Reference Soeiro, Boen and Wagner 23 ). Data from the Brazilian National Health Surveillance Agency showed that 87 % of maize flour samples had Fe content lower than that established by mandatory fortification( 24 ). Thus, strict control over food industries is also necessary to ensure that they follow the established fortification range.
The limitations of the present study are related to the cross-sectional design and are the same as those of any study based on reported data on consumption; in particular, under-reporting of intake. There is no reason to believe that under-reporting could be different between the groups evaluated. Also, there are not any biomarkers of folic acid and Fe intake capable of estimating what the under-reporting in the population might be. Even with a high percentage of under-reporting, the absolute value would not be significant for the study’s conclusions, given the population’s low dietary folate and Fe intakes.
Conclusion
In conclusion, the proposed strategy to estimate Fe from fortification programmes indicates that the intake of Fe from fortified flour observed in Brazil does not justify the current use of electrolytic Fe as the mandatory flour fortificant. Thus, it appears to be necessary to reassess the mandatory flour fortification programme in Brazil, in order to adjust the programme to achieve better outcomes in terms of anaemia prevention.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: Q.d.S. and R.S. performed the statistical analyses, interpreted the data and wrote the paper; E.V.J. and E.A.F.N. performed the interpretation of the data and wrote the paper. All authors actively participated in the manuscript preparation and they all read and approved the final version of the manuscript. Ethics of human subject participation: The research protocol was approved by the Ethics Committee of the Medicine Social Institute of the State University of Rio de Janeiro (CAAE 0011.0.259.000-11).




